2017 Volume 40 Issue 3 Pages 352-356
2017 Volume 40 Issue 3 Pages 352-356
Osteoclasts represent the only bone resorbing cells in an organism. In this study, we investigated the effect of glucosamine (GlcN), a nutrient used to prevent joint pain and bone loss, on the osteoclastogenesis of murine macrophage-like RAW264 cells. GlcN supplementation suppressed the upregulation of osteoclast-specific genes (tartrate-resistant acid phosphatase (TRAP), cathepsin K, matrix metallopeptidase 9, and nuclear factor of activated T cell c1 (NFATc1)), receptor activator of nuclear factor-κB ligand (RANKL)-dependent upregulation of TRAP enzyme activity, and the formation of TRAP-positive multinuclear cells more effectively than N-acetylglucosamine (GlcNAc), which we have previously shown to inhibit osteoclast differentiation. To clarify the mechanism by which GlcN suppresses osteoclastogenesis, we further investigated the effect of GlcN on O-GlcNAcylation by Western blotting and on other types of glycosylation by lectin blotting. We found that, upon addition of GlcN, the O-GlcNAcylation of cellular proteins was increased whereas α2,6-linked sialic acid modification was decreased. Therefore, these glycan modifications in cellular proteins may contribute to the suppression of osteoclastogenesis.
Bone homeostasis is regulated by the balance between bone formation and resorption; their imbalance leads to pathological bone disease such as osteoporosis. The only cell in an organism capable of resorbing bone is the osteoclast, which is derived from the monocyte/macrophage lineage.1–3) Osteoclasts are tartrate-resistant acid phosphatase (TRAP)-positive multinuclear cells and their differentiation is regulated by macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL). During the differentiation process, M-CSF induces the expression of RANK, the receptor of RANKL. Following receptor binding, RANKL stimulates its downstream signaling pathways and activates nuclear factor of activated T cell c1 (NFATc1), which is the master transcriptional regulator of osteoclast differentiation and induces the expression of osteoclast specific genes such as TRAP, cathepsin K, and matrix metallopeptidase 9 as well as the NFATc1 gene itself.4)
Recently, we reported that the extracellular addition of N-acetylglucosamine (GlcNAc) suppresses osteoclast differentiation in part through the promotion of O-GlcNAcylation.5) This may occur through the incorporation of extracellular GlcNAc into the cells by pinocytosis whereupon it may be converted intracellularly to uridine diphosphate (UDP)-GlcNAc, which could be utilized for O-GlcNAcylation and also other types of glycosylation such as N-glycosylation.6) Notably, it has been reported that GlcNAc or glucosamine (GlcN) feeding attenuated bone loss caused by menopause and that the numbers of TRAP-positive osteoclasts differentiated from bone marrow stem cells isolated from GlcNAc- or GlcN-fed ovariectomized mice were lower than those from control ovariectomized mice.7) Furthermore, extracellular GlcN, which is also converted intracellularly to UDP-GlcNAc, is considered to be more effectively incorporated into cells than GlcNAc because unlike GlcNAc, GlcN is taken up by the cells via a glucose transporter.8) Therefore, it could be hypothesized that GlcN suppresses osteoclastogenesis as well and to a more effective degree.
In this study, we investigated the effect of GlcN on osteoclastogenesis using RANKL-dependent osteoclastogenic differentiation of murine RAW264 cells, which is known to suitably reflect the physiological differentiation process,9) as well as mouse primary bone marrow cells.
The mouse macrophage-like RAW264 cell line was obtained from the RIKEN Cell Bank (Tsukuba, Japan) and maintained in modified Eagle’s medium alpha (Wako Pure Chemical Industries, Ltd., Osaka, Japan) containing 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, U.S.A.) and 1×penicillin/streptomycin (Wako Pure Chemical Industries, Ltd.) under a humidified atmosphere containing 5% CO2 at 37°C.
Osteoclast Differentiation, TRAP Enzyme Activity Assay, and TRAP StainingRAW264 cells were seeded on a 96-well plate (1000 cells/well) and cultured for 1 d. Thereafter, the cells were treated with 250 ng/mL glutathione S-transferase (GST)-tagged soluble RANKL (sRANKL) (Oriental Yeast, Tokyo, Japan) in the presence of GlcN (glucosamine hydrochloride) or GlcNAc (N-acetylglucosamine) (Wako Pure Chemical Industries, Ltd.) and allowed to differentiate for 4 d. The sugars were dissolved in phosphate-buffered saline (PBS). RANKL (GST-tagged sRANKL) concentration used in this study was optimized by our preliminary experiments (data not shown). Differentiated cells were subjected to TRAP enzyme activity assay or TRAP staining as described previously5) and TRAP-positive cells that were stained red and contained three or more nuclei were counted.
Real-Time PCRRAW264 cells were allowed to differentiate for 4 d as described. Total RNA extraction and cDNA synthesis were performed using the Power SYBR® Green Cells-to-CT™ Kit (Life Technologies, Carlsbad, CA, U.S.A.) according to the manufacturer’s instruction. Real-time PCR was performed with the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, U.S.A.) and the Power SYBR® Green PCR Master Mix (Life Technologies) as described previously.5) PCR was performed with a preliminary incubation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 65°C for 1 min. The primers used for PCR were as follows: Hprt: 5′-GCT CGA GAT GTC ATG AAG GAG-3′ and 5′-CAG CAG GTC AGC AAA GAA CTT-3′; TRAP: 5′-AGG GAG TGG CAG GGC AGG AA-3′ and 5′-TTG TAG GCC CAG CAG CAC CA-3′; cathepsin K: 5′-GGC TGT GGA GGC GGC TAT-3′ and 5′-AGA GTC AAT GCC TCC GTT CTG-3′; matrix metallopeptidase 9 (MMP9): 5′-AAA GAC CTG AAA ACC TCC AAC CT-3′ and 5′-GCC CGG GTG TAA CCA TAG C-3′; and NFATc1, 5′-TTC AGC TGG AGG ACA CCC CAT-3′ and 5′-CGT GAT CCG GTG GAC CTG GTA-3′.
Immunoblotting and Lectin BlottingRAW264 cells were seeded on a 6-well plate (20000 cells/well) and cultured for 1 d. Thereafter, cells were stimulated with RANKL and allowed to differentiate for 4 d. Differentiated cells were washed with PBS and lysed in 200 µL sample buffer (50 mM Tris–HCl, pH 6.8, 1% sodium dodecyl sulfate, 10% glycerol, 0.01% bromophenol blue, and 2% 2-mercaptoethanol) with sonication. After boiling and centrifugation, the resulting supernatants were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the separated proteins were transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Berkeley, CA, U.S.A.) using a wet electroblotting system. Immunoblotting and lectin blotting were performed on an iBind™ Western Device (Life Technologies) according to the manufacturer’s instructions. Horseradish peroxidase (HRP)-conjugated anti-O-GlcNAc (CTD110.6) mouse monoclonal antibody (Cell Signaling Technology, Danvers, MA, U.S.A.) was used for immunoblotting. Biotin-conjugated Sambucus sieboldiana agglutinin (SSA), Maackia amurensis agglutinin (MAM), HRP-conjugated Ricinus communis agglutinin I (RCA120), Phaseolus vulgaris agglutinin (PHA-E4), Canavalia ensiformis agglutinin (Con A), and Lens culinaris agglutinin (LCA) (J-Oil Mills, Tokyo, Japan) were used for lectin blotting. The blots were visualized using a Luminata Crescendo (Merck Millipore, Darmstadt, Germany) and signals were detected using ChemiDoc XRS+ (Bio-Rad). Equal protein loading was confirmed by Coomassie brilliant blue staining. Bovine serum albumin (approximately 30 ng/lane), which contains no glycan, was used as a negative control for lectin blotting. The intensities of total O-GlcNAcylated proteins or glycoproteins detected by each lectin were measured using NIH ImageJ software.10)
Statistical AnalysisData are presented as the mean±standard deviation (S.D.). Statistical analysis was carried out using StatMate 3 software (ATOMS Inc., Tokyo, Japan). The significance of variance was determined by one-way ANOVA followed by Tukey’s multiple comparison test. The probability level used to determine statistical significance was p<0.05. Each experiment was repeated at least two times with similar results.
To investigate the effect of GlcN on osteoclastogenesis, we stimulated RAW264 cells with RANKL in the presence of GlcN or GlcNAc. After 4 d, cells were lysed and their TRAP enzyme activity was measured (Fig. 1A). Treatment with GlcNAc (20 mM) or GlcN (2 mM) significantly suppressed RANKL-dependent induction of TRAP enzyme activity without affecting cell viability (data not shown). Although 20 mM GlcN treatment also suppressed TRAP enzyme activity, this treatment exhibited cytotoxicity (data not shown). Therefore, we used sugars at 2 mM or lower concentration for the subsequent experiments. These concentrations are higher than that observed in vivo, because pharmacokinetic studies in humans have shown that the Cmax after recommended GlcN dosing (1500 mg/d) is approximately 10 µM11); thus, we used these concentrations to clearly observe the effect of GlcN on in vitro osteoclastogenesis. We next examined the effect of GlcN and GlcNAc on the formation of multinuclear TRAP-positive osteoclasts. After 4 d of RANKL stimulation in the presence of sugars, cells were stained and TRAP-positive multinuclear cells containing more than 3 nuclei/cell were counted (Fig. 1B). The results indicated that the numbers of formed osteoclasts were more effectively reduced by treatment with GlcN than GlcNAc.
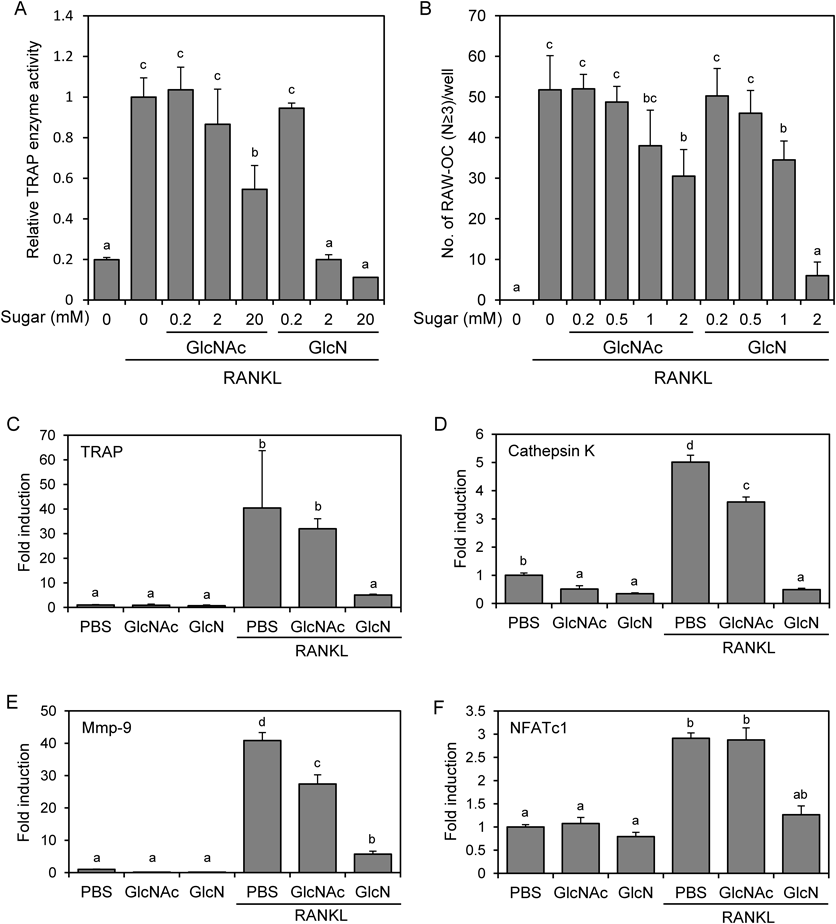
(A, B) RAW264 cells were treated with RANKL and GlcN or GlcNAc at the indicated concentrations. Differentiated RAW264 cells were subjected to TRAP enzyme activity assay (A) or TRAP staining followed by counting of TRAP-positive multinuclear cells (RAW-OCs) (B). (C–F) RAW264 cells were treated with RANKL and 2 mM of GlcN or GlcNAc. Real-time PCR analysis of the osteoclast marker genes, TRAP (C), cathepsin K (D), matrix metallopeptidase 9 (MMP9) (E), and NFATc1 (F) were performed using cDNA prepared from differentiated RAW264 cells. Data are expressed as the mean±S.D. Values with different letters are significantly different.
To confirm the effect of the sugars at the molecular level, the expression of osteoclast marker genes was analyzed by real-time PCR. RAW264 cells were stimulated with RANKL in the presence of 2 mM GlcNAc or GlcN. After 4 d, the mRNA expression of TRAP, cathepsin K, MMP9, and NFATc1 was analyzed (Figs. 1C–F). RANKL stimulation significantly induced the expression of these genes, whereas GlcN effectively suppressed their expression and GlcNAc also tended to suppress them. Furthermore, we observed that GlcN tended to suppress osteoclastogenesis of primary-cultured mouse bone marrow cells (data not shown). Together, these results showed that GlcN suppresses osteoclast differentiation more effectively than GlcNAc, presumably because extracellular GlcN is more efficiently taken up by the cells via transporters.
GlcN Treatment Increases O-GlcNAcylated Proteins and Decreases Glycoproteins Containing α2,6-Linked Sialic AcidGlcN and GlcNAc are intracellularly metabolized and converted to UDP-GlcNAc, which is used for O-GlcNAcylation and glycosylation.6,12) As GlcNAc treatment increases global O-GlcNAcylation, which in turn has been shown to suppress osteoclast differentiation,5) GlcN may also suppress osteoclastogenesis through O-GlcNAcylation. Therefore, to clarify the effect of GlcN on O-GlcNAcylation, RAW264 cells were treated with RANKL in the presence of sugars at 2 mM for 4 d and the cells were then lysed and subjected to Western blotting using an anti-O-GlcNAc antibody. The total band intensity of each lane was measured because numerous proteins were modified with O-GlcNAc; the relative intensities are shown in the bottom of the panel in Fig. 2A. We found that GlcN treatment resulted in a global increase of O-GlcNAcylation regardless of RANKL stimulation. Conversely, GlcNAc treatment showed little effect on the amount of total O-GlcNAcylated proteins, presumably because GlcNAc is less efficiently taken up by the cells than GlcN. However, given that 2 mM GlcNAc treatment affected osteoclast differentiation to a slight degree (Fig. 1), it is possible that although GlcNAc was incorporated in the cells and increased O-GlcNAcylation to some extent, this increase may not have been substantial enough to be detected in this experiment owing to limited sensitivity.
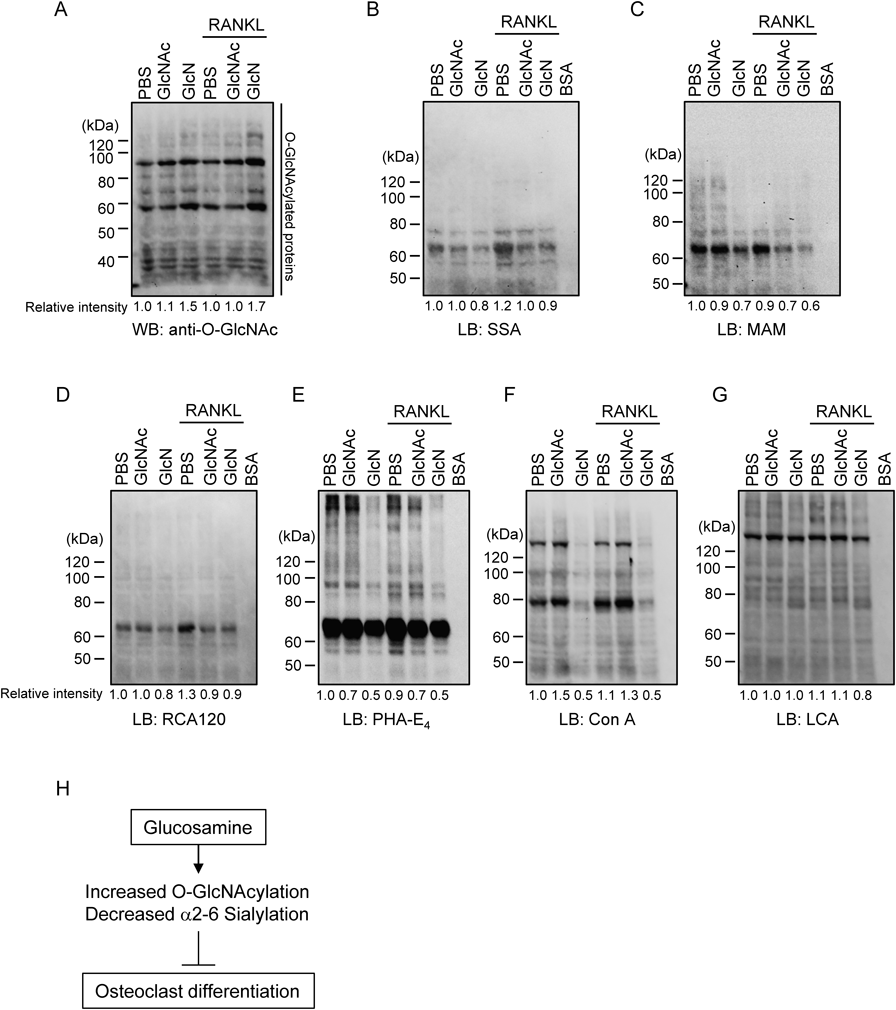
RAW264 cells were treated with RANKL and 2 mM GlcN or GlcNAc. Differentiated cells were lysed and subjected to Western blotting with an anti-O-GlcNAc antibody (A) or lectin blotting with SSA (B), MAM (C), RCA120 (D), PHA-E4 (E), Con A (F), or LCA (G). Equal protein loading was confirmed by Coomassie brilliant blue staining. Bovine serum albumin, which contains no glycan, was used as a negative control for lectin blotting. (H) Effect of GlcN on osteoclastogenesis. GlcN suppresses osteoclastogenesis at least through upregulation of O-GlcNAcylation and downregulation of α2,6 sialylation. The relative intensities of total O-GlcNAcylated proteins or glycoproteins detected by each lectin were measured using NIH ImageJ software.
In addition, it is possible that GlcN affects osteoclastgenesis not only through the upregulation of O-GlcNAcylation but also by the modification of other types of glycosylation. This may occur because UDP-GlcNAc could be used as a donor substrate for glycosylation and is exchangeable for certain other nucleotide sugars including cytidine monophosphate (CMP)-Neu5Ac, a donor substrate for the sialylation of glycans.6) In turn, α2,6-linked sialylation of cell surface glycoconjugates has been shown to be essential for osteoclastogenesis as the knockdown of α2,6 sialyltransferase suppressed osteoclastgenesis.13) In addition, sugar-binding proteins such as siglec, which interact with sialic acid, and galectins, which interact with Galβ1-4GlcNAc, also have important roles in osteoclastogenesis.14–19) Therefore, in order to clarify the potential for GlcN-mediated glycosylation modification, we investigated the effect of GlcN treatment on the pattern of glycosylation using lectin blotting of cell extracts with SSA, MAM, RCA120, PHA-E4, Con A, and LCA, which mainly recognize α2,6-linked sialic acid, α2,3-linked sialic acid, Galβ1-4GlcNAc, bisecting GlcNAc, high mannose type N-glycan, and N-glycan core modified with fucose, respectively (Figs. 2B–G).
When BSA, a negative control that has no glycans attached to its polypeptide, was subjected to lectin blotting, no positive signals were detected using any of the lectins. In contrast, signals detected by some lectins tended to decrease following GlcN treatment. In particular, as signals detected by SSA were decreased, GlcN might affect osteoclastogenesis through the decrease of α2,6-linked sialic acid. GlcN treatment also decreased signals detected by MAM. Although the role of α2,3-linked sialic acid on osteoclastic differentiation has not yet been reported, it has been demonstrated that α2,3-linked sialic acid affects osteogenesis,20) suggesting that it may be of functional relevance in osteoclasts. Notably, among all the lectins we used, the effect of GlcN treatment was most significant on signals detected by PHA-E4 and Con A. The signals detected by Con A were not decreased by GlcNAc treatment; however, those detected by PHA-E4 were decreased not only by GlcN but also by GlcNAc. These results suggest the possibility that both GlcN as well as GlcNAc suppress osteoclastogenesis through the decrease of glycans detected by PHA-E4. PHA-E4 is reported to bind oligosaccharides containing bisecting GlcNAc by affinity chromatography,21,22) which could be supported by the result of lectin blotting in conjunction with mass spectrometric analysis of cellular oligosaccharides.23) It has been reported that bisecting GlcNAc, which is recognized by PHA-E4, modulates the functions of integrins,24) although the exact effect of the decrease of bisecting GlcNAc on the function of integrin αvβ3, which plays an important role in osteoclastogenesis, remains to be determined. Therefore, the role of bisecting GlcNAc in osteoclastogenic differentiation and its effect on integrin αvβ3 in particular require further investigation.
In conclusion, the results of the present study demonstrate that GlcN treatment suppressed osteoclastogenesis, possibly through an increase of O-GlcNAcylation and decrease of α2,6-linked sialic acid modification (Fig. 2H). As the increase of O-GlcNAcylation in osteoblasts, which could be induced by GlcN treatment, promotes osteoblast differentiation,25) GlcN and O-GlcNAcylation likely have important roles in the regulation of the balance between bone formation and resorption. Furthermore, given that GlcN supplementation attenuates bone loss,7) the supplementation of GlcN and chemicals with comparable function might have beneficial effects on bone destructive diseases such as osteoporosis.
We are grateful to Mr. Fuyuhiko Yoshimura and Ms. Toshiko Sekiguchi (Josai University Faculty of Pharmaceutical Sciences) for technical assistance.
The authors declare no conflict of interest.