2017 Volume 40 Issue 3 Pages 318-326
2017 Volume 40 Issue 3 Pages 318-326
Zinc (Zn) is a trace element with anti-diabetes mellitus (anti-DM) effects. Zn complexes exhibit stronger insulin-like activity than Zn ions. Bis(hinokitiolato)zinc complex ([Zn(hkt)2]) was recently reported to be a potent anti-DM candidate. We examined the effects of [Zn(hkt)2] on insulin resistance and pancreatic islet cells through in vivo long-term ingestion studies. In an in vivo study, we performed 4-month long-term [Zn(hkt)2] administration experiments in KK-Ay mice as a type 2 DM animal model. Ingestion of [Zn(hkt)2] resulted in lower blood glucose levels compared with the non-treated KK-Ay mice (control group). Additionally, [Zn(hkt)2] treatment decreased plasma insulin concentration compared with that of the non-treated KK-Ay group. [Zn(hkt)2] treatment resulted in a significant suppression of islet cell enlargement and a significantly decreased number of insulin-positive cells compared with the non-treated KK-Ay control group. The [Zn(hkt)2] treatment group showed the increasing tendency in the amount of Zn levels in peripheral organs; liver, muscle, adipose, and pancreas, compared with the non-treated KK-Ay control group. However, the Zn level in the pancreas of the [Zn(hkt)2] treatment group did not show the significant increase compared with the non-treated KK-Ay control group. This accumulation of Zn in pancreas suggested that [Zn(hkt)2] mainly effects on the peripheral tissue, and [Zn(hkt)2] has the less effect on the pancreas directly. Thus, we concluded that [Zn(hkt)2] exerted the main effect on peripheral organs by ameliorating insulin resistance.
Zinc (Zn) is an essential trace element. In adults, total Zn content is estimated to be 2 grams. Total Zn content is maintained at a constant level in the body and plays an important role in various biological functions.1–3) Several reports support the notion that Zn possesses anti-diabetes mellitus (anti-DM) effects.4–7) In 1992, ZnCl2 was reported to exhibit insulin-like activity and improve symptoms of hyperglycemia in animal models of DM.8) DM is a metabolic disease characterized by chronic hyperglycemia, and the number of new DM cases is increasing every year.9) In more serious cases, patients with DM are at risk of developing DM complications which reduce the quality of life as disease progresses. Therefore, it is essential to not only suppress the progression of DM, but also prevent the onset of the disease itself. Moreover, current therapies do not cure DM, and novel agents are required. DM is mainly divided into two groups; type 1 DM and type 2 DM. Type 1 DM is the one of the autoimmune disease in pancreas, and there is a fault in the pancreas, especially insulin production. Type 2 DM occurs from the insufficient of insulin secretion or the hypersecretion of insulin, resulting from the insulin resistance. Both type 1 and type 2 DM result in the pancreas dysfunction. Ideally, a new type of anti-DM drug is critically needed to protect pancreatic islet cells and to prevent progressive islet cell loss in both type 1 and type 2 DM.
A novel finding was reported in 2000 that Zn complexes exhibit stronger insulin-like activity than Zn ions; since then, we have reported that various types of Zn complexes exert anti-DM effects.10–13) We aimed to identify Zn complexes with high anti-DM effects at low doses. Bis(hinokitiolato)Zn complex ([Zn(hkt)2]) contains hinokitiol, a compound derived from natural products. We previously reported that [Zn(hkt)2] exhibits anti-DM blood glucose lowering effects in KK-Ay type 2 DM mouse model by intraperitoneal (i.p.) injection.14) Using cultured 3T3-L1 adipocytes, we recently demonstrated that [Zn(hkt)2] activates the insulin signaling pathway by inducing Akt phosphorylation in an insulin-independent manner.15,16) These results suggested that [Zn(hkt)2]-inducing activation of the insulin signaling pathway has the potential to ameliorate insulin resistance in peripheral organs such as adipose tissue and muscles.
Pancreatic duodenal homeobox-1 (PDX-1) is a transcription factor consisting of 283 amino acids that it is predominantly expressed in β cells of adult pancreatic islets. Following glucose influx into β cells, PDX-1 translocates into the nucleus and induces transcription of various genes, including insulin and glucose transporter type 2 (GLUT2).17) PDX-1 activity is associated with insulin gene expression and secretion as well as and the enlargement of β cells caused by insulin resistance.18,19) Lower PDX-1 activity decreases insulin synthesis.20) Thus, it is important to study PDX-1 to fully understanding insulin secretory capacity and β cell homeostasis.
Previously, we reported that Zn complexes with various coordination modes have the anti-DM effect by i.p. injection or oral administration with KK-Ay mice. These results showed that Zn complexes have the higher bioavailability and more potent activity than Zn2+ ion.4,5,21–24) In this study, we investigated the effects of [Zn(hkt)2] on Akt phosphorylation using the RIN-5F cell line in in vitro, which is a secondary clone of the rat islet tumor cell line RIN-m and the in vivo anti-DM effects of [Zn(hkt)2] using the KK-Ay mice via the oral route. We conducted a long-term feeding study in mice fed a high-fat diet (HFD) containing [Zn(hkt)2] for 4 months using pioglitazone, which is the one of the thiazolidine derivatives, as a positive control which we have used before in the previous study.23) Although we previously described the anti-DM effects of Zn complexes, we did not investigate the effects of their long-term intake on various organs. Thus, in the current study, we examined the effects of the [Zn(hkt)2] compound on pancreatic islets, the principal tissue responsible for insulin secretion.
Pioglitazone (PIO) was obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Hinokitiol and all other chemical reagents were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). RIN-5F cells were purchased from DS Pharma Biomedical Co., Ltd. (Osaka, Japan). Fetal bovine serum (FBS) was purchased from Equitech-Bio, Inc. (Kerrville, TX, U.S.A.). RPMI-1640, Dulbecco’s modified Eagle’s medium (DMEM), and bovine serum albumin (BSA, protein standard) were obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). Antibiotics/antimycotics were obtained from Nakalai Tesque, Inc. (Kyoto, Japan). Specific antibodies against phospho-Akt (Ser473), Akt, and horseradish peroxidase (HRP)-conjgated anti-rabbit immunoglobulin G (IgG) were obtained from Cell Signaling Technologies (Danvers, MA, U.S.A.). Anti-mouse/rat PDX-1/IPF1 antibody was purchased from R&D Systems (AF2517, Minneapolis, MN, U.S.A.). HRP-conjugated anti-goat IgG was obtained from Bethyl Laboratories (A50-200P, Montgomery, TX, U.S.A.). Immobilon™ Western Chemiluminescent HRP substrate was obtained from Millipore (Billerica, MA, U.S.A.).
Preparation and Characterization of [Zn(hkt)2]The [Zn(hkt)2] complex was prepared in deionized water/ethanol by mixing Zn(CH3COO)2 and hinokitiol at a 1 : 2 molar ratio, and the solution was stirred for 12 h at room temperature. The resulting pale yellow precipitate was collected by vacuum filtration, washed several times with pure ethanol and dried overnight in vacuo.14) These prepared complexes were characterized by elemental analysis, IR absorption (Shimadzu FT-IR 8100 A on KBr pellet, Shimadzu Co., Kyoto, Japan), and low-resolution mass spectrometry (JEOL JMS-SX 102AQQ, JEOL Ltd., Tokyo, Japan). Elemental analyses were performed at the Analytical Center of Kyoto Pharmaceutical University (KPU). Low-resolution mass spectra were measured in electron ionization (EI)(+) mode at the Analytical Center of KPU. Elemental analysis (Found/Calcd); C 59.40/59.14%, H 5.91/5.86%. Infrared spectra (complex/ligand); νC=O=1591/1609 cm−1. EI(+) MS m/z; 390 [M]+.
Cell CultureRIN-5F cells are rat insulinoma cell line, derived from rat pancreatic β cells, and secret insulin. RIN-5F cells were cultured in 60-mm Petri dishes under 5% CO2 at 37°C in RPMI-1640 supplemented with 10% FBS and antibiotics/antimycotics. Media were changed every two days. Experiments were performed when cells were approximately 80–90% confluent.
Immunoblotting AnalysisAn immunoblotting analysis against phospho-Akt and total Akt was performed following previously described methods.25) The 80–90% confluent RIN-5F cells were starved in serum-free RPMI-1640 for 4 h at 37°C and stimulated with insulin, ZnSO4, or [Zn(hkt)2] for 10 min using low glucose (11 mM) RPMI-1640 or high glucose (25 mM) DMEM. After incubation, the cells were washed twice with ice-cold PBS, and scraped in cell lysis buffer (10 mM Tris–HCl, pH 7.5, 0.875% Brij-97, 0.125% nonidet P-40 (NP-40), 150 mM NaCl, 2.5 mM ethylenediaminetetraacetic acid (EDTA), 10 mM NaF, 0.1 mM Na3VO4, 200 µM phenylmethylsulfonyl fluoride (PMSF), and 5 µg/mL leupeptin) at 4°C for 30 min. The lysates were centrifuged at 15000×g at 4°C for 20 min. The supernatant was used to detect Akt phosphorylation by a Western blot analysis. The protein concentration was measured by a BCA assay (Thermo Scientific, Logan, UT, U.S.A.), using BSA as the standard. The whole lysates (5 µg) were separated using a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel. The resolved proteins were transferred to polyvinylidene difluoride (PVDF) membranes. The blotted membranes were incubated in 5% non-fat dry milk in Tris-buffered saline (TBS) containing 0.1% (v/v) Tween-20 (TBS/T) in order to block the nonspecific absorption of antibodies. The membranes were reacted with primary antibodies in 5% non-fat dry milk-TBS/T at 4°C for overnight, followed by washing and incubation with the secondary antibody (HRP-conjugated anti-rabbit IgG). Specific immunoreactions were visualized using the Immobilon™ Western Chemiluminescent HPR substrate and by exposure to Hyperfilm™ ECL (GE Healthcare Bio-Science, Piscataway, NJ, U.S.A.).
AnimalsMale KK-Ay mice (4 weeks old) with type 2 DM weighing 25–30 g and control C57BL/6J mice (4 weeks old) were purchased from CLEA Japan, Inc. (Tokyo, Japan). Animals were maintained on a 12 h light/dark cycle in our central animal facility, and KK-Ay mice were housed individually in one cage. All animals were allowed free access to original food (Table 1) and tap water. All animal experiments were approved by the Experimental Animal Research Committee of KPU and were performed according to the Guidelines for Animal Experimentation of KPU.
| Normal and CNT | PIO | [Zn(hkt)2] | |||
|---|---|---|---|---|---|
| 10 mg Zn | 20 mg Zn | 30 mg Zn | |||
| Casein | 18.2 | 18.2 | 18.2 | 18.2 | 18.2 |
| Sucrose | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Lard | 18.2 | 18.2 | 18.2 | 18.2 | 18.2 |
| Vitamin mix. AIN 93N | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 |
| Mineral mix. AIN 93N | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 |
| Cellulose | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 |
| L-Cystine | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Choline bitartrate | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| t-Butylhydroquinone | 0.0007 | 0.0007 | 0.0007 | 0.0007 | 0.0007 |
| Cornstarch | 15.5 | 15.5 | 15.5 | 15.4 | 15.3 |
| Sample | 0.00 | 0.02 | 0.04 | 0.08 | 0.19 |
| Water | 9.1 | 9.1 | 9.1 | 9.1 | 9.1 |
| Total (%) | 100 | 100 | 100 | 100 | 100 |
All animals had free access to water and semi-synthetic HFDs that were high in sugar, and therefore hypercaloric (composition of the basal diet [%]: sucrose, 30%; lard fat, 18.2%; casein, 18.2%; Kobe Women’s University special diets, Kobe, Japan). Diets were prepared for all groups using AIN-93N provided with a mixture of a standard diet (Oriental East Co., Ltd., Tokyo, Japan) as shown in Table 1. For the preparation of the diet consisting 30 mg PIO/kg body weight (BW) for the PIO group, the dose of PIO was determined by the previous studies.26–28) We maintained the ratio of PIO in the HFD as 0.02%, the intake of PIO concentration were varied because of the changes of food intake and BW in PIO group. For the preparation of the diet consisting of 30 mg Zn for the [Zn(hkt)2] group, the Zn sample ratio was adjusted to maintain Zn intake levels (30 mg Zn/kg BW/d) in consideration of the changes in BW and food intake.
Animal ExperimentsAll animals used for the in vivo study were 6 weeks old. C57BL/6J mice were used as non-diabetic control mice (Normal). Male KK-Ay mice were divided into three groups, fed either a basal HFD (CNT), a basal HFD with added PIO, or a basal HFD supplemented with [Zn(hkt)2]. The doses of PIO were 15–35 mg PIO/kg BW. The doses of [Zn(hkt)2] were 10 mg Zn/kg BW for 0–29 d, 20 mg Zn/kg BW for 30–80 d, and 30 mg Zn/kg BW for 81–120 d.
C57BL/6J mice were allowed free access to a solid HFD and tap water for 4 months (Normal). KK-Ay mice with type 2 DM were allowed free access to a solid HFD for CNT, and HFD with PIO or [Zn(hkt)2], and tap water for 4 months. Blood glucose levels were measured once per week and BW, food intake, and water consumption were monitored every 3 days. Blood samples for analysis of blood glucose concentration were obtained from the tail vein of the C57BL/6J normal mice and the KK-Ay mice, and measurements were obtained using the glucose oxidase method (Glucocard, Arkray, Kyoto, Japan).
Upon completion of PIO or [Zn(hkt)2] administration, blood was collected from orbital exsanguinations of mice under anesthesia with ether, centrifuged for 10 min at 650×g in 4°C. Serum samples for analyses of blood urea nitrogen (BUN), glutamic pyruvic transaminase/alanine aminotransferase (GPT/ALT), glutamic oxaloacetic transaminase/aspartate aminotransferase (GOT/AST), triglyceride (TG), total cholesterol (TCHO), high density lipoprotein (HDL), insulin, and adiponectin were separated. Serum levels of BUN, GPT/ALT, GOT/AST, TG, TCHO, and HDL were measured using a Fuji Dry Chem system (FUJIFILM Medical Co., Ltd., Tokyo, Japan), and those of insulin and adiponectin were determined using a Morinaga Mouse Insulin Kit (Morinaga Institute of Biological Science, Inc., Yokohama, Japan) and adiponectin Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D systems, Minneapolis, MN, U.S.A.), respectively. HbA1c levels in the blood following PIO or [Zn(hkt)2] administration were determined using a DCA 2000 system (Bayer Medical Co., Leverkusen, Germany). The values of HOMA-R as the index of insulin resistance were calculated as: (fasting blood insulin concentration×fasting blood glucose)/405.
Oral Glucose Tolerance Test (OGTT)After administration of PIO or [Zn(hkt)2] for 4 months, oral glucose tolerance tests were performed. KK-Ay mice were fasted for 12 h and glucose (1 g/kg BW) was given orally. Blood samples were obtained from the tail vein at 0, 0.25, 0.5, 1.0, 1.5, and 2.0 h after glucose administration. Blood glucose concentrations were measured using a Glucocard system (Arkray Inc., Kyoto, Japan) with the glucose oxidase method.
Tissue Fixation and ProcessingAt 4 months of age, pancreas tissue from C57BL/6J and KK-Ay mice was removed, fixed in 10% buffered formalin, sectioned at a thickness of 3 µm, and stained with hematoxylin and eosin (H&E). Double immunostaining for insulin and glucagon was performed using an indirect immunoenzyme method with a cocktail containing an anti-insulin rabbit polyclonal antibody (Santa Cruz Biotechnology, Dallas, TX, U.S.A.) and an anti-glucagon mouse monoclonal antibody (Novus Biologicals, Littleton, CO, U.S.A.). Immunoreactivities of insulin and glucagon were visualized with diaminobenzidine (DAB; brown color) and Fuchsin+ (red color) solutions (Dako, Glostrup, Denmark), respectively. Immunostaining for PDX-1 was performed using the universal immunoenzyme polymer method (Nichirei Bioscience, Tokyo, Japan) with an anti-PDX-1 goat polyclonal antibody (R&D Systems, Minneapolis, MN, U.S.A.), and the signal was developed with a DAB solution (Dako). HE-stained sections and immunostaining sections were recorded by a light microscope (BX-51; Olympus, Tokyo, Japan) at 200× magnification. The size of islets was measured using NIS-Elements for D document (Olympus, Tokyo, Japan).
Determination of Zn Concentration in Organs of C57BL/6J and KK-Ay Mice Treated with PIO or [Zn(hkt)2]After the administration period, mice were sacrificed and the liver, kidneys, muscle tissue, adipose tissue, and pancreas were collected. After washing the organs, approximately 30 mg of tissue was obtained, heated to 180°C, and supplemented with 2 mL of 60% HNO3, 2 mL of 60% HClO4, and 2 mL of 30% H2O2. This procedure was repeated until all organic material was removed. After cooling the samples to room temperature, residues were re-suspended in 9 mL of 5% HNO3. Zn concentrations were determined using inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7700x/Mass Hunter; Agilent Technologies, Inc., Santa Clara, CA, U.S.A.).
StatisticsData are expressed as the mean±standard deviation (S.D.). The significance of differences among groups was determined using one-way ANOVA and Dunnett multiple comparison tests. Differences were considered significant at p<0.05 or p<0.01.
We examined the effects of [Zn(hkt)2] on induction of Akt phosphorylation with respect to the glucose concentrations. RIN-5F cells were stimulated with 20 µM [Zn(hkt)2] for 10 min. ZnSO4 did not induced Akt phosphorylation, but [Zn(hkt)2] induced Akt phosphorylation significantly in a low-glucose concentration medium (11 mM glucose) (Fig. 1A). When the cells were treated with 20 µM [Zn(hkt)2] for 10 min in a high-glucose concentration medium (25 mM glucose), ZnSO4 did not show any change. In this case, [Zn(hkt)2] tended to increase Akt phosphorylation, but not significantly (Fig. 1B).
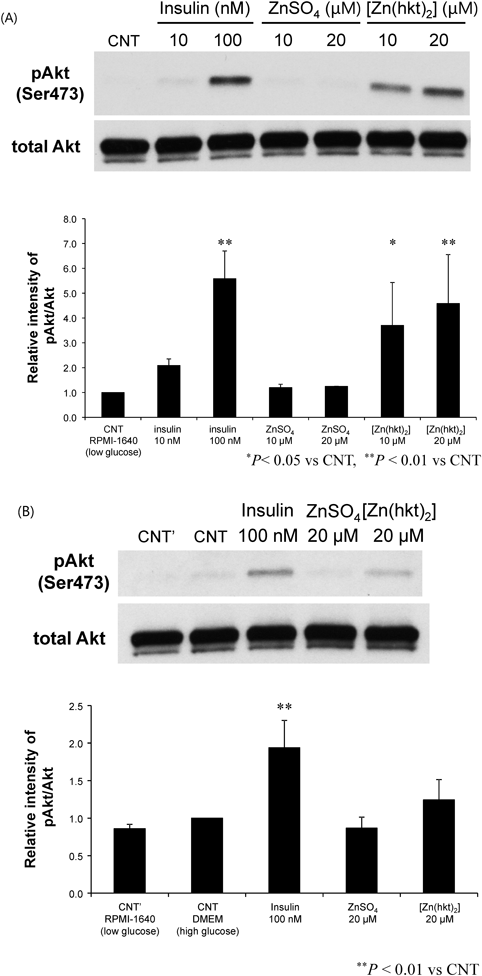
Serum starved RIN-5F cells were treated with 100 nM insulin or 20 µM Zn samples for 10 min in (A) low glucose (11 mM, 200 mg/dL) RPMI-1640 and (B) high glucose (25 mM, 450 mg/dL) DMEM. The cell lysates were separated with 10% SDS-PAGE and immunoblotted with phospho-Akt (pAkt) and Akt antibodies. Data are expressed as the mean±S.D. for three independent experiments. * Significance at p<0.05 vs. CNT. ** Significance at p<0.01 vs. CNT.
We did not investigate the effects of long-term Zn complex intake on various organs, and then the effects of [Zn(hkt)2] were examined in vivo using the KK-Ay type 2 DM mouse model. During the feeding period, a gradual increase in BW was observed in the Normal group. A striking increase in BW was observed in the PIO group; increased BW is a reported side effect of PIO treatment, consistent with this finding. Compared with the Normal group, BW was higher in the CNT and Zn groups but did not differ between the latter two groups (Fig. 2A). In terms of blood glucose concentration, significant normoglycemic effects were observed in the PIO group compared with the CNT group, immediately after the initiation of the dosing period (p<0.01); the blood glucose concentration was maintained at approximately the same level as in the Normal group (Fig. 2B). However, in the Zn group, blood glucose concentration did not decrease significantly but was maintained at a lower level compared to that of the CNT group. In particular, the gap in blood glucose concentration between the CNT group and the Zn group became wider after day 93. The significant decrease in blood glucose concentration in the Zn group was observed at days 42, 93, and 111 (p<0.05 or p<0.01) (Fig. 2B). The Normal group showed the small amount of food intake compared with the CNT, PIO, and Zn groups in terms of food intake; there were no differences among the latter three groups (Fig. 2C). The Normal and PIO groups had approximately the same water intake, which was slightly lower than that in the other two groups (Fig. 2D).

* Significance at p<0.05 vs. control KK-Ay mice. ** Significance at p<0.01 vs. control KK-Ay mice.
After the administration period, we evaluated lipid metabolism, kidney function, and liver function (Table 2). No differences were observed among groups in terms of BUN, an index of kidney function. In terms of liver function, GPT/ALT and GOT/AST were increased in the CNT group, indicating impaired liver function. The PIO and Zn groups showed a trend of decreasing and improvement in terms of GPT/ALT. We also examined lipid metabolism markers such as TG, TCHO, and HDL. There was no significant variation in TG among any of the four groups. However, in terms of TCHO and HDL, the PIO group showed the significant decrement and improvements (p<0.01 for TCHO, p<0.05 for HDL); the Zn group showed a trend of decreasing HDL. These findings suggested that [Zn(hkt)2] intake promoted restoration of liver function. Levels of HbA1c—an index of long-term blood glucose variability—significantly decreased in the PIO group (p<0.01), consistent with the blood glucose data obtained during the feeding period. No significant change in the HbA1c level was observed in the Zn group compared with the CNT group.
| BUN | TG | TCHO | HDL | GPT/ALT | GOT/AST | HbA1c | Insulin | HOMA-R | Adiponectin | |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg/dL) | (U/L) | (%) | (ng/mL) | (µg/mL) | ||||||
| Normal | 21.2±1.7 | 108±20 | 135±35* | 101±28 | 16±6** | 43±5** | ND | 0.7±0.3** | 8.6±4.8* | 15.1±6.2** |
| CNT | 21.5±1.0 | 151±29 | 184±42 | 132±31 | 95±40 | 100±7 | 8.1±0.8 | 5.0±1.7 | 39.5±22.0 | 3.5±1.0 |
| PIO | 20.2±4.7 | 138±36 | 121±24** | 96±9* | 61±20* | 68±10** | 4.5±0.3** | 1.0±0.2** | 5.6±3.3* | 23.3±2.4** |
| Zn | 18.6±4.9 | 165±47 | 175±39 | 108±28 | 53±13** | 92±22 | 7.1±1.4 | 3.1±1.1** | 20.1±11.5* | 4.6±1.9 |
* Significance at p<0.05 vs. CNT. ** Significance at p<0.01 vs. CNT.
After the administration period, we performed OGTT—a method for measuring insulin resistance—and measured plasma concentrations of insulin and adiponectin. Blood glucose level decreased in the Zn group at 0.25, 0.5, and 1.0 h compared with the CNT group (p<0.05 or p<0.01) (Fig. 3A). For the change in area under the curve (ΔAUC), the Zn group showed a significant decrease compared with the CNT group (p<0.05) (Fig. 3B). Significant decreases in plasma insulin concentration compared with that in the CNT group were observed in the other groups (p<0.01) (Table 2). The values of HOMA-R, the index of the insulin resistance, also showed decrement tendency like the results in plasma insulin concentration. The CNT group showed hypoadiponectinemia, but the adiponectin concentrations in the Zn group were not significantly different from those in the CNT group (Table 2). However, the serum adiponectin concentration in the PIO group was restored to a similar concentration as that in the Normal group. There was a trend of decreased insulin resistance in the Zn group. The observed effects of [Zn(hkt)2] differed from the adiponectin-mediated effects of PIO.

After being fasted for 12 h, mice were given an oral glucose solution at a dose of 1 g/kg body weight (A). Area under the blood glucose concentration–time curve (AUC) of normal C57BL/6J mice, control KK-Ay mice, and KK-Ay mice after daily oral ingestion of PIO or [Zn(hkt)2] (B). * Significance at p<0.05 vs. control KK-Ay mice (CNT). ** Significance at p<0.01 vs. control KK-Ay mice (CNT).
The finding of improved plasma insulin concentration led us to perform a histopathological examination of the pancreas. Striking enlargement of pancreatic islets was observed in the CNT group (Fig. 4A). However, in the PIO and Zn groups, a significant inhibition of such enlargement was observed (p<0.01) (Fig. 4B). Thereafter, we quantified the numbers of insulin-positive and glucagon-positive cells within pancreatic islets using double immunostaining (Fig. 5A). The insulin-positive cell count within pancreatic islets was significantly decreased in the PIO group (p<0.01) and in the Zn group (p<0.05) compared with that of the CNT group (Fig. 5B).

H&E staining (scale bar=100 µm) (A) and pancreatic islet area (B) in normal C57BL/6J mice (Normal), control KK-Ay mice (CNT), KK-Ay mice daily treated with PIO or [Zn(hkt)2] (Zn). The numbers of analyzed images are as follows: for the Normal group n=35, the CNT group n=42, the PIO group n=33, and the Zn group n=52. ** Significance at p<0.01 vs. control KK-Ay mice (CNT).

Double immunostaining for insulin (brown color) and glucagon (red color) (scale bar=100 µm) (A) and insulin positive cell counts (B) in normal C57BL/6J mice (Normal), control KK-Ay mice (CNT), KK-Ay mice daily treated with PIO or [Zn(hkt)2] (Zn). The numbers of insulin-positive cells were counted using the hematoxylin-stained nuclear as the cell index. The numbers of analyzed images are as follows: for the Normal group n=59, the CNT group n=90, the PIO group n=48, and the Zn group n=100. * Significance at p<0.05 vs. control KK-Ay mice (CNT). ** Significance at p<0.01 vs. control KK-Ay mice (CNT).
In addition, we quantitatively evaluated PDX-1-positive cells (Fig. 6A). The PDX-1-positive cell count in pancreatic islets showed a similar trend as that of the insulin-positive cell count. The CNT group displayed an increased cell count compared with that of the Normal group. The PIO and Zn groups displayed a trend of decreased cell count compared with that of the CNT group but there were no significant differences (Fig. 6B).

Immunostaining for PDX-1 (scale bar=100 µm) (A) and PDX-1 positive cell counts (B) in normal C57BL/6J mice (Normal), control KK-Ay mice (CNT), KK-Ay mice daily treated with PIO or [Zn(hkt)2] (Zn). The numbers of analyzed images are as follows: for the Normal group n=31, the CNT group n=33, the PIO group n=36, and the Zn group n=59. * Significance at p<0.05 vs. control KK-Ay mice (CNT).
The amount of Zn in the liver, kidneys, muscle tissue, adipose tissue, and pancreas of C57BL/6J and KK-Ay mice was determined after the treatment period using the ICP-MS method. As shown in Table 3, the CNT group showed a significant decrease in hepatic Zn concentration compared with the Normal group (p<0.05). The renal Zn concentration in the Zn group significantly decreased compared with that in the CNT group (p<0.05). Moreover, Zn levels in the Zn group were notably increased in the liver, moderately in muscle and adipose tissue, and slightly in the pancreas compared with the CNT group. In particular, the Zn level in the liver of the Zn group showed a distinct increment compared to that in the CNT group. However, Zn levels in the pancreas and kidney of the PIO group were not different, while the Zn levels in the liver, muscle, and adipose tissue of the PIO group tended to increase. These results suggested that the therapeutic effects of PIO could be related to the restoration of Zn levels in insulin resistance-associated organs.
| Liver | Kidney | Muscle | Fat | Pancreas | |
|---|---|---|---|---|---|
| (µg/g organ) | |||||
| Normal | 62±14* | 67±4 | 30±7 | 1.9±0.7 | 44±6 |
| CNT | 20±6 | 71±3 | 41±10 | 5.6±2.3 | 37±5 |
| PIO | 39±8 | 62±8 | 57±13 | 8.4±6.0 | 36±8 |
| Zn | 49±21 | 56±10* | 52±18 | 7.4±3.7 | 44±8 |
* Significance at p<0.05 vs. CNT.
We investigated the effects of [Zn(hkt)2] on DM-associated biochemical and physiological parameters using in vitro and in vivo approaches. As we showed that [Zn(hkt)2] induced Akt phosphorylation in 3T3-L1 adipocytes,15,16) we examined [Zn(hkt)2] for inducing Akt phosphorylation in RIN-5F cells. We revealed that RIN-5F showed the different cell reactivity in Akt phosphorylation under different two types of medium; low-glucose (11 mM, 200 mg/dL) RPMI-1640, or high-glucose (25 mM, 450 mg/dL) DMEM (Figs. 1A, B). Next, we evaluated mRNA expression of PDX-1 and insulin in high-glucose (25 mM) DMEM considering hyperglycemic state. When cells were treated for 2 h in high-glucose (25 mM) DMEM, insulin and [Zn(hkt)2] significantly increased the PDX-1 and insulin mRNA expressions (Supplementary Fig. S1). These in vitro data suggested that 1) the cells shows the different reactivity depending on the extracellular glucose concentrations, and that 2) [Zn(hkt)2] may protect pancreas when [Zn(hkt)2] is distributed to pancreas. Then, we examined the in vivo study using diabetic KK-Ay mice. In the previous study, we had revealed the effects of short-term [Zn(hkt)2] administration on KK-Ay mice.14) On the other hand, we did not obtain any data of the long-term [Zn(hkt)2] intake influences on various organs. We assessed the in vivo effects of long-term [Zn(hkt)2] administration on DM improvement. Neither striking normoglycemic effects nor increased adiponectin secretion was observed after 4 months of [Zn(hkt)2] administration (Fig. 2B, Table 2). The administration routes of [Zn(hkt)2] were different between the previous and the present study. In the previous study of short-term [Zn(hkt)2] administration, the KK-Ay mice were given the [Zn(hkt)2] by i.p. administration.14) [Zn(hkt)2] could be distributed to the blood and organs in high concentration by the i.p. administration. Considering the gastrointestinal absorption, the ingestion of [Zn(hkt)2] in HFD could not make [Zn(hkt)2] to be distributed to the blood and organs as high concentration as the i.p. administration of [Zn(hkt)2]. However, plasma insulin concentration and oral glucose tolerance significantly improved (Table 2, Fig. 3). These findings suggested that the anti-hyperglycemic effects of [Zn(hkt)2] were distinct from those of PIO. Thiazolidine derivatives have been reported to possess adipocyte differentiation-inducing activity, and they directly bind to PPARγ to regulate transcription.29) Serum adiponectin concentration is positively controlled by thiazolidine derivatives.30) In the present study, long-term PIO intake resulted in increased plasma adiponectin and decreased plasma insulin concentrations, improving insulin resistance (Table 2). [Zn(hkt)2] intake showed the possibility for ameliorating the insulin resistance in the results of plasma insulin concentration in this study. Previous studies using Zn complexes showed the amelioration of the insulin resistance.4,5,7) We also found that PIO increased BW, which was probably caused by edema, one of the side effects of PIO intake.31) Notably, this BW gain was not observed with [Zn(hkt)2] treatment. Thus, lower BW gain is a potential advantage of [Zn(hkt)2] usage over PIO usage.
In our histopathological evaluation, [Zn(hkt)2] intake significantly suppressed the enlargement of pancreatic islets and decreased the number of insulin-positive cells (Figs. 4, 5). Similar results were obtained for PDX-1 positive cells, but the difference was not significant (Fig. 6). In addition, PIO reduced the insulin-positive cell count in islets, as well as plasma insulin levels via indirect effects. PIO displays strong effects in peripheral organs such as adipose tissue.32) For example, PIO induces the secretion of adiponectin, which ameliorates insulin resistance and normalizes blood glucose levels.
We recently reported that [Zn(hkt)2] treatment modified 3T3-L1 adipocytes via the activation of Akt phosphorylation.15,16) However, from our results in this study, the in vivo effect of [Zn(hkt)2] on HFD-fed type 2 DM mice was not sufficient to ameliorate the daily blood glucose levels compared to PIO intake. [Zn(hkt)2] exerted its effects on adipocytes by inducing insulin signaling pathway activation and glucose uptake. However, in the present study, the dose of [Zn(hkt)2], 10–30 mg Zn/kg BW, was a relatively small amount to decrease blood glucose levels.
The Zn level in the liver of the Zn group tended to increase compared to that of the CNT group (Table 3). AdipoR1 and AdipoR2 are adiponectin receptors expressed in many organs; AdipoR1 is expressed in skeletal muscle, and AdipoR2 in the liver.33) Tanabe et al. reported that a Zn ion is bound in a coordinated form (three His residues and one Asp residue) within the seven-transmembrane domains of the AdipoR1 and AdipoR2 structures.34) They also reported that these receptors require Zn to exert those functions as the adiponectin receptors, and that Zn is involved in the structure stabilization of the receptors by using the AdipoR1, AdipoR2 and the mutants for each receptor. Thus, the activities of those receptors could decrease when Zn levels in the liver decrease. In this study, the Zn group had increased Zn content in the liver, suggesting that the [Zn(hkt)2] intake could ameliorate the sensitivities of AdipoR receptors and potentially enhance the effects of adiponectin. Our previous studies reported that oral Zn complex administration significantly increased the Zn levels in the blood and organs compared to Zn2+ ions such as ZnCl2, or ZnSO4.5,21–24) We also reported that the Zn complexes labeled by radioisotope 65Zn were distributed to the organs and they exhibited the significantly higher Zn levels in the organs than Zn ion. Then, it is suggested that the increment of Zn levels in the organs of the [Zn(hkt)2] group was fundamentally due to the [Zn(hkt)2] ingestion. The Zn level in the pancreas of the Zn group did not show the significant increase compared with the CNT group. This accumulation of Zn in pancreas suggested that [Zn(hkt)2] mainly effects on the peripheral tissue, and [Zn(hkt)2] has the less effect on the pancreas directly. The Zn accumulations in the Zn group were observed on the increase in liver, muscle, and fat tissues compared to the CNT group. The previous studies revealed that [Zn(hkt)2] induced Akt phosphorylation in adipocytes and also inhibited PTP1B and PTEN, which are the negative regulators of insulin signaling.15,16) From these results, the possible mechanism of [Zn(hkt)2] is the induction of Akt phosphorylation in the insulin target tissues; liver, muscle, and adipose, and this induction brings the amelioration of the insulin resistance and the pancreas protection.
Additional studies are required to determine the optimal dose and administration methods for [Zn(hkt)2] to decrease blood glucose concentrations. Detailed analyses are also necessary to clarify the [Zn(hkt)2] mechanisms associated with the amelioration of insulin resistance in Zn-targeted organs.
In conclusion, we considered that the observed anti-hyperglycemic effects of [Zn(hkt)2] intake occurred via an improvement of peripheral insulin resistance, as [Zn(hkt)2] ameliorated insulin resistance. The anti-hyperglycemic effects of [Zn(hkt)2] and the degree of amelioration in insulin resistance were different from those observed with PIO treatment, which induced adiponectin secretion. [Zn(hkt)2] exhibited islet-protective effects in an indirect manner on the basis of ameliorating insulin resistance in the periphery. It could be possible to obtain the stronger hypoglycemic effect combined with the effect on the peripheral tissue, if new Zn complexes could be identified that display a greater uptake in the pancreas.
The authors are grateful to the members of the Analytical Center of KPU for the elemental analysis and mass spectra measurements. This study was financially supported in part by a Grant from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. Supported Program for the Strategic Research Foundation at Private Universities, 2012–2016 (S1201008). This work was also supported by the JSPS KAKENHI; Grant numbers 25460048 (to YY) and 26460636 (to HY).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.