2017 Volume 40 Issue 11 Pages 1894-1902
2017 Volume 40 Issue 11 Pages 1894-1902
Previously, we first reported the identification of four p-coumaroyl anthocyanins (petanin, peonanin, malvanin, and pelanin) from the tuber epidermis of colored potato (Solanum tuberosum L. cv JAYOUNG). In this study, we investigated the anti-oxidative and anti-inflammatory effects of a mixture of peonanin, malvanin, and pelanin (10 : 3 : 3; CAJY). CAJY displayed considerable radical scavenging capacity of 1, 1-diphenyl-2-picryl-hydrazyl (DPPH), increased mRNA levels of the catalytic and modulatory subunit of glutamate cysteine ligase, and subsequent cellular glutathione content. These increases preceded the inhibition of lipopolysaccharide (LPS)-induced intracellular reactive oxygen species (ROS) production. CAJY inhibited inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in a concentration-dependent manner at the protein, mRNA, and promoter activity levels. These inhibitions caused attendant decreases in the production of prostaglandin E2 (PGE2). CAJY suppressed the production and mRNA expression of tumor necrosis factor (TNF)-α and interleukin (IL)-6. Molecular data revealed that CAJY inhibited the transcriptional activity and translocation of nuclear factor κB (NF-κB) and phosphorylation of signal transducer and activator of transcription 1 (STAT1) and STAT3. Taken together, these results suggest that the anthocyanin mixture exerts anti-inflammatory effects in macrophages, at least in part by reducing ROS production and inactivating NF-κB and STAT 1/3.
Reactive oxygen species (ROS) are important in various biological processes at the levels of gene expression, protein translation, post-translational modification, and protein interactions.1) Cell-derived ROS independently or cooperatively regulate cellular signaling in response to environmental cues.1) A sustained pro-inflammatory state characterized by excessive ROS production is the common aspect in the development, progression, and complication of obesity, infections, cardiovascular and neurodegenerative diseases, and cancer.2–4) The shift in the balance between oxidants and anti-oxidants in favor of oxidants is termed oxidative stress. This stress contributes to many pathological conditions.
Aerobic organisms have integrated anti-oxidant systems, which include enzymatic and non-enzymatic antioxidants, that are usually effective in blocking harmful effects of ROS.5) Anti-oxidant defense systems comprise glutathione (GSH) and its synthesis, phase II detoxifying enzymes, and ROS inactivating enzymes, which play key roles in protecting cells upon oxidative damage.6) GSH effectively protects cells from various oxidative stresses caused by scavenging free radicals, suppression of lipid peroxidation, and removal of hydrogen peroxides in cells.7) Glutamate cysteine ligase (GCL) is the rate-limiting enzyme for de novo GSH synthesis and comprises a heterodimer formed of a catalytic subunit (GCLC) and a modulatory subunit (GCLM).8)
Since inflammation and oxidative stress are closely associated pathophysiological features involved in many incurable diseases, drugs with anti-inflammatory and anti-oxidative effects are in demand.9) The pathogenesis of inflammation is a complex process that is regulated by cytokine networks and the inductions of many pro-inflammatory genes, such as prostaglandin E2 (PGE2), nitric oxide (NO), tumor necrosis factor (TNF)-α, and interleukin (IL)-6.10) These factors participate in the main cytotoxic and pro-apoptotic mechanisms involved in the innate response in macrophages.11) Macrophages are central in orchestrating the immune response through phagocytosis and pro-inflammatory mediator secretions against foreign agents that include lipopolysaccharide (LPS).12) The overproduction of inflammatory cytokines by activated macrophages has been implicated in rheumatoid arthritis, arteriosclerosis, septic shock, and other chronic inflammatory diseases.13) Activation of toll-like receptor 4 (TLR4) on LPS-induced macrophages modulates two branches of downstream signaling pathway—the myeloid differentiation primary response gene 88 (MyD88) and the Toll/interleukin-1 receptor (TIR)-domain-containing adaptor inducing interferon-β (TRIF)-dependent pathways—culminating in the expression of inflammatory gene products including cytokines and chemokines. These activate downstream kinases and then induce the activation of transcription factors, such as nuclear factor κB (NF-κB), activation protein-1 (AP-1), signal transducer and activator of transcription (STATs), and interferon response factors (IRFs).14,15)
A dark purple-fleshed potato cultivar designated JAYOUNG originally bred in the Republic of Korea contains substantial amounts of polyphenols, such as anthocyanin and phenolic acid.16) Phenolic acids like chlorogenic acid, caffeic acid, protocatechuic acid, and p-coumaric acid have been identified in purple- and red-fleshed potatoes.17,18) Small amounts of rutin, quercetin, myricetin, kaempferol, naringenin, and some other flavonoids have also been detected.17) The main anthocyanin pigments in purple-fleshed potatoes are petunidin- and malvidin-3-retinoside-5-glucosides acylated with p-coumaric or ferulic acid. p-Coumaric acid acylated derivatives from petunidin, malvidin, pelargonidin, and peonidin are called petanin, malvanin, pelanin, and peonanin, respectively. Food-derived anthocyanins have a variety of biological effects, such as anti-oxidant, anti-inflammatory, anti-hypertensive, anti-mutagenic, anti-proliferative, and pro-apoptotic properties.19)
In a previous study, we obtained a mixture of three p-coumaroyl anthocyanins (peonanin, malvanin, and pelanin) from the tuber epidermis of JAYOUNG and this mixture was designated CAJY. Although anthocyanins have many health-beneficial properties, there are few reports on the detailed molecular mechanisms on the anti-inflammatory activity of p-coumaroyl anthocyanins. Therefore, as a part of our ongoing screening program to evaluate the anti-inflammatory potentials of natural compounds, we investigated the anti-oxidative and anti-inflammatory activities of CAJY and the molecular mechanisms in activated macrophages.
Dulbecco’s modified Eagle medium (DMEM) were obtained from Gibco™ Thermo Fisher Scientific (IL, U.S.A.). Fetal bovine serum (FBS), penicillin and streptomycin were obtained from GE Healthcare Hyclone™ (Logan, UT, U.S.A.). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), sulfanilamide, phenylmethylsulfonylflouride (PMSF), protease inhibitor cocktail (PIC), dithiothreitol (DTT), LPS, sodium bicarbonate, N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES), sodium dodecyl sulfate (SDS), and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Antibodies against inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), p65, inhibitor of kappaB (IκB), poly(ADP-ribose) polymerase-1 (PARP-1), and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Antibodies against phosphor-STAT1 and -STAT3 were purchased from Cell Signaling Technology (Danvers, MA, U.S.A.). Horseradish peroxidase (HPR) conjugated anti-mouse, anti-rabbit, and ant-goat immunoglobulin (Ig) were purchased from Thermo Fisher Scientific (IL, U.S.A.). SYBR green ex Taq were obtained from TaKaRa (Shiga, Japan). iNOS, COX-2, TNF-α, IL-6, and β-actin oligonucleotide primers were purchased from Bioneer (Seoul, Korea). Enzyme linked immunosorbent assay (ELISA) kits for TNF-α and IL-6 were obtained from R&D Systems (Minneapolis, MN, U.S.A.).
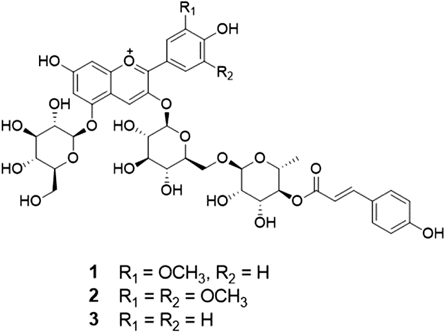
Plant Material, Extraction and IsolationThe p-coumaroyl anthocyanin mixture used in this study were isolated from the tuber epidermis of a colored potato S. tuberosum cv JAYOUNG as reported previously.20) The structures of the three anthocyanins in the mixture (Fig. 1) were identified as peonanin (1), malvanin (2), and pelanin (3) by spectroscopic data (UV, 1D-NMR, 2D-NMR, and MS) measurement and by comparison with published values.21,22) The composition of 1–3 in the mixture was determined as ca. 10 : 3 : 3, respectively, by integration of the peaks in the 1H-NMR spectrum and intensity of molecular ion peaks in the MS (see supplementary materials).
RAW264.7 macrophage cell line was obtained from the American Type Culture Colleciton (ATCC, Rockbille, MD, U.S.A.). These cells were cultured in DMEM supplemented with 10% FBS, penicillin (100 units/mL) and streptomycin sulfate (100 µg/mL). Cell cultures were maintained at 37°C in a humidified atmosphere of 5% CO2. Cells were incubated with CAJY (10, 25, or 50 µg/mL) or an appropriate positive control for 1 h and then stimulated with 10 ng/mL LPS from Escherichia coli, serotype 0111:B4 (Sigma-Aldrich, St. Louis, MO, U.S.A.) for the indicated time.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging AssayThe antioxidant activity of CAJY was assessed on the basis of the radical scavenging effect of the stable DPPH radical. CAJY ranging from 3.125 to 100 µg/mL was added to 100 µL DPPH radical in methanol solution. After a 30 min incubation at room temperature in the dark, the absorbance was measured at 517 nm. Data are expressed as the percent decrease in the absorbance compared to the control.
GSH AssayRAW264.7 macrophages were incubated in 6-well plate (5×105 cells/mL) for 12 h. Cells were harvested using a cell scraper and collected by centrifugation at 2000×g for 10 min at 4°C. The cell pellet was homogenized or sonicated in 1–2 mL of cold buffer and centrifuged at 10000×g for 15 min at 4°C. Levels of GSH in the supernatants were quantified using Glutathione Assay Kit, according to the manufacturer’s instructions (Cayman, Ann Arbor, MI, U.S.A.).
Measurement of ROSIntracellular accumulation of ROS was determined using the fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). RAW264.7 macrophage cells (2×105 cells/mL) were seeded on a 6-well plate for 24 h and pretreated with CAJY (50 µg/mL) and N-acetyl-L-cysteine (NAC, 10 mM) for 1 h. The cells were stimulated with LPS (1 µg/mL) for 4 h and treated with H2DCFDA (20 µM) for 30 min. After staining, cells were resuspended in phosphate buffered saline (PBS) and monitored using a flow cytometer (Beckman Coulter Inc., Brea, CA, U.S.A.) and the results were analyzed using CELL QUEST software (Beckman Coulter Inc.).
MTT Assay and Determination of PGE2, TNF-α, and IL-6 AssaysRAW264.7 macrophages were plated at 2×105 cells/mL in 24-well plates and incubated overnight. Following treatment with various concentrations of CAJY for 1 h, cells were treated with LPS (10 ng/mL) for 24 h. The levels of PGE2, TNF-α, and IL-6 in the cell cultured medium (supernatant) were quantified by a colorimetric competitive ELISA kit (Enzo Life Science, NY, U.S.A.) or mouse DuoSet kit (R&D Systems, MN, U.S.A.) according to the manufacturer’s instructions. Cells were then incubated in MTT solution for 4 h at 37°C under 5% CO2. The medium was removed, and the formazan blue that formed in the cells were solubilized in 200 µL of DMSO. Absorbances of each well was measured at 540 nm using a microplate reader (Molecular Devices Inc., Sunnyvale, CA, U.S.A.).
Western Blot AnalysisProtein extracts from CAJY-treated cells were lysed using PRO-PREP™ protein extraction solution (Intron Biotechnology, Seoul, Korea) supplemented with protease inhibitor cocktail (Sigma-Aldrich) and incubated for 30 min at 4°C. Debris was removed by microcentrifugation, followed by quick freezing of the supernatants. The protein concentration was determined using protein assay reagent (Bio-Rad, Hercules, CA, U.S.A.) according to the manufacturer’s instructions. Cellular protein extracts were mixed with 5× sodium dodecyl sulfate (SDS) sample buffer, boiled for 5 min, and then were separated by 10–12% SDS-polyacrylamide gel electrophoresis. The resolved proteins were electroblotted onto a PVDF membrane. The membrane was incubated for 1 h with blocking solution (5% skim milk) at room temperature, followed by incubation overnight with a primary antibody at 4°C (1 : 1000). Blots were washed three times with Tween 20/Tris-buffered saline (T/TBS) and incubated with a 1 : 2000 dilution of HRP-conjugated secondary antibody for 2 h at room temperature. Blots were again washed three times with T/TBS, and then developed using an ECL chemiluminescence substrate (Santa Cruz Biotechnology, CA, U.S.A.). The protein bands were visualized by LAS-4000 luminescent image analyzer (FUJIFILM, Tokyo, Japan). Quantity One® Software (Bio-Rad) was used for the densitometric analysis.
RNA Isolation and Real-Time Quantitative PCR (qRT-PCR)Total RNA was extracted from cells using by Easy Blue®kits (Intron Biotechnology, Seoul, Korea). RNA (1 µg) was reverse-transcribed (RT) using TOPscript™ RT DryMIX (Enzynomics, Daejeon, Korea) and 0.5 mg/mL random primer. PCR amplification was performed using the incorporation of SYBR green using SYBR Premix Ex Taq (TaKaRa). The PCR primers used in this study are listed below and were purchased from Bioneer (Seoul, Korea): for GCLM designed from mouse were GGG AAC CTG CTC AAC TGG GG (forward) and CTG CAT GGG ACA TGG TGC ATT (reverse); for GCLC designed from mouse were TCC GGC ATC GGA GAG GAG A (forward) and AGC AGT TGC CCA TCC CGA AT (reverse); for iNOS designed from mouse were AAC ATC CTG GAG GAA GTG GG (forward) and GCT GTG TGG TGG TCC ATG AT (reverse); for COX-2 designed from mouse were TGC TGT ACA AGC AGT GGC AA (forward) and GCA GCC ATT TCC TTC TCT CC (reverse); for TNF-α designed from mouse were AGC ACA GAA AGC ATG ATC CG (forward) and CTG ATG AGA GGG AGG CCA TT (reverse); for IL-6 were CCC CAG GAG AAG ATT CCA AA (forward) and TTG TTT TCT GCC AGT GCC TC (reverse). The oligonucleotide primers for β-actin used as a house-keeping gene designed from mouse were ATC ACT ATT GGC AAC GAG CG (forward) and TCA GCA ATG CCT GGG TAC AT (reverse). Steady-state mRNA levels were determined by real time qPCR using the TaKaRa thermal cycler device. A dissociation curve analysis of iNOS, COX-2, TNF-α, IL-6, and β-actin showed a single peak for each. Mean Ct values of genes of interest were calculated from triplicate measurements and normalized versus the mean Ct of β-actin.
Transient Transfection and Luciferase AssayRAW264.7 macrophages (1×105 cells/mL) were co-transfected with pGL3-iNOS, pGL3-COX-2, or pNF-κB-Luc plasmid (Clontech, Shiga, Japan) plus the phRL-TK plasmid (Promega, WI, U.S.A.) using Nuclefector™ kit (Lonza, Basel, Switzerland) as instructed by the manufacturers. After 6 h of transfection, cells were pretreated with CAJY (10, 25, or 50 µg/mL) for 1 h and then stimulated with LPS (10 ng/mL) for 6 or 12 h. Each well was washed with cold-PBS and cells were lysed. Luciferase activities were measured using the dual-luciferase reporter assay system (Promega, Madison, WI, U.S.A.), according to the manufacturer’s instructions.
Nuclear ExtractionNuclear extracts were prepared as described previously with slight modifications. Cells were resuspended in hypotonic buffer (10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.1 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 0.5 mM PMSF, 1 mM DTT, and protease inhibitor cocktail (PIC)) and incubated on ice for 15 min. Cells were then lysed by adding 10% NP-40 and vortexed vigorously for 10 s. The nuclei were pelleted by centrifugation at 12000×g for 1 min at 4°C and resuspended in high salt buffer (20 mM HEPES (pH 7.9), 400 mM NaCl, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, and PIC).
Statistical AnalysisResults are presented as the mean±standard deviations (S.D.s) of triplicate experiments. Statistically significant values were compared using ANOVA and Dunnett’s post hoc test, and p-values <0.05 were considered statistically significant.
ROS function as signaling molecules, as they can induce cell injury and cell death at higher intracellular concentrations.23) Since anthocyanin has anti-oxidant effects,24) we first examined the radical scavenging activities of CAJY using the DPPH assay. CAJY scavenged free radicals in a concentration-dependent manner (Fig. 2A). GSH is an important non-enzymatic antioxidant present in cells.25) To determine the cellular antioxidant activity of CAJY, we measured the effects of CAJY on cellular GSH levels. GSH levels time-dependently increased with 50 µg/mL CAJY (Fig. 2B). GCL is the rate-limiting enzyme in GSH synthesis and controls the biosynthesis of reduced GSH. We examined whether GCLC and GCLM gene expression were associated with GSH synthesis in CAJY-induced GSH increase. Treatment with 50 µg/mL CAJY significantly increased GCLC and GCLM mRNA expression (Figs. 2C, D). These results suggested that significant increases in GSH content by CAJY involved gene inductions of GCLC and GCLM. Next, we investigated the effect of CAJY on LPS-induced intracellular ROS production using DCFDA-based flow cytometry. Intracellular ROS was substantially increased in LPS-induced cells compared to control cells (80.6% vs. 50.8%). Pretreatment with 50 µg/mL CAJY significantly suppressed LPS-induced intracellular ROS generation (66.7%). NAC was used as a positive control (Fig. 2E).
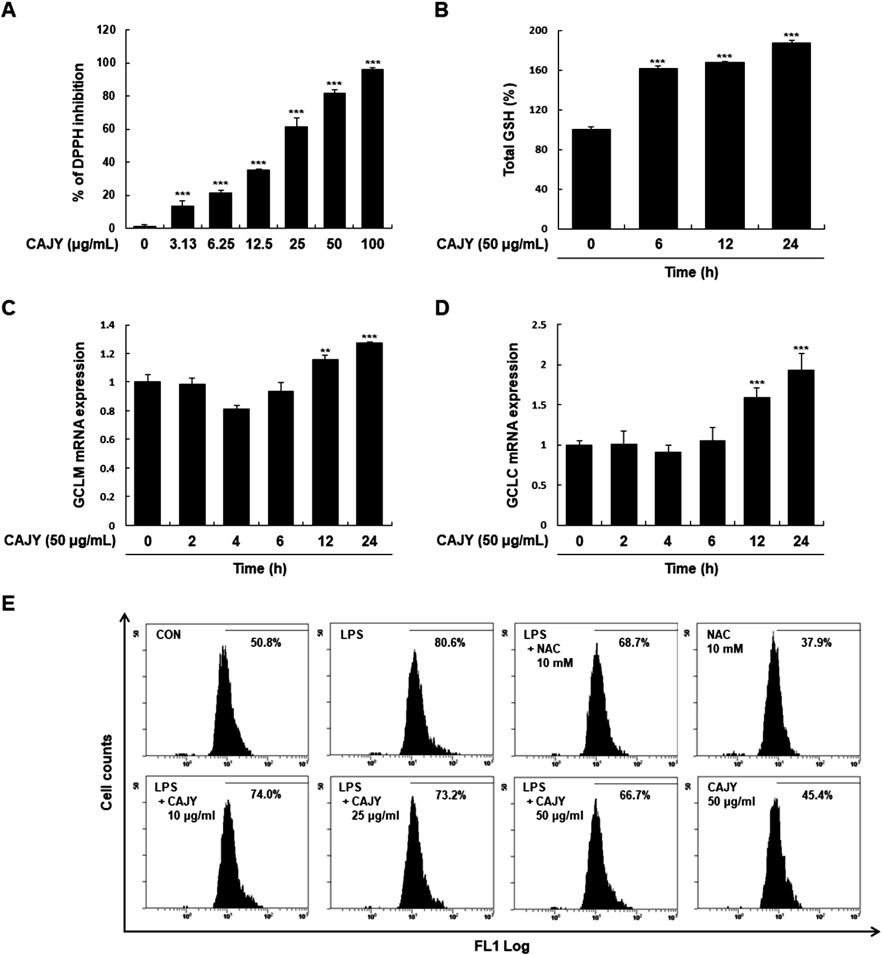
(A) Scavenging activity of DPPH radical was measured as described in Material and Methods. (B) Cells were pretreated with or without CAJY (50 µg/mL) for the indicated times and intracellular GSH was measured using an ELISA kit. (C) Total RNA was prepared for qRT-PCR analysis of GCLM and GCLC in cells with/without CAJY (50 µg/mL) for the indicated times. The mRNA levels of GCLM and GCLC were determined using gene specific primers. (E) Intracellular ROS was measured by flow cytometry. Cells were incubated with H2DCFDA (20 µM) for 30 min in the presence of CAJY (50 µg/mL) or NAC (10 mM) in LPS-stimulated cells. Data are presented as the mean±S.D. of three independent experiments. ** p<0.01, *** p<0.001 vs. the control group.
To investigate the anti-inflammatory activities of CAJY, we firstly determined NO and PGE2 production in LPS-induced RAW264.7 macrophages with CAJY at 10, 25, or 50 µg/mL. LPS-induced PGE2 generation was significantly attenuated in a concentration-dependent manner by CAJY, with attenuation evident even at 10 µg/mL (Fig. 3A). NS-398 (3 µM) was a selective inhibitor of COX-2 and was used as a positive inhibitor. However, we could not determine the production of nitrite (a surrogate of NO production) from Griess reagent because of the interference caused by the same absorption spectrum of CAJY itself and nitrite. Next, we determined whether inhibition of PGE2 production by CAJY correlated with COX-2 expression. CAJY concentration-dependently inhibited the LPS-induced expression of iNOS protein and mRNA in RAW264.7 macrophages although NO production was not measured (Figs. 3B, C). Moreover, LPS significantly enhanced the transcriptional activities of the iNOS and COX-2 gene promoters. CAJY inhibited these inductions in a concentration-dependent manner (Fig. 3D). These inhibitory effects of CAJY were not caused by nonspecific cytotoxicity, because CAJY had no effect on cell viability as determined by MTT-based assay at concentrations from 10 to 50 µg/mL (Fig. 3A).
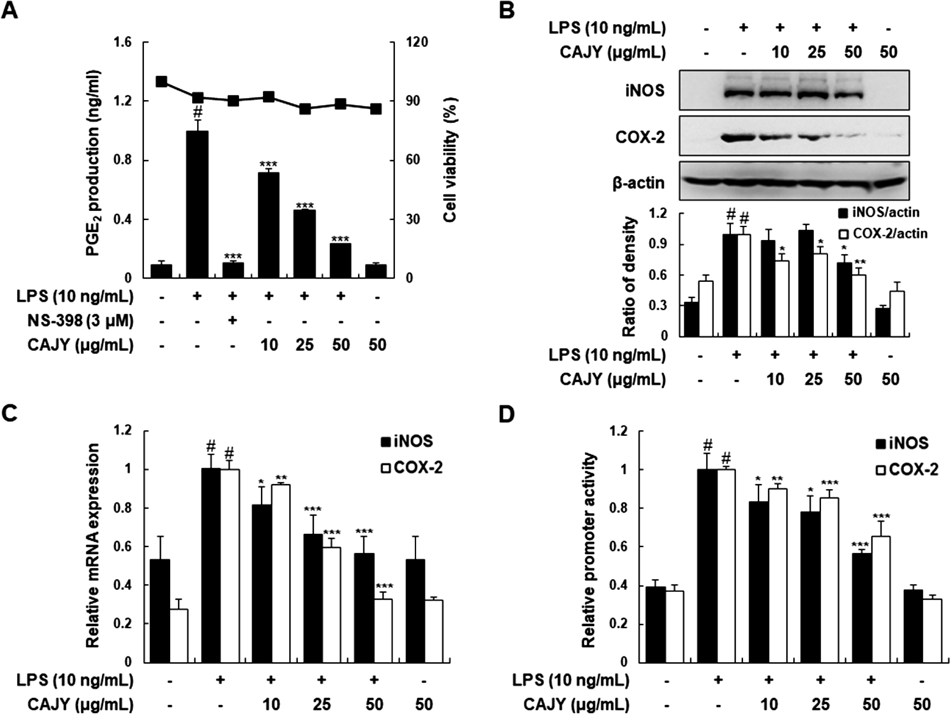
(A) Following pretreatment with CAJY (10, 25, or 50 µg/mL) for 1 h, cells were treated with LPS (10 ng/mL) for 24 h. PGE2 production in the culture media was quantified using an EIA kit. Negative controls were not treated with LPS and CAJY. NS-398 (3 µM) was used as the positive control for PGE2 production. (B) Total cellular proteins were obtained from the cells stimulated with LPS (10 ng/mL) for 24 h in presence or absence of CAJY (10, 25, or 50 µg/mL) resolved by SDS-PAGE, and iNOS and COX-2 were detected using specific antibodies. β-Actin was used as an internal control. (C) Total RNA was prepared for qRT-PCR analysis of iNOS and COX-2 in cells stimulated with LPS (10 ng/mL) with or without CAJY (10, 25, or 50 µg/mL) for 6 h. The mRNA levels of iNOS and COX-2 were determined using gene specific primers. (D) Cells were transiently transfected with a pGL3-iNOS or a pGL3-COX-2 promoter vector, and phRL-TK vector was used as an internal control. Cells were treated with or without CAJY (10, 25, or 50 µg/mL) for 1 h and stimulated with LPS (10 ng/mL) for 6 h. Cells were harvested and luciferase activity levels were determined. Data are presented as the mean±S.D. of three independent experiments. # p<0.05 vs. the control group; * p<0.05, ** p<0.01, *** p<0.001 vs. LPS-simulated cells.
Next, we examined the effects of CAJY on LPS-induced TNF-α and IL-6 production, and their mRNA expressions in LPS-induced RAW264.7 macrophages. Pretreatment with CAJY reduced the LPS-induced TNF-α and IL-6 production (Figs. 4A, B) and their mRNA expression (Figs. 4C, D) in concentration-dependent manner, showing that suppressive effect of CAJY on the expression of these inflammatory genes was exerted at the transcriptional level.
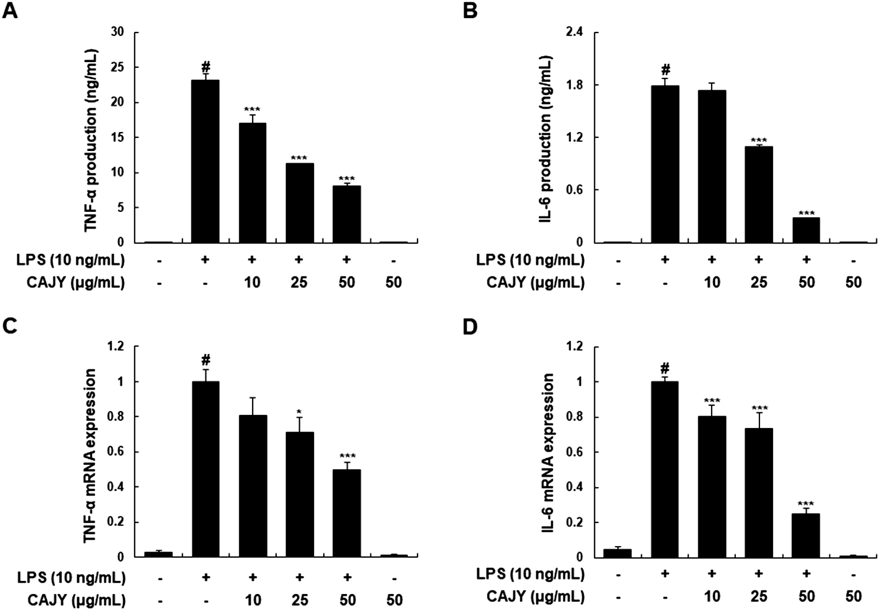
(A and B) Following pretreatment with CAJY (10, 25, or 50 µg/mL) for 1 h, cells were treated with LPS (10 ng/mL) for 24 h. Levels of TNF-α and IL-6 in the culture media were quantified using an EIA kit. Negative controls were not treated with LPS and CAJY. (C and D) Total RNA was prepared for qRT-PCR analysis of TNF-α and IL-6 in cells stimulated with LPS (10 ng/mL) with or without CAJY (10, 25, or 50 µg/mL) for 6 h. The mRNA levels of TNF-α and IL-6 were determined using gene specific primers. Data are presented as the mean±S.D. of three independent experiments. #p<0.05 vs. the control group; *** p<0.001 vs. LPS-simulated cells.
The critical transcription factors for LPS-induced pro-inflammation are NF-κB and AP-1.26) Thereore, we examined whether CAJY affected on LPS-induced NF-κB and AP-1 dependent reporter gene activities. The results demonstrated the concentration-dependent inhibition of CAJY in NF-κB-dependent luciferase activity, but not AP-1-dependent luciferase activity (Fig. 5A). Translocation of NF-κB into the nucleus is essential for the transcriptional activation of target genes. Accordingly, we investigated whether CAJY could prevent the nuclear translocation of the p65 NF-κB subunit. Pretreatment with CAJY attenuated the LPS-induced nuclear translocation of p65 at 10 min (Fig. 5B). Next, in order to understand the inhibitory mechanism of CAJY in LPS-induced NF-κB activation, we examined the effect of CAJY on LPS-induced phosphorylation and degradation of IκBα. As shown in Fig. 5C, CAJY reduced LPS-induced IκBα phosphorylation and prevented IκBα dagtadation. In addition, STAT1 and 3 is pivotal transcription factors regulating the expression of genes encoding pro-inflammatory proteins and cytokines activated by tyrosine and serine phosphorylation at particular residues.27) CAJY markedly reduced LPS-induced phosphorylation of STAT1 (Y701) and STAT3 (Y705) in macrophages, suggesting that CAJY inhibits STAT1 and STAT3 activation (Fig. 5D).

(A) Cells were co-transfected with pNF-κB-Luc reporter with phRL-TK vector. Cells were pretreated with or without CAJY (10, 25, or 50 µg/mL) for 1 h, followed by stimulation with LPS (10 ng/mL) for 18 h. Cells were then harvested and luciferase activity levels were determined. (B) Cells were pretreated with CAJY (50 µg/mL) for 1 h and stimulated with LPS (10 ng/mL) for indicated times. Nuclear (N) and cytosolic (C) extracts were isolated and the levels of p65 in each fraction were determined using specific antibody. (C) Cells were pretreated with CAJY (10, 25, or 50 µg/mL) for 1 h, and stimulated with LPS (10 ng/mL) for 5 min. Total cellular proteins were resolved by SDS-PAGE, and detected using specific p-IκBα and IκBα antibodies. (D) Cells were pretreated with CAJY (10, 25, or 50 µg/mL) for 1 h, and stimulated with LPS (10 ng/mL) for 2 h. Total cellular proteins were resolved by SDS-PAGE, and detected using specific p-STAT1 (Y701), p-STAT3 (Y705), and STAT1 and STAT3 antibodies. PARP and β-actin were used as internal controls. The experiment was repeated three times, and similar results were obtained. Data are presented as the means±S.D. of three independent experiments. # p<0.05 vs. the control group; ** p<0.01, *** p<0.001 vs. LPS-simulated cells.
The anthocyanin-rich fraction from several kinds of fruits and vegetables including berry, cranberry, and grape has various pharmacological effects that include anti-oxidant, anti-proliferative, and anti-inflammatory properties.28–30) Colored rice extract suppresses LPS-induced inflammation by inhibition of the mitogen-activated protein kinase (MAPK) signaling pathway, which leads to decreased NF-κB and AP-1 translocation.31) The red bean (Phaseolus radiatus L. var. aurea) significantly suppresses the inflammatory responses in LPS-stimulated macrophages by reducing cellular NO and down-regulating the gene expressions of iNOS, COX-2, TNF-α, and IL-6.32) We and others have recently discovered that color potato ‘JAYOUNG’ has anti-oxidative activity33) and anti-inflammatory properties in LPS-induced macrophages and dextran sodium sulfate-induced colitis mice.34) In this study, we further defined the anti-oxidative and anti-inflammatory activities of p-coumaroyl anthocyanins mixture from JAYOUNG in LPS-induced RAW264.7 macrophages. CAJY exhibited potent anti-oxidant activities involving DPPH radical scavenging, GSH enhanced activity, and reduced cellular ROS production. A theoretical study evaluated the anti-oxidant character of three common anthocyanidins (cyanidin, delphinidin, and malvidin)35) according to different parameters (bond dissociation enthalpy, ionization potential, proton affinity, and electron transfer enthalpy) and the atomic charges corresponding to the O atoms of the hydroxyl groups. In addition, the anti-oxidant effect of anthocyanins involves free radical scavenging by OH groups.36) Based on these previous studies, the DPPH assay was presently used to demonstrate the potent radical scavenging activity of CAJY. Excessive ROS production damages essential cellular macromolecules including lipids, proteins, and DNA. Consequences include inflammation, cancer, atherosclerosis, rheumatoid arthritis, and neurodegenerative diseases.37) Therefore, ROS clearance and inhibition of oxidative stress could be profoundly important in preventing numerous diseases, including inflammatory diseases. GSH is a major anti-oxidant that protects cells against oxidative stress by scavenging ROS.38) GSL, which is composed of a modifier subunit (GCLM) and a catalytic subunit (GCLC), is involved in the endogenous synthesis of GSH, and is important as the rate-limiting enzyme.39) In the present study, CAJY up-regulated intracellular GSH levels by inducing GCLC and GCLM gene expression. GSH is involved in immune and inflammatory responses. Oxidative stress and inflammation triggers, such as LPS, lead to reduction in cellular GSH levels and could have consequences in gene expressions of pro-inflammatory mediators and antioxidant molecules.40) Accordingly, decreases in LPS-induced ROS by CAJY might be involved with free radical scavenging and/or GSH enhancing activity of CAJY. Our results suggest for the first time that the increase of endogenous anti-oxidant enzyme activities might be one of the important mechanisms of CAJY against oxidative stress damage in LPS-induced stress in RAW264.7 macrophages.
Another important finding is that CAJY inhibited various pro-inflammatory genes, such as iNOS, COX-2, TNF-α, and IL-6, in LPS-induced RAW264.7 macrophages. LPS is a bacterial endotoxin that activates macrophages and leads to increased pro-inflammatory cytokines and related enzymes through the activation of various cellular signaling pathways including MAPKs, phosphoinositide 3-kinase (PI3K)/Akt, NF-κB, AP-1, and STATs.41) The anti-inflammatory activities of anti-oxidants are presented through several mechanisms involving the modulation of inflammatory signals, reduction in the production of inflammatory molecules, diminished recruitment and activation of inflammatory cells, and regulation of cellular function.42) Multiple lines of evidence have suggested that anthocyanins inhibit pro-inflammatory mediators by modulating transcription factors including the inhibition of NF-κB, AP-1, cAMP response element-binding protein (CREB), and CCA AT/enhancer-binding protein (CAAT-) activation.43,44) Prompted by these findings, we examined whether CAJY inhibits NF-κB activity in RAW264.7 macrophages using reporter gene assays. CAJY inhibited the LPS-induced transcriptional activity of NF-κB in a concentration- dependent manner. In particular, as reported in a previous study, when NF-κB is stimulated with inflammatory agents such as LPS, activated NF-κB dimers translocate to the nucleus and interact with target DNA recognition sites to activate the transcription of diverse pro-inflammatory genes.45) To identify the mechanisms involved in the inhibition of NF-κB activity by CAJY, we tested the effect of CAJY on activation signals. Presently, CAJY was found to inhibit the LPS-induced phosphorylation and degradation of I IκBα and reduce the amount of p65 in nuclear fractions. These findings suggest that the inhibition of NF-κB activation by CAJY is the result of the inhibition of IκBα phosphorylation and degradation and thus of the nuclear translocation of p65.
STAT1 and STAT3 also participate in the regulation of inflammatory mediators in response to diverse stimuli.46) In particular, STAT signal pathways are crucial in iNOS expression.47) Blocking the activation of the STATs pathway is beneficial in inhibiting LPS-induced inflammatory responses.48) Therefore, we determined whether the anti-inflammatory effect of CAJY was related to the activation of STATs as revealed by the phosphorylation of STATs proteins in RAW264.7 macrophages. CAJY markedly reduced LPS-induced phosphorylation of STAT1 and STAT3, suggesting that CAJY inhibits activation of these STATs. Because the NF-κB and STAT1/3 signaling pathways are redox sensitive, the inhibitory effects of CAJY might be associated with its ROS scavenging activities, although the exact mechanism remains to be defined. Further studies need to unravel the molecular mechanism in NF-κB and STAT1/3 transcriptional regulation. Based on these results, we suggest that the inhibition of the expression of pro-inflammatory mediators by CAJY might be due to the suppression of NF-κB and STAT1/3 activation.
In summary, we demonstrate that CAJY has anti-oxidative activity that includes radical scavenging activity, increased intracellular GSH through induction of cellular defense genes including GCLC and GCLM, and recovery of intracellular ROS. Moreover, CAJY decreases LPS-induced expression of iNOS, COX-2, and inflammatory cytokines by NF-κB and STAT1/3 inactivation in macrophages. These findings implicate CAJY as a potential treatment option for inflammatory diseases.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, Information & Communication Technology, and future Planning [Grant NRF-2015R1A2A2A01003459].
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.