2017 Volume 40 Issue 10 Pages 1724-1729
2017 Volume 40 Issue 10 Pages 1724-1729
Aging leads to functional changes in the brain and decreases ability of learning and memory. Neurite outgrowth is important in learning and memory, therefore regulation of neurite outgrowth might be a candidate for treating aged brain. Echinocystic acid (EA), a pentacyclic triterpene, has shown to exert various neurological effects. However, the effect of EA on neurite outgrowth has not been studied. In this study, we examined if EA is effective on neurite outgrowth and memory in aged mice. The effect of EA on neurite outgrowth was observed by examining neurite processes of Neuro2a cells treated with EA. Western blot analysis was conducted to examine possible mechanisms. Morris water maze test was used to examine the effect of EA on learning and memory in aged mice. Immunohistochemistry was conducted to observe the effect of EA on neurite outgrowth in the hippocampus. EA was shown to induce neurite outgrowth in a concentration dependent manner without affecting cell viability. Moreover, EA treatment increased phosphorylation of c-jun N-terminal kinase (JNK) and JNK inhibitor, SP600125, blocked the effect of EA on neurite outgrowth. These results demonstrated that EA treatment promotes neurite outgrowth through the JNK signaling pathway. In in vivo experiments, EA treatment increased neurite outgrowth in aged mouse hippocampus. Moreover, EA treatment enhanced spatial learning and memory in aged mice. These results suggest that EA can be developed as a new, naturally occurring drug to treat ageing-related neurological diseases.
The ability to remember certain types of information declines with aging.1,2) Aging leads to functional changes in the hippocampus, which is an important brain region for spatial learning and memory.3) On the synaptic level, synaptic size, strength, and plasticity decline with aging.4,5) Moreover, hippocampal neurogenesis is reduced in the aged brain.6,7) Several studies have reported hippocampal atrophy in elderly humans.8,9) These studies suggest that morphological changes lead to functional changes in aged brain.
Neurite outgrowth connects neurons to one another and plays an essential role during neuronal differentiation.10) Neurite outgrowth is important when repairing nerve system damage due to nerve injury or neurological disorders11); it is regulated by various signaling pathways and supported by several extracellular matrix proteins, including collagen, laminin, and fibronectin.12,13) Neurotrophic factors stimulate neurite outgrowth, and neurite outgrowth is regulated through pathways such as the extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase, and phosphatidylinositol 3-kinase (PI3K),14) all of which are associated with neuronal survival.15) Recent finding demonstrated age-related impairment in neurite outgrowth,16) suggesting that regulation of neurite outgrowth might be a target for treating aging-induced neurological dysfunctions.
Echinocystic acid (EA) is a pentacyclic triterpene. Recent studies revealed that EA isolated form Codonopsis lanceolata showed various neurological effects including anti-amnesic effect and anti-depressant effect.17,18) Moreover, EA has been shown to have several positive effects such as anti-inflammation, antiviral, anticancer, and cardioprotection.19–22) These results suggest that EA can penetrate blood brain barrier and can do positive effects to the brain function. However, the effect of EA on neurite outgrowth and aged brain function has not been studied. Therefore, in this study, we investigated the effects and mechanisms of EA on neurite outgrowth in mouse neuroblastoma Neuro2a cells and tested EA on spatial learning and memory in aged mice.
Echinocystic acid and retinoic acid (RA) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). SP600125, which was stocked in dimethyl sulfoxide (DMSO) (100 mM), was purchased from Tocris Bioscience (Ellisville, MO, U.S.A.). Primary antibodies were purchased from Cell Signaling Technology (Danvers, Mass, U.S.A.). BCA protein assay kit was purchased from Thermo (Rockford, IL, U.S.A.).
Cell CultureNeuroblastoma Neuro2a cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; WelGENE Co., Daegu, Korea) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin antibiotic (PSA) and grown in a 5% CO2 humidified incubator at 37°C. DMEM with 10% FBS was changed with DMEM supplemented with 1–2% FBS for differentiation. DMEM and PSA were purchased from WelGENE Co.
Cell Viability AssayTo determine cell viability, EZ-cytox cell viability assay kit (DaeilL Abservice, Seoul, Korea) was used. Neuro2a cells were plated in 24-well culture plates (3×104 cells/well) and grown for 24 h. The cells were then treated with EA (0–25 µM). After 6 or 12 h incubation, cells were incubated with EZ-cytox solution for 2 h at 37°C. The formazan salt dissolved in the cell media incubated with EZ-cytox solution was determined by measuring the optical density at 450 nm using an enzyme-linked immunosorbent assay (ELISA) plate reader (Bio-Rad, Hercules, CA, U.S.A.). Cell viability was shown relative to the control in a graph.
Measurement of Neurite OutgrowthNeuro2a cells were captured and counted under a phase contrast microscope (magnification of 200×). Neurite-bearing cells containing neuritic processes longer than cell body were scored from 3–5 random fields of cells. The neurite length of each cell was measured by Image J software. Each well was evaluated using four different fields under a microscope. The average neurite length and the number of neurite of nearly 200 cells are presented as the mean±standard error of the mean (S.E.M.)
Protein Extraction and Western Blot AnalysisCells treated with EA were lysed using M-PER buffer (Thermo, Rockford, IL, U.S.A.), a containing protease inhibitor, and phosphatase inhibitor cocktail (Thermo). Proteins from whole-cell lysates were quantified using a BCA protein assay kit according to the manufacturer’s instructions. Each signal was quantified by image analysis software (ImageJ; National Institute of Mental Health, Bethesda, MD, U.S.A.).
AnimalsMale CD-1 mice (26–30 g, 6 weeks old) were purchased from the Orient Co., Ltd., a branch of the Charles River Laboratories (Gyeonggi-do, Korea), and maintained in the University Animal Care Unit for 17 months. All experimental protocols were approved by the Institutional Animal Care and Use Committee. The mice, housed five per cage, were provided food and water ad libitum and maintained on a 12 h light/dark cycle (the light was on from a.m. 07:30–p.m. 7:30) at a constant temperature (23±1°C) and relative humidity (60±10%). Animal treatment and maintenance were conducted in accordance with the Animal Care and Use Guidelines issued by Kyung Hee University, Korea. A total of 20 mice were used.
Drug AdministrationTo determine the effects of EA on cognitive performance, EA (10 mg/kg, per os (p.o.)) was administered to the mice for 3 d. The mice in the control group received the same volume of vehicle (10% Tween 80 solution). One hour after the final drug administration, animals were tested in the Morris water maze test.
Morris Water Maze TestThe Morris water maze, which is used for evaluation of hippocampal-dependent spatial learning and memory,23,24) is a circular pool with a featureless inner surface with a diameter of 90 cm and height of 45 cm. The pool was filled to a depth of 30 cm with water (24±1°C) containing 500 mL of black pigment. The tank was placed in a dimly lit, soundproof test room containing four visual cues. A black platform (6 cm in diameter and 29 cm in height) was placed in one of the pool quadrants. The trials were composed of a swimming training on day one, training trials on days 2–5, and a subsequent probe trial on day 6. Swimming training took place for 60 s in the absence of the platform. The mice were then given two trials per day for five days with the platform in place. When a mouse located the platform, it was permitted to remain on it for 10 s. If the mouse did not locate the platform within 60 s, it was placed on the platform for 10 s. The time interval between each trial per session was 30 min (Paxinos and Franklin, 2001). During each trial session, the time required (latency time) and the distance traveled to find the hidden platform were recorded using a video camera-based Ethovision System (Nodulus, The Netherlands). On day 6, mice were subjected to a probe trial session in which the platform was removed from the pool, and allowed to swim for 60 s to search for it. The swim time in the pool quadrant where the platform had previously been placed (the target zone) was recorded.
Tissue PreparationImmediately after probe trial of Morris water maze test, the mice were anesthetized using a mixture of N2O and O2 (70 : 30) containing 2% isoflurane. The animals were perfused transcardially with phosphate buffer (50 mM, pH 7.4) followed by ice-cold 4% paraformaldehyde for prefixation. The brains were removed and post-fixed overnight in phosphate buffer (50 mM, pH 7.4) containing 4% paraformaldehyde. The samples were then immersed in a 30% sucrose solution (in 50 mM phosphate buffered saline (PBS)) and stored at 4°C until sectioning. The frozen brains were sectioned in the coronal plane (30 µm) using a cryostat (Leica, Nussloch, Germany) and maintained in a storage solution (300 mL of ethylene glycol, 250 mL of glycerol, 450 mL of phosphate buffer) at 4°C.
ImmunohistochemistryFive sections, at 10-section intervals (300 µm), were selected throughout the hippocampus from −1.50 mm posterior to bregma as defined in the mouse brain atlas.25) For doublecortin (DCX) immunostaining, the sections were incubated with a blocking solution (5% normal rabbit serum in 0.3% Triton X-100 PBS) for 2 h and then incubated with goat anti-DCX antibody (1 : 1000) overnight at room temperature. After 3 times of 5-min period washing steps, the sections were then incubated in secondary antibody solution containing biotinylated rabbit anti-goat immunoglobulin G (IgG) (1 : 200 dilution, Vector, Burlingame, CA, U.S.A.) followed by ABC complex (1 : 100 dilution, Vector) for 1 h at room temperature, and reacted with 0.02% 3,3′-diaminobenzidine and 0.01% H2O2 for about 3 min. After finishing every steps, slices were mounted on gelatin-coated slides, dehydrated in an ascending alcohol series, and cleared in xylene. The length of dendrite of DCX-positive cells in the DG region was measured with ImageJ software (freeware from the NIH).
Statistical AnalysisData comparisons between groups were performed by one-way ANOVA with Tukey’s multiple comparisons test. In in vivo experiments, Student’s t-test was conducted. Data from Morris water maze test was analyzed with two-way ANOVA with Bonferroni’s post hoc test. Statistical significance set at p<0.05. Data are expressed as means±S.E.M.
To investigate the influences of EA (Fig. 1) on neurite outgrowth, Neuro2a cells were treated with EA in a concentration-dependent manner (0, 5, 10, 15 and 20 µM) for 6 h. RA (15 µM) has been used as control stimuli of neuronal differentiation in cells. As shown in Fig. 2A, morphological changes were observed by phase contrast microscope (magnification of 200×). Cells bearing neurites were captured from 3 random fields of the treated cells and neurite-bearing cell was determined if dendrite was at least longer than the cell body. EA promoted neurite outgrowth in a concentration-dependent manner (neurite-bearing cell: F5, 12=15.52, p<0.05, n=3/group, Fig. 2B, length of neurite: F5, 12=25.98, p<0.05, n=3/group, Fig. 2C, the number of neurite: F5, 12=17.24, p<0.05, n=3/group, Fig. 2D). Next, to examine the effects of EA on cell cytotoxicity, we determined cell viability using EZ-Cytox cell viability assay kit. EA (0, 5, 10, 15, 20, 25 µM) has no influence on cell viability at the designated incubation time (6 h: F5, 12=0.8491, p>0.05, n=3/group; 12 h: F5, 18=1.143, p>0.05, n=4/group, Fig. 3). This result suggests that EA has no cytotoxic effect in Neuro2a cells and the effect of EA on neurite outgrowth is not due to its effect on cell viability.

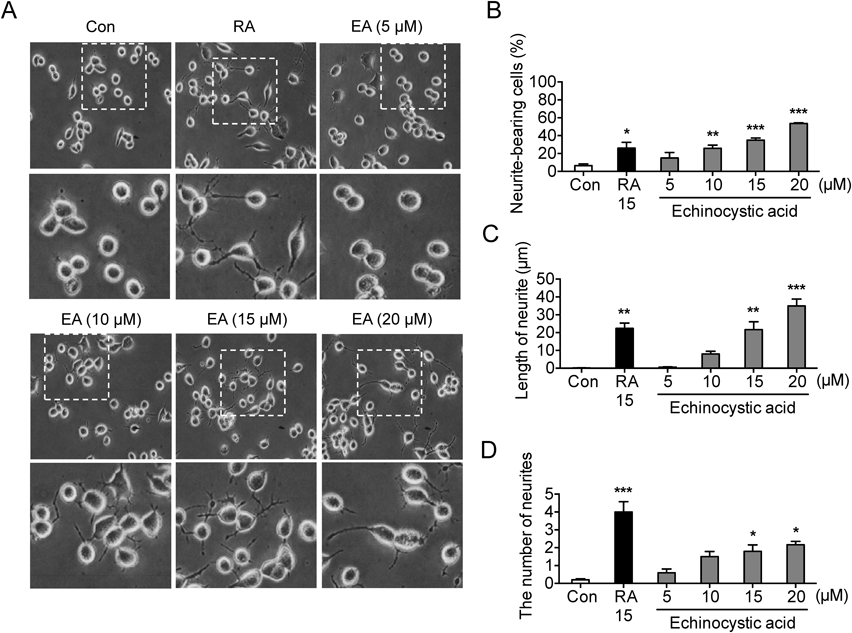
Neuro2a cells were treated with 15 µM of RA or various concentrations of EA (5, 10, 15, 20 µM) for 6 h. Morphological images of cells were observed by phase contrast microscope (magnification of 200×) (A) and then neurite-bearing cells were counted (B). (C) Length of neurite. (D) The number of neurite. Data are presented as the mean±S.E.M. * p<0.05, ** p<0.01 and *** p<0.001 compared to control. # p<0.05 compared to RA treated-group. EA, echinocystic acid. RA, retinoic acid.

Effects of EA on cell viabilities in Neuro2a cells. Neuro2a cells were treated with EA in a concentration-dependent manner for different incubation time (6 and 12 h). Cell viabilities were measured using EZ-Cytox cell viability assay kit. Data are presented as the mean±S.E.M. EA, echinocystic acid. RA, retinoic acid.
Akt-GSK-3β and JNK signaling pathways are involved in neurite outgrowth.10,26) To investigate whether PI3K-Akt-3β and/or JNK signaling pathways underlie EA-induced neuronal differentiation, a Western blot assay was performed in a time-dependent manner using protein extracts of EA (20 µM)-treated cells. Phosphorylation of JNK was significantly increased (F4, 10=3.605, p<0.05, n=3/group, Figs. 4A, B) by EA treatment for 1–3 h. The level of phosphorylation of JNK at 6 h was reduced compared to 3 h but was higher than control levels. The Akt-GSK-3β pathway was not affected by EA treatment (Akt: F4, 10=0.1721, p<0.05, n=3/group; GSK-3β: F4, 10=0.4088, p<0.05, n=3/group, Figs. 4A, B). These results suggested that EA treatment promotes JNK phosphorylation in Neuro2a cells and this may be the mechanism of EA-induced promotion of neurite outgrowth. To test whether activation of JNK signaling is a mechanism of the effect of EA on neurite outgrowth, we conducted blocking study with JNK inhibitor, SP600125. As shown in Figs. 4C–F, EA (20 µM) promoted neurite outgrowth and SP600125 (10 µM) completely the effect of EA (neurite bearing cells: F3, 8=9.107, p<0.05, n=3/group, Fig. 4D; length of neurite: F3, 8=54.12, p<0.05, n=3/group, Fig. 4E; the number of neurites: F3, 8=18.36, p<0.05, n=3/group, Fig. 4F). This result suggests that EA facilitates neurite outgrowth through activation of JNK signaling.

Neuro2a cells were treated with 20 µM of EA in time-dependent manner and then protein extracts were prepared for Western blot analysis. (A) Western blot analysis was performed with specific antibodies against phospho-JNK, JNK, phospho-Akt, Akt, phospho-GSK-3β and GSK-3β. (B) Quantitative analysis of Western blot data. Data are presented as the mean±S.E.M. * p<0.05 vs. 0 h. (C, D) JNK inhibition blocks facilitating effect of EA on neurite outgrowth. Neuro2a cells were treated with EA (20 µM) and/or SP600125 (10 µM) for 6 h. Morphological images of cells were observed by phase contrast microscope (C) (magnification of 200×) and then neurite-bearing cells (D), length of neurites (E) and the number of neurites (F) were counted. Data are presented as the mean±S.E.M. * p<0.05. EA, echinocystic acid. SP, SP600125.
The effect of EA (10 mg/kg/d for 3 d, p.o.) on spatial learning and memory of aged mice was evaluated using the Morris water maze task. As shown in Fig. 5A, EA-treated group showed shorter escape latency compared to the vehicle-treated group (two way ANOVA: day, F3, 72=6.537, p<0.05; group, F1, 72=7.280, p<0.05; interaction, F3, 72=1.609, p>0.05, n=10/group, Fig. 5A) without affecting swimming speed (two way ANOVA: day, F3, 72=0.1075, p>0.05; group, F1, 72=0.01695, p>0.05; interaction, F3, 72=0.1493, p>0.05, n=10/group, Fig. 5B). Moreover, during the probe trial session, the EA-treated group spent significantly longer time in the target zone (t18=2.023, p<0.05, n=10/group, Fig. 5C) and crossed the platform zone significantly more times (t18=1.883, p<0.05, n=10/group, Fig. 5D) compared to vehicle-treated group. These results suggest that EA enhances spatial learning and memory in aged mice. To determine whether EA also increases neurite outgrowth in the hippocampus, we measured the length of neurites of DCX-positive cells in the subgranular zone of the DG. Neurite length in DCX-positive cells in EA-treated group was significantly longer compared to that of vehicle-treated group (t8=2.332, p<0.05, n=5/group, Figs. 5E, F). These results suggest that EA promotes neurite outgrowth in aged mice hippocampus.

To test the effects of EA (10 mg/kg/d for 3 d, p.o.) on spatial memory, Morris water maze testing was conducted. During training trials for 4 d, escape latency (A) and swimming speed (B) were measured. In the probe trial, swimming time in the target quadrant where the platform was located (C) and the number of crossing the platform zone (D) were measured. (E, F) EA increased length of neurite processes of DCX-positive cells. (E) Representative of microphotographs of DCX-positive cells in the hippocampal DG region (bar=200 µm). (F) Length of dendrite of DCX-positive cells. Data are presented as the mean±S.E.M. n=10/group. * p<0.05. EA, echinocystic acid.
In this study, we found that EA concentration-dependently promoted neurite outgrowth. In addition, EA had no severe cell toxicity by 25 µM, the highest concentration investigated. EA treatment also enhanced spatial learning and memory in aged mice. Moreover, EA promoted neurite outgrowth in aged mouse hippocampus. These results suggest that EA has positive effect to aged brain function and this might be due to its neurite outgrowth effect.
EA has been reported to have various functional effects. EA inhibits pro-inflammatory cytokines, tumor necrosis factor (TNF)-α, inducible nitric oxide synthase, cyclooxygenase-2, and nuclear factor-kappaB (NF-κB) activation in lipopolysaccharide-stimulated alveolar macrophages19); additionally, it has an inhibitory effect on ear inflammation induced by 12-O-tetra decanoylphorbol-13-acetate in mice.27) EA induces apoptosis in human promyelocytic leukemia cells.20,28) Further, EA has been reported to produce anti-human immunodeficiency virus (HIV) an antifungal activities29) and cardioprotective effects.21) Recent studies reported that EA has neurological effects including antidepressive and anti-amnesic effects.17,18) These studies suggest that EA may be beneficial various age-related brain deficits. Accordingly, in the present study, we found that EA improved spatial memory in aged mice.
Neurite outgrowth is regulated by growth factors, cytokines, transcription factors and membrane-bound receptor30) and is mediated by a number of mechanisms. Mitogen-activated protein kinase (MAPK) and Akt signaling are crucial for neurite outgrowth and are activated by natural compounds,31,32) and are associated with neural protective effects after drug-induced injury.33) JNK mediates not only cell survival.15) JNK is induced during neuronal differentiation in the developing brain in PC 12 cells, as well as in other neuronal cell lines.34) In Neuro2a cells, JNK activation leads to the activation of stat3 and neurite outgrowth.35) JNK activity has been shown to increase at early times, returns to basal levels, and then increase again later.34) In current study, a similar phenomenon was observed with EA treatment and JNK inhibition blocked the effect of EA on neurite outgrowth; this finding suggests that EA induces neurite outgrowth through JNK pathway.
Age attacks brain functions through various mechanisms including oxidative stress. Decrease of the branches and length of immature neuronal dendrites in the hippocampus is one of age-related phenomenon, which can be restored by drugs.36) Recent researches revealed that exercise improves learning and memory in senescence-accelerated mouse prone 8 mice with increase of leucine zipper transcription factor-like protein 1 mRNA and protein, which is reported to promote neurite outgrowth.37) This means that drugs promoting neurite outgrowth could enhance learning and memory of aged subject. In the present study, EA promoted neurite outgrowth, which was confirmed by the fact that EA-treated DCX-positive immature neurons showed longer neurite than that of vehicle-treated immature neurons in the hippocampus. Moreover, EA enhanced spatial memory in aged mice. These results demonstrated that EA enhanced spatial memory and neurite outgrowth in immature neuron in old mice.
In conclusion, this study demonstrates that EA promotes neurite outgrowth through the JNK signaling pathway in Neuro2a cells and improve spatial memory in aged mice, suggesting that EA can be developed as a new natural drug to promote neuronal differentiation.
This study was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MEST) (2016R1A5A2007009).
The authors declare no conflict of interest.