2017 Volume 40 Issue 11 Pages 1983-1989
2017 Volume 40 Issue 11 Pages 1983-1989
The expression phase of cocaine-induced conditioned place preference (CPP) represents a cocaine-seeking behavior triggered by contextual cues associated with the rewarding effects of cocaine. However, the exact mechanisms underlying the cocaine CPP expression remain unclear. Here, we investigated the role of dopaminergic (DAergic) transmission in the medial prefrontal cortex (mPFC) for the expression of cocaine CPP. An intra-ventral tegmental area (VTA) injection of a cocktail of γ-aminobutyric acid (GABA)B and GABAA receptor agonists (baclofen and muscimol, respectively) immediately before the posttest inhibited the expression of cocaine CPP. An intra-mPFC injection of a dopamine D1 but not D2 receptor antagonist before the posttest significantly attenuated CPP expression. Moreover, after the posttest, the number of cFos-positive mPFC neurons in rats that were conditioned with cocaine was significantly larger than that with saline. Additionally, photostimulation of channelrhodopsin-2 expressing fibers derived from the VTA induced cFos expression in the mPFC, and this induction was reduced by a prior systemic injection of a D1 receptor antagonist. These findings indicate that during the expression of cocaine CPP, enhanced DAergic transmission from the VTA to the mPFC stimulates D1 receptors; this results in the activation of mPFC neurons, further leading to the expression of cocaine CPP.
The conditioned place preference (CPP) is widely used in evaluating the motivational and rewarding effects of addictive drugs, including cocaine.1) In the conditioning session (acquisition phase), animals learn the association between the rewarding effects of cocaine and the environmental context in which they have received the cocaine injections. In the posttest session (expression phase), animals exhibit a preference for the environment where they experienced cocaine, probably because of the retrieval of memories associated with the rewarding effects of cocaine that motivate the animals to seek the drug in that environment. This process may be similar to the drug-seeking behaviors of cocaine abusers, which are triggered by environmental cues, such as the circumstances in which they have previously administered cocaine. Therefore, understanding the neural mechanisms of cocaine CPP expression is crucial for the development of therapeutic strategies to treat cocaine addicts.
Accumulating evidence indicates that the mesocorticolimbic circuitry plays a critical role in the cocaine CPP expression.2–4) Systemic injection of a dopamine (DA) D1 and a DA D2 receptor antagonist, respectively, tends to reduce and significantly attenuates the cocaine CPP,5) indicating an enhanced activity of dopaminergic (DAergic) neurons during the posttest of CPP. This is supported by our previous study showing that a blockade of cholinergic transmission from the laterodorsal tegmental nucleus (LDT), a brainstem nucleus implicated in reward-associated behavior,6–8) to the ventral tegmental area (VTA) attenuates the cocaine CPP expression,9) most likely due to the inhibition of acetylcholine-induced excitation of VTA DAergic neurons.10)
The medial prefrontal cortex (mPFC) is implicated in memory retrieval, decision making, and cocaine-seeking behavior,3,11,12) and one of the main projection targets of VTA DAergic neurons. Although the mPFC has been associated with the cocaine CPP expression,2) the relationship among mPFC neuronal excitation, DAergic signaling and CPP expression remains unclear. Therefore, in the present study, we have tested the hypothesis that DAergic signaling, and subsequent changes in neuronal excitability in the mPFC, are involved in the cocaine CPP expression.
Male Sprague–Dawley rats (weighing 180–260 g at the beginning of behavioral tests) were maintained in a temperature-controlled (22±1°C) room under a 12-h light/dark cycle with food and water available ad libitum. All experiments were conducted in accordance with the National Institutes of Health guidelines, and performed with the approval of the Institutional Animal Care and Use Committee at Hokkaido and Kanazawa University. All efforts were made to minimize the number and suffering of animals used in the experiments.
DrugsCocaine hydrochloride was purchased from Takeda Pharmaceutical (Osaka, Japan), muscimol, baclofen, and R(+)-SCH23390 hydrochloride were from Sigma-Aldrich, and raclopride was from Tocris Bioscience. All drugs were dissolved in 0.1 M phosphate buffered saline (PBS) (pH 7.4) or saline (Cocaine and SCH23390 for systemic injection). The doses of these drugs were as follows: cocaine (20 mg/kg, intraperitoneal (i.p.)), baclofen/muscimol cocktail (0.065, 0.0034 µg/0.5 µL/side13,14)), SCH23390 (0.3, 1 µg/0.5 µL/side15,16) or 1.0 mg/kg, i.p.17,18)), and raclopride (3.0 µg/0.5 µL/side19,20)).
Surgery for Drug MicroinjectionUnder sodium pentobarbital anesthesia (50 mg/kg, i.p.), rats were implanted bilaterally with 25-gauge stainless-steel guide cannulae (o.d., 0.5 mm; i.d., 0.22 mm) above the VTA (5.8 mm caudal, 1.0 mm lateral, 8.5 mm ventral to bregma), or mPFC (3.0 mm rostral, 0.67 mm lateral, 4.0 mm ventral to bregma).21) Then, rats were housed individually in their home cages, allowed to recover for 6–9 d, and handled each day for 3 consecutive days before the behavioral experiments. For microinjection, 33-gauge stainless-steel injection cannulae (o.d., 0.2 mm; i.d., 0.08 mm) were inserted bilaterally into the guide cannulae. The injection cannulae protruded 1.5 mm from the tip of the guide cannulae to reach the VTA or mPFC. Bilateral infusions were performed with a volume of 0.5 µL in each side at a rate of 0.5 µL/min, and the injection cannulae were left in place for an additional 1 min after microinjection to prevent backflow.
Conditioned Place PreferenceCPP tests were conducted as described previously.6,9) The CPP chambers consisted of two equally-sized compartments (30×30×30 cm), with distinct tactile and visual cues. One compartment had a black floor and walls with an equally spaced stainless-steel stripe-like grid on the floor, and the other had a white floor and walls with a stainless-steel grid on the floor. These were separated by a removable partition. On days 1 (habituation) and 2 (pretest), rats freely explored the two compartments for 900 s, and the time spent in each compartment during the exploratory period and locomotor activity were measured using infrared sensors (Supermex, Muromachi Kikai, Tokyo, Japan) that were positioned on the top cover of each compartment. We used a bias-like protocol1) and designated the compartment in which each rat spent less time on day 2 (pretest) as their cocaine-paired compartment. On days 4–9 (conditioning), rats were given alternating injections of cocaine or saline (1 mL/kg, i.p.) and were confined to one compartment for 30 min for 6 consecutive days. On day 11 (posttest), rats were allowed to explore the two compartments freely for 900 s, and the time spent in each compartment during the exploratory period and their locomotor activity were measured. Rats were given a bilateral intra-VTA or intra-mPFC microinjection 15 min before the start of posttest. The CPP scores were calculated by subtracting the time spent in the cocaine-paired compartment during the pretest from that during the posttest.
HistologyAfter the CPP tests, the brains were rapidly removed and frozen in powdered dry ice. Coronal sections (50 µm) of the VTA or mPFC were prepared using a cryostat, thaw-mounted onto slides, stained with thionin, and examined under a microscope.
ImmunohistochemistryRats were perfused with 0.1 M PBS followed by 4% paraformaldehyde (PFA) 90 min after the end of posttest session. The brains were removed, post-fixed overnight with 4% PFA, placed in a 10% sucrose solution, and switched to a 30% sucrose solution. Coronal sections (50 µm) were collected at levels corresponding to the mPFC, washed with 0.1 M PBS containing 0.3% Triton-X (PBS-T) and blocked in 3% bovine serum albumin. The sections were then incubated overnight with a primary antibody (Rabbit anti-cFos antibody, sc-52, 1 : 1000, Santa Cruz). The following day, the sections were washed with PBS-T, and then incubated for 1 h with a secondary antibody (AlexaFluor-488 labeled donkey anti-rabbit antibody, A21206, 1 : 200, Invitrogen). The sections were washed with PBS, mounted onto glass slides, and coverslipped using the Vectashield mounting medium (Vector). Staining was visualized and captured using a Bio-zero BZ-9000 microscope (Keyence, Osaka, Japan). We counted the cFos-positive cells manually within a 1×1 mm2 grid placed unilaterally over the mPFC sections. Numbers of cFos-positive cells for individual rats were calculated by averaging the counts from three sections.
OptogeneticsUnder sodium pentobarbital anesthesia, rats were injected unilaterally into the VTA with 0.5 µL of an adeno-associated virus (AAV) vector expressing enhanced yellow fluorescent protein (eYFP) with or without channelrhodopsin-2 (ChR2) under a neuronal-specific human synapsin (hSyn) promoter (AAV-hSyn-eYFP or AAV-hSyn-ChR2-eYFP) obtained from the vector core at the University of North Carolina. The viral titers were 4.6–6.6×1012 vg/mL. A 2 µL Neuros syringe (Hamilton) with a 30 gauge beveled metal needle was used to infuse the viral vectors with a microsyringe pump (UMP3; WPI) and its controller (Micro4; WPI). Following infusion, the needle was left in place for 10 min. At least 4 weeks after injection, rats were implanted unilaterally with an optic cannula (400 µm diameter; CFMC14L10, Thorlabs) into the mPFC. After surgery, rats were housed individually in their home cages, allowed to recover for 6–7 d, and then handled and habituated to the environment (plastic box, 30×30×30 cm) for 3 consecutive days, in which optical stimulation was delivered to rats. Five minutes after systemic injection of saline or SCH23390, optical stimulations consisted of 30 Hz bursts of eight 5 ms pulses of 473 nm light (COME2-LB473 model; Lucir) were conducted every 5 s for 5 min.22) Rats were perfused 90 min after the optical stimulation. Their tissues were immunostained with a rabbit anti-cFos antibody and AlexaFluor-568 labeled goat anti-rabbit antibody (A11031, 1 : 200, Invitrogen), and cFos-positive cells in the mPFC were quantified as described above. To investigate the density of eYFP-positive fibers in the mPFC, each image was converted to grayscale, and the mean gray value within the 1×1 mm2 grid placed unilaterally over the mPFC, within which cFos-positive cells were quantified, was measured as an index of the density of positive fibers.
Statistical AnalysesData are expressed as means±standard error of the mean (S.E.M.) and were compared using a one-way or a two-way ANOVA followed by the Holm–Sidak post hoc test when comparing more than two groups, or a Student’s t-test when comparing two groups.
To examine the contribution of VTA neuronal activity to the cocaine CPP expression, we microinjected a cocktail of baclofen and muscimol (B/M), γ-aminobutyric acid (GABA)B and GABAA receptor agonists, respectively, or vehicle into the VTA immediately before the posttest (Fig. 1A). The CPP score obtained after intra-VTA B/M microinjection (n=6) was significantly smaller than that after the vehicle injection (n=9, t13=4.983, p=0.0003, Student’s t-test; Fig. 1B). The B/M microinjection did not affect locomotor activities (t13=1.662, p=0.1205; Fig. 1C), confirming that the reduced CPP score was not due to the modulation of locomotor activity. These results indicate that VTA neuronal activity is critical for the cocaine CPP expression.
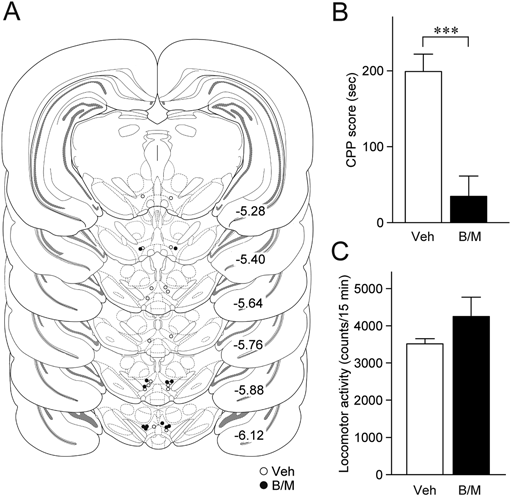
(A) The placements of the tips of the microinjection cannulae for the injection of a cocktail of baclofen and muscimol (B/M, black circles, n=6) or vehicle (Veh, white circles, n=9) into the VTA. The numbers beside each panel represent the approximate AP distance (mm) from bregma. (B) Summary graph of CPP scores. *** p<0.001, Student’s t-test. (C) Summary graph of locomotor activity counts during the posttest session. Data in B and C are expressed as means±S.E.M.
We next examined the possible involvement of DAergic transmission to the mPFC in the cocaine CPP expression. For this, SCH23390 (0.3 or 1.0 µg), raclopride (3.0 µg), or vehicle was injected into the mPFC immediately before the posttest (Fig. 2A). A one-way ANOVA revealed a significant difference in CPP scores among the vehicle-, 0.3 µg SCH23390-, and 1.0 µg SCH23390-injected groups (F2,15=4.27, p=0.0341; Fig. 2B). SCH23390 dose-dependently decreased the CPP score (0.3 µg, p=0.1417; 1.0 µg, p=0.0216, post hoc Holm–Sidak test; Fig. 2B). On the other hand, intra-mPFC raclopride injection did not affect the CPP score (vehicle, 216.9±55.8 s, n=7, raclopride, 123.3±74.2 s, n=7, t12=1.064, p=0.3083, Student’s t-test). Intra-mPFC injection of SCH23390 (F2,15=0.0418, p=0.9592; Fig. 2C) or raclopride (t12=1.601, p=0.1354) had no effect on locomotor activities. These results indicate that DAergic transmission to the mPFC via D1 receptors is involved in the cocaine CPP expression.
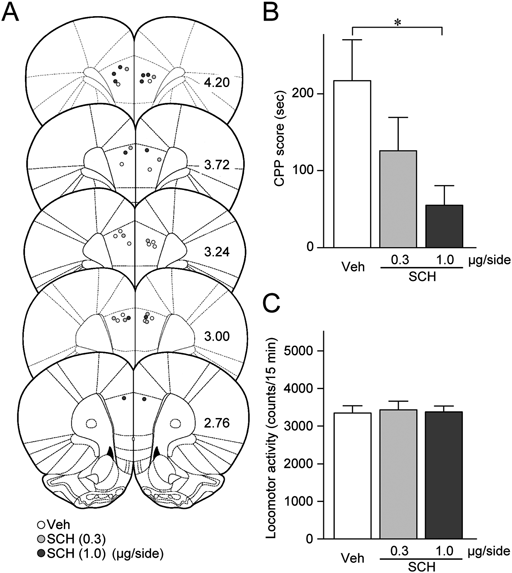
(A) The placements of the tips of the microinjection cannulae for the injection of 0.3 µg/side SCH23390 (SCH (0.3), light gray circles, n=6), 1.0 µg/side SCH23390 (SCH (1.0), dark gray circles, n=6), or vehicle (Veh, white circles, n=7) into the mPFC. The numbers beside each panel represent the approximate AP distances (mm) from bregma. (B) A summary graph of CPP scores. * p<0.05, one-way ANOVA with post hoc Holm–Sidak test. (C) A summary graph of locomotor activity counts during the posttest session. Data in B and C are expressed as means±S.E.M.
We next addressed whether mPFC neuronal activity is associated with cocaine CPP expression by monitoring cFos expression after the posttest in rats conditioned using saline or cocaine. We confirmed that the CPP score of the cocaine-conditioned rats was significantly higher than that of the saline-conditioned rats (t8=3.538, p=0.0076, Student’s t-test; Fig. 3A). Locomotor activities during the posttest were not significantly different between the groups (t8=0.5403, p=0.6037; Fig. 3B). Figures 3C and D summarize the cFos expression in the mPFC. The number of cFos-positive cells in the cocaine-conditioned rats was significantly larger than that in saline-conditioned rats (t8=10.97, p<0.0001, Student’s t-test). These data indicate that the increased mPFC neuronal activity during the posttest, which is possibly associated with DAergic transmission to the mPFC, is related to the cocaine CPP expression.
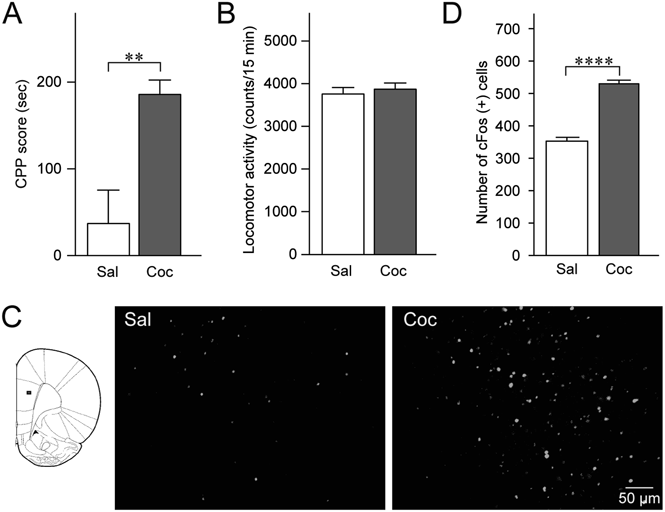
(A) Summary graph of CPP scores of rats conditioned with saline (Sal, n=5) and cocaine (Coc, n=5). ** p<0.01, Student’s t-test. (B) Summary graph of locomotor activity counts during the posttest session. (C) Representative photomicrographs of cFos-positive cells in the mPFC after the posttest session. Small rectangle on the left panel shows the region depicted in the photomicrographs. (D) Summary graph of the number of cFos-positive cells in the mPFC after the posttest session. **** p<0.0001, Student’s t-test. Data in A, B, and D are expressed as means±S.E.M.
The findings above suggest that D1 receptor-mediated DAergic transmission in the mPFC activates mPFC neurons. To test this hypothesis more directly, we injected AAV-hSyn-eYFP or AAV-hSyn-ChR2-eYFP into the VTA (Fig. 4A), and photostimulated eYFP-positive axon terminals in the mPFC (Fig. 4B) of the rats that had received a systemic injection of vehicle or SCH23390. Figure 4C shows eYFP and cFos expressions in rats that received a systemic vehicle injection with either an intra-VTA AAV-hSyn-eYFP injection (ChR2(−)/Veh, n=5) or an intra-VTA AAV-hSyn-ChR2-eYFP injection (ChR2(+)/Veh, n=7), and a systemic SCH23390 injection with either an intra-VTA AAV-hSyn-eYFP (ChR2(−)/SCH, n=5) or an intra-VTA AAV-hSyn-ChR2-eYFP injection (ChR2(+)/SCH, n=5). As shown in Fig. 4D, a two-way ANOVA revealed that the effects of ChR2 photostimulation and SCH23390 treatment on the number of cFos-positive cells were significant (ChR2 effect: F1, 18=18.34, p=0.0004; SCH23390 effect: F1, 18=10.56, p=0.0044), although there was no significant interaction between the effects of ChR2 photostimulation and SCH23390 treatment (F1, 18=0.7092, p=0.4107). The number of cFos-positive cells in the ChR2(+)/Veh was significantly higher than that in the ChR2(−)/Veh (p=0.0070, two-way ANOVA with post hoc Holm–Sidak test), indicating that photostimulation of ChR2(+) axons increases cFos-positive cells. Although there was no significant difference in the number of cFos-positive cells between the ChR2(−)/Veh and ChR2(−)/SCH (p=0.2225), the ChR2(+)/SCH exhibited a significantly lower number of cFos-positive cells than that in the ChR2(+)/Veh (p=0.0296). These results demonstrate that SCH23390 treatment decreased the number of cFos-positive cells, which was increased by photostimulation of ChR2-positive axons in the mPFC. We also found that there was no significant difference in the density of eYFP-expressing fibers in the mPFC between the ChR2(+)/Veh and ChR2(+)/SCH (t10=0.9118, p=0.3833), confirming that the lower number of cFos-positive cells in the ChR2(+)/SCH may not be due to a lower expression of ChR2 in these rats. Taken together, activation of mPFC neurons via D1 receptor stimulation most likely plays an important role in the cocaine CPP expression.
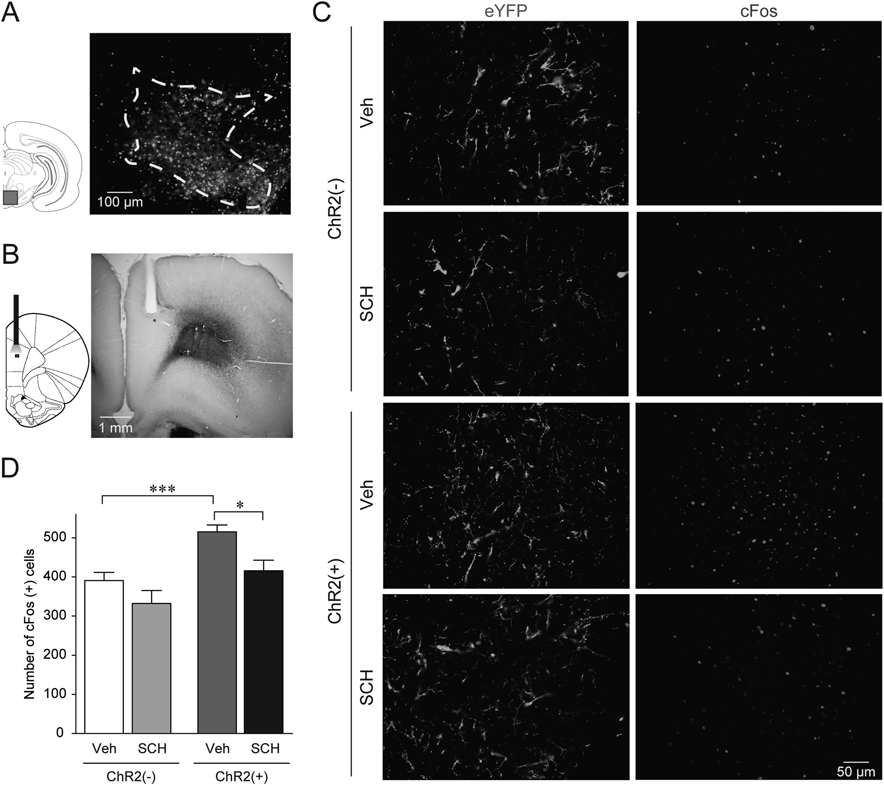
(A) Representative photomicrograph of ChR2-eYFP-expressing cells in the VTA. Rectangle on the left panel shows the region depicted in the photomicrograph. (B) Representative photomicrograph of the optical fiber placement in the mPFC. Small rectangle on the left shows the region depicted in the photomicrograph expanded in panel C. (C) Representative photomicrographs of the mPFC after photostimulation. Left and light panels show eYFP-positive fibers and cFos-positive cells, respectively. Upper (ChR2(−)) and lower (ChR2(+)) two panels were obtained from rats that received an intra-VTA infusion of AAV-hSyn-eYFP and AAV-hSyn-ChR2-eYFP, respectively. In each case, rats are injected with vehicle (Veh) or 1.0 mg/kg SCH23390 (SCH) before the photostimulation. (D) Summary graph of the number of cFos-positive cells in the mPFC after photostimulation. The number of animals in each group is as follows: ChR2(−)/Veh, n=5; ChR2(−)/SCH, n=5; ChR2(+)/Veh, n=7; ChR2(+)/SCH, n=5. *, *** p<0.05, 0.001, two-way ANOVA with post hoc Holm–Sidak test. Data are expressed as means±S.E.M.
We found that the intra-VTA B/M injection and the intra-mPFC injection of SCH23390 but not raclopride significantly reduced cocaine CPP scores, indicating that an enhanced VTA DAergic neuronal activity and subsequent DAergic transmission in the mPFC via D1 receptor stimulation are critical for the cocaine CPP expression. The requirement of DAergic neuronal activity has been suggested by previous studies showing that the blockade of nicotinic and muscarinic acetylcholine receptors in the VTA9) and inhibition of D1 receptors in the VTA that leads to a reduction in extracellular glutamate levels23) also attenuate the cocaine CPP expression.24) Although, to the best of our knowledge, this is the first direct evidence showing a role for mPFC D1 receptors in the cocaine CPP expression, accumulating evidence indicates that mPFC D1 receptors are associated with the reinstatement of extinguished cocaine CPP25) and cocaine self-administration.26) Thus, although the neural mechanisms for the cocaine CPP expression may be different from those involved in the reinstatement of cocaine CPP or self-administration, these findings indicate that D1 receptor-mediated transmission in the mPFC is generally critical for context- and cue-induced initiation of cocaine-seeking behaviors.
The number of cFos-positive neurons in the mPFC after the posttest in rats conditioned using cocaine was higher than that observed with saline, indicating that mPFC neurons are excited during the posttest of cocaine CPP only when rats had previously experienced the rewarding effects of cocaine. Considering the data showing that D1 receptor blockade attenuated the cocaine CPP scores (Fig. 2), these findings suggest that DAergic transmission most likely contributes to cFos expression in the mPFC. In support of this, the photostimulation of VTA-derived ChR2 fibers increased the number of cFos-positive mPFC neurons; further, this increase was inhibited by a prior systemic SCH23390 administration, indicating the DAergic but not glutamatergic27) transmission-dependent cFos induction. This is also supported by a recent study showing that selective photostimulation of VTA DAergic neurons expressing ChR2 in TH-Cre mice increases the cFos-positive mPFC neurons.22) Therefore, these findings, albeit indirect, strongly suggest that D1 receptor-mediated excitation of mPFC neurons during the posttest might induce cocaine-seeking behavior. Consistent with this, it has been reported that a reduction in mPFC neuronal activity attenuates the expression of cocaine CPP.3)
Previous studies demonstrated the increased expression of cFos in mPFC GABAergic but not pyramidal neurons after the CPP expression.2) This is consistent with findings that DA increases inhibitory synaptic transmission in pyramidal cells,28) and that VTA stimulation results in excitation of fast-spiking interneurons.29) By contrast, it has been reported that DA depolarizes and excites mPFC pyramidal cells.30,31) Moreover, a D1 receptor agonist has been shown to enhance pyramidal cell excitability, potentiate N-methyl-D-aspartate (NMDA)-induced excitation, and induce persistent activation of pyramidal cells.32,33) Additionally, the optogenetic inhibition of projections from the mPFC to the NAc prevents the reinstatement of cue-induced cocaine self-administration,34) suggesting a critical role of output neurons in the mPFC in cue-induced cocaine-seeking behavior. Further studies to investigate the functional role of mPFC GABAergic and pyramidal neurons in the expression of cocaine CPP using cell type-specific manipulation of neuronal activity would be necessary to clarify this issue.
VTA DAergic neurons are divided into subpopulations depending on the input-output relationships.8,35,36) One subpopulation responds to rewarding stimuli, whereas the other responds to aversive events. The former mainly projects to the NAc lateral shell, while the latter innervates the mPFC.8,35,36) Our results indicate that mPFC projecting DAergic neurons may be activated during the posttest, indicating that these DAergic neurons may respond not only to aversive stimuli but also to rewarding stimuli. Alternatively, these DAergic neurons might also be activated when emotional memory, irrespective of it being a rewarding or aversive stimulus, is retrieved,37) or when novel cues for animals are shown.38)
In the present study, we found that VTA DAergic neuronal activity is necessary for the cocaine CPP expression, which may be at least partly regulated by cholinergic input from the LDT because this input has been reported to increase DAergic neuronal activity.10) However, other inputs such as glutamatergic transmission from the LDT8) and orexinergic transmission from the lateral hypothalamus39) might also contribute to activating VTA DAergic neurons. Additionally, we have reported that repeated cocaine administration enhances the excitatory synaptic transmission to LDT cholinergic neurons7) and induces membrane excitation through an increase in persistent sodium conductance in those neurons.6) Given that VTA DAergic neurons also exhibit excitatory synaptic plasticity after cocaine exposure,40) these findings indicate that LDT-VTA signaling might be readily enhanced in cocaine-associated situations. As we found that D1 receptor-mediated signaling in the mPFC is critical for the cocaine CPP expression, the LDT-VTA-mPFC circuitry may trigger an executive function in context-dependent cocaine-seeking behaviors.
This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas on “Elucidation of the neural computation for prediction and decision making” (K.K., 26120702) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, a Grant-in-Aid for Scientific Research (C) (K.K., 15K06765) from the Japan Society for the Promotion of Science (JSPS), and Grants from Hoansha Foundation (K.K.).
The authors declare no conflict of interest.