2017 Volume 40 Issue 2 Pages 169-173
2017 Volume 40 Issue 2 Pages 169-173
Shivering associated with spinal anesthesia during Cesarean delivery is an uncomfortable experience for the parturient, which may also cause adverse effects. In this prospective, randomized, double-blind, placebo-controlled study, we sought to evaluate the effect of intrathecal dexmedetomidine, administered as an adjunct to hyperbaric bupivacaine for Cesarean delivery, on the incidence and severity of shivering associated with spinal anesthesia. Patients undergoing Cesarean delivery were randomly allocated to three groups of 30 patients each. Experimental treatments were added to hyperbaric bupivacaine as follows: Patients in group I (control) were administered isotonic saline. Patients in groups II and III received dexmedetomidine (2.5, 5 µg, respectively), mixed with isotonic saline. Shivering was observed in 11, 10 and 2 patients in groups I, II and III, respectively. The incidence of shivering in group III was significantly lower than that in groups I (p=0.005) and II (p=0.01). The severity of shivering was significantly different between the three groups (p=0.01). There were no significant inter-group differences with respect to mean arterial pressure and heart rate at any time point after administration of intrathecal local anesthesia (p>0.05). Intrathecal dexmedetomidine (5 µg) administered as an adjunct to hyperbaric bupivacaine during Cesarean delivery significantly reduced the incidence and intensity of shivering associated with spinal anesthesia.
Spinal anesthesia (SA) is widely used for covering elective or emergency Cesarean delivery as it provides adequate anesthesia and analgesia for this purpose while avoiding risks associated with general anesthesia.1–3) Shivering is a common and distressing side effect which is associated with increased oxygen consumption, carbon dioxide production, and metabolic rate. Shivering may also interfere with intraoperative monitoring of electrocardiogram, blood pressure (BP), and pulse oxygen saturation.4,5) Moreover, it is an uncomfortable experience for the parturient.6) Multiple studies have demonstrated that intravenous (i.v.) dexmedetodine, 0.5 or 1.0 µg/kg body weight provides effective prophylaxis against post-anesthetic shivering.7,8) However, a recent meta-analysis of clinical trials demonstrated that intravenous dexmedetomidine was not optimal for prevention of shivering due to its potential side effects.9) More importantly, the use of i.v. dexmedetomidine to facilitate Cesarean delivery in the parturient population has been reported in several case reports, including in a woman with primary pulmonary hypertension,10) and in another with spinal muscular atrophy.11) On the contrary, many studies showed that dexmedetomidine was an effective adjuvant to intrathecal local anesthetics for prolonging the duration of analgesia for lower abdominal surgeries, lower limb surgeries and Cesarean delivery.12,13) However, the preventive effect of intrathecal dexmedetomidine on shivering has not been evaluated.
We designed a double-blind, randomized, placebo-controlled study to determine whether intrathecal dexmedetomidine, administered along with hyperbaric bupivacaine for covering Cesarean delivery decreases the incidence of shivering associated with spinal anesthesia.
The study protocol was approved by the ethics committee at the Second Xiangya Hospital, Central South University, China. Written informed consent was obtained from all subjects prior to their enrolment in the study. The trial is registered with the Chinese clinical trial registry (registration number ChiCTR-IIR-15007548).
Ninety obstetric patients (singleton pregnancy; American Society of Anesthesiologists (ASA) physical status I or II; age range 18–40 years; gestational age ≥37 weeks) who were scheduled for Cesarean delivery under spinal anesthesia were enrolled in the study. Parturients with contraindications to regional anesthesia, hypersensitivity to amide local anesthetics or dexmedetomidine, cases of severe preeclampsia, hyperthyroidism, cardiopulmonary disease, and those with a known history of alcohol or substance abuse were excluded from the study. Patients were randomly allocated to three groups (I, II and III; N=30 each) using a computer-generated random number table.
Anesthesia ProcedureMedication was prepared by an anesthesiologist who was not involved in the study. All therapeutic interventions were standardized. Prior to administration of spinal anesthesia, patients were placed under standard monitoring, and received 4 mL/kg hydroxyethyl starch 130/0.4 sodium chloride injection. Patients were subsequently maintained on crystalloid fluid infusion at the rate of 10 mL/kg body weight per hour. Oxygen was administered during anesthesia; patients were covered with drapes but not actively warmed.
The preservative-free dexmedetomidine 100 µg/mL was loaded into a 40 unit insulin syringe (2.5 µg/unit). All patients were administered spinal anesthesia with 2.5 mL of 0.5% hyperbaric bupivacaine. In addition to hyperbaric bupivacaine, patients in groups II and III received 1 unit and 2 units of dexmedetomidine, respectively; patients in group I received an equivalent volume of normal saline. The total volume was 3.0 mL in all the groups (by adding appropriate amount of preservative-free 0.9% saline where necessary).
Spinal anesthesia was administered in the left lateral position, at the L3–4 intervertebral space using a midline approach, with a 25-gauge Quincke spinal needle. The intrathecal injection was given after confirming free flow and positive aspiration for cerebrospinal fluid (CSF). Injection speed in all the groups was maintained at 0.1 mL/s. After spinal injection, parturients were immediately placed in a supine position with left uterine displacement.
The level of sensory and motor block was monitored by an independent anesthesiologist who was blinded to the anesthetic regimen administered; any shivering episode was documented. Maternal BP was non-invasively measured at the forearm every 2 min subsequent to the administration of spinal anesthesia. Any episode of hypotension (systolic BP <90 mmHg or >30% drop from the baseline BP) was treated with intravenous ephedrine (10–15 mg). Decrease in the heart rate <50 beats per min was treated with incremental doses of intravenous atropine (0.3–0.5 mg). Respiratory depression (pulse oxygen saturation <90% or >10% drop from the baseline) was treated with face mask oxygen inhalation.
The level of sensory block (defined as the loss of pinprick sensation), was recorded bilaterally at the mid-clavicular line every 2 min. The peak sensory block level was defined as the highest cephalad sensory level that persisted for four consecutive testing (temperature, pinprick, or pinch). The onset of sensory blockade at highest level was recorded. Shivering was graded using a four-point scale (none=no perceptible tension of muscles observed; mild=slight muscle tonus of masseter muscle; moderate=shivering of proximal muscles; severe=generalized shivering of the whole body).14) Intravenous fluids infused were at room temperatures and ambient temperature of the operating room was maintained at 22–28°C. Occurrence of pruritus, nausea, vomiting, dizziness and bradycardia was documented. The duration of surgery was recorded. Apgar scores were recorded at 1 and 5 min. A blinded observer was involved in the data collection.
Based on our previous experience, the incidence of shivering was estimated to be 6% after administration of intrathecal dexmedetomidine versus 35% in the absence of dexmedetomidine. Assuming a <5% probability of a type I error, (i.e., significance level α=0.05) and <20% for that of a type II error (i.e., accepting a null hypothesis when it is false, β=0.20), the required sample size in each group was estimated to be 27. However, we recruited 30 patients in each group.
Statistical AnalysisStatistical analyses were performed using Statistical Product for Social Sciences (SPSS) software v. 18.0. Data pertaining to normally distributed continuous variables (age, weight, height, baseline tympanic temperature, highest segment blocked, time to reach highest block, time of onset of motor block, lowest heart rate (HR), lowest systolic arterial pressure (SAP) and Apgar scores) are expressed as the mean±standard deviation (S.D.); inter-group differences were assessed using one-way ANOVA. Inter-group differences with respect to the incidence and maximal intensity of shivering was assessed using χ2 test or ridit test, as appropriate. Data are expressed as the mean±S.D. or median (range). A value of p<0.05 was considered indicative of a statistically significant between-group difference. In the event of any discrepancy, a post-hoc test using Bonferroni correction was performed for paired comparison, and a corrected p-value of 0.05/3 was considered statistically significant.
A total of 98 patients were assessed for eligibility. Eight patients were not included in the trial because of their refusal to participate (Fig. 1). No statistically significant between-group differences were observed between the three groups with respect to age, weight, height, baseline tympanic temperature, gestational age, or ASA class. Further, no significant inter-group differences were observed with respect to the time to achieve the highest level of sensory block, the maximal number of segments blocked, duration of surgery and the Apgar scores at 1 and 5 min between the three groups (p>0.05, Table 1). The incidence of respiratory depression, pruritus, nausea, vomiting, dizziness and bradycardia was comparable in all the 3 groups with no statistic ally significant between-group differences.
| Variables* | Group I | Group II | Group III |
|---|---|---|---|
| Age (year) | 29.5±3.8 | 30.0±3.1 | 31.1±3.7 |
| ASA (I : II) | 20 : 10 | 21 : 9 | 20 : 10 |
| Weight (kg) | 69.9±7.2 | 69.9±6.0 | 70.1±6.5 |
| Height (cm) | 161.7±5.3 | 159.4±3.8 | 160.9±4.4 |
| Gestational age (week) | 38 (37–41) | 38 (37–40) | 38 (37–41) |
| Baseline tympanic temperature (°C) | 36.7±0.24 | 36.9±0.19 | 36.8±0.20 |
| Highest segment sensory blocked (dermatome) | T4 (T4–T5) | T4 (T4–T5) | T4 (T4–T5) |
| Time to reach highest sensory block (min) | 7.3±1.4 | 6.8±1.3 | 6.7±1.1 |
| Apgar 1 min | 10 (8–10) | 10 (8–10) | 10 (8–10) |
| Apgar 5 min | 10 (9–10) | 10 (9–10) | 10 (9–10) |
| Duration of surgery (min) | 53.3±6.3 | 52.1±7.1 | 55.1±6.8 |
| Nausea/vomiting | 1/1 | 2/1 | 1/1 |
| Dizziness/bradycardia/pruritus | 1/0/0 | 1/0/0 | 1/0/0 |
| Respiratory depression | 0 | 0 | 0 |
ASA, American Society of Anesthesiologists. * Data expressed as the mean±S.D.
Shivering was observed in 11 (36.7%), 10 (33.3%) and 2 (6.7%) patients in group I, II and III, respectively. The incidence of shivering in group III was significantly lower than that in group I (p=0.005) and II (p=0.01) (Fig. 2). In group II, 2, 4 and 4 subjects experienced mild, moderate, and severe shivering, respectively. In group III, mild shivering was observed in 2 subjects, while none of the subjects experienced shivering of moderate or severe intensity. The intensity of shivering in group III was significantly lower than that in group I (p=0.000) and II (p=0.000) (Fig. 2).
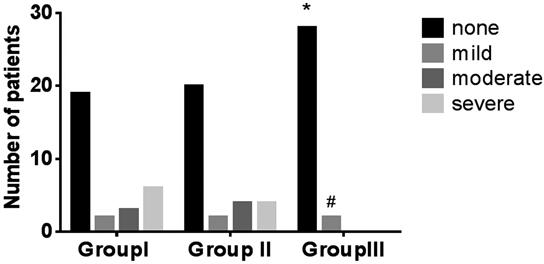
* Compared with group I, p<0.001 (incidence of shivering). # Compared with group I, p<0.001 (severity of shivering).
There were no significant inter-group differences with regard to mean arterial pressure (MAP) or HR at any time-point after administration of intrathecal local anesthesia (p>0.05, Fig. 3).
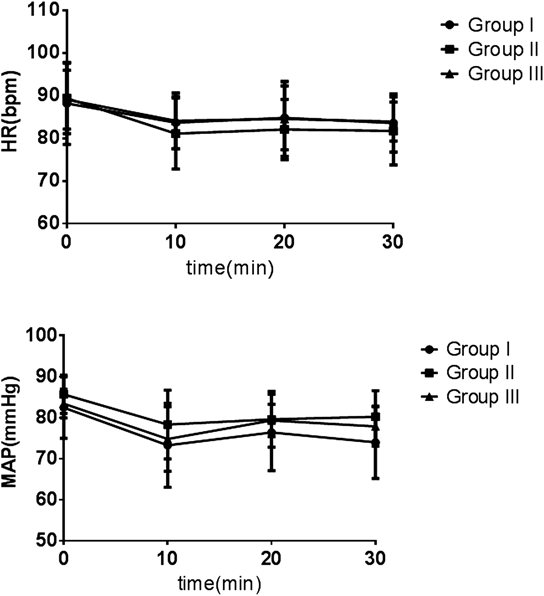
No statistically significant inter-group differences were observed.
In this prospective, randomized, double-blind, placebo-controlled study, we sought to evaluate the effect of intrathecal different dose dexmedetomidine in reducing the incidence and severity of intraoperative shivering associated with spinal anesthesia. We observed that the addition of 5 µg dexmedetomidine to hyperbaric bupivacaine for spinal anesthesia in parturients reduced the incidence of intraoperative shivering in a dose-dependent manner, and in the absence of any noticeable side-effects, while supplementation of bupivacaine with 2.5 µg dexmedetomidine did not decrease the incidence of shivering.
Various pharmacological therapies aim to prevent or treat shivering include opioids, clondine, meperidine, granisetron and tramadol, but some adverse side-effects may occur during drug administration.9) Dexmedetomidine, a highly selective α-2-adrenoreceptor agonist, is a novel drug for suppression of shivering associated with regional as well as general anesthesia.15–17) The mechanism of action by which dexmedetomidine inhibits shivering is complex. Several studies have demonstrated that dexmedetomidine alleviated shivering mainly through its central α2-adrenoreceptor agonist effect.18,19) Alpha-2 adrenergic receptors, which are widely distributed in the hypothalamus, are thought to mediate thermoregulatory inhibition. In many studies, dexmedetomidine was shown to increase the temperature range without triggering thermoregulatory defenses through stimulation of central α2-adrenergic receptors, thereby decreasing the central thermoregulatory threshold for shivering.20–22) It is possible that the anti-shivering effect of intrathecal dexmedetomidine may be caused by decreasing shivering threshold.
The route of administration of dexmedetomidine in previous studies was intravenous.20–23) Moreover, robust data on safety of use of IV dexmedetomidine in parturients is largely lacking. Callaway et al. reported a decreased incidence of shivering with use of i.v. dexmedetomidine, 1 µg/kg, in normal volunteers; however, this effect was associated with decreased systolic blood pressure.24) Prevention of shivering by administration of intravenous dexmedetomidine in a parturient may be limited by its principal side-effects, i.e., hypotension and bradycardia.9) Several studies have shown that use of intrathecal dexmedetomidine as an adjuvant to hyperbaric bupivacaine is associated with prolonged motor and sensory block, while maintaining the hemodynamic stability.25–27) Recent studies have shown that dexmedetomidine may be safely used as an intrathecal supplement in Cesarean delivery.12,28) Respiratory depression is a potential side effect of dexmedetomidine. But, the side effect is just reported to occur by using high doses or prolonged intravenous pumping dexmedetomidine.29) Many studies demonstrated intravenous optimal dose (0.5–1 µg/kg) or intrathecal 2.5 or 5 µg dexmedetomidine didn’t result respiratory depression.30,31)
Therefore, intrathecal dexmedetomidine might be more appropriate than i.v. dexmedetomidine in reducing the incidence of shivering associated with spinal anesthesia for Cesarean delivery.
Many animal studies have demonstrated that dexmedtomidine has a neuroprotective effect. In clinical research, no adverse neurological outcomes have been reported with intrathecal use of dexmedetomidine as an adjunct to local anesthetic.32–35) Recently, many studies have demonstrated that intrathecal ropivacaine or bupivacaine-dexmedetomidine may increase the duration and efficacy of analgesia and decrease postoperative analgesic use, without notable adverse effects.11,12,36)
The use of dexmedetomidine as an adjuvant to bupivacaine for covering Cesarean delivery provided better intra-operative analgesia and did not affect Apgar scores or caused any noticeable side effects.13,28) The intrathecal doses of dexmedetomidine used in our study were based on previous human studies wherein no neurotoxic effects were documented at these doses.25–27,33–35) A limitation of this study was that a dose–response experiment was not performed to determine the optimal dose of dexmedetomidine required for optimal suppression of shivering, but without causing any significant side effects. In the present study, dexmedetomidine was used as an adjuvant to hyperbaric bupivacaine in a dose of 5 µg as this dosage is reported to be associated with prolonged duration of sensory and motor block without significant side effects.26,27)
In conclusion, intrathecal dexmedetomidine (5 µg) as an adjuvant to hyperbaric bupivacaine for spinal anesthesia in patients undergoing Cesarean delivery appears to be safe and effective in decreasing the incidence and severity of shivering.
The authors declare no conflict of interest.