2017 Volume 40 Issue 4 Pages 531-534
2017 Volume 40 Issue 4 Pages 531-534
The possibility of using dissolving microneedles (DMs) as a skin allergy test device was studied in rats. Poly-L-arginine was used as a model allergen. Dextran was used to prepare three kinds of DM array chips containing different doses of poly-L-arginine: 17.1±0.5 µg (low-dose DM), 42.2±0.8 µg (medium-dose DM), and 87.4±1.1 µg (high-dose DM); each 1.0 cm2 chip contained 300 DMs. The mean lengths of the low-, medium-, and high-dose DM were 489±3, 485±3, and 492±1 µm and mean diameters of the base were 301±2, 299±1, and 299±2 µm, respectively. Furthermore, for the low-, medium-, and high-dose DM, the administered doses of poly-L-arginine were estimated to be 9.3±1.9, 31.1±1.3, and 61.9±4.7 µg and the scratching behavior per 30 min was 9.8±3.4, 60.4±8.3, and 95.7±10.6 times, respectively. These results demonstrate the dose dependence of the immunoreactivity of the poly-L-arginine DMs, suggesting that DMs can be used an alternative skin allergy device.
Allergy is an important immunological disease and, in Japan, about 20% of the population suffers from an allergic disease known as cedar pollinosis.1,2) Allergy testing is used to diagnose allergic diseases. Skin and/or blood tests are carried out to identify the substance that triggers an allergic response. Clinically, the skin test is used more than the blood test, because it is rapid, reliable, and cheaper than the blood test. For a skin test, a small amount of the suspected allergen is administered to the skin, and this site is then observed for development of immune reaction. When the skin becomes itchy and appears red and swollen, the skin allergy test is read as positive. The standard skin allergy tests used are the skin prick test, the intradermal test, and/or the skin patch test.3–6) Skin prick test is performed by dropping an allergen solution on the skin. To enhance the absorption of the allergen into the skin, scratches or needle pricks are made on the skin. However, the skin prick test is not commonly used because of the risk of exposure to infectious diseases.7) In the skin patch test, the allergen solution is adsorbed onto a pad which is fixed to the skin for 1 to 3 d. In both, the skin prick test and the skin patch test, the allergen is absorbed into the skin through the stratum corneum and is recognized by the Langerhans cells in the epidermis and the dendritic cells in the dermis. The function of these cells is to detect the environmental antigens, process antigens, and present specific epitopes to T cells. However, the identity of the cell type that responds to the antigen is still under debate. Several groups have proposed the Langerhans cells8–10) while others the dendritic cells11,12) as the targets of the antigen. An intradermal allergy test is performed after a negative skin prick test when there is a clinical suspicion for a particular allergen. A dermal syringe is used to inject small amounts of the allergen solution into the dermis.13) The intradermal test is more sensitive than the skin prick and patch test. However, it often shows false–positive results in patients who do not react to the allergen. The reason why allergic testing is performed on the skin is that the skin is densely populated with antigen presenting cells (APCs). Langerhans cells and dendritic cells are abundant in the epidermis and dermis, respectively. The function of these immune cells is to detect environmental antigens and present specific epitopes to T cells. Our previous study using 2- and 3-layered dissolving microneedles (DMs) containing fluorescent-labeled ovalbumin (FITC-OA) as a model antigen showed that three-layered DMs delivered more FITC-OA to the epidermis and induced 2.5–7.0- and 5.4-fold higher total immunoglobulin (Ig) (G+A+M) secretion than did two-layered DMs and subcutaneous injection of OA solution, respectively.14) Recently Lee et al. reported the tuberculin skin testing with microneedle MicronJet600™, which was consisted of hollow microneedle for intradermal injection and the antigen was delivered to the dermis.15) The DMs used chondroitin sulfate as a base. The DMs physically broke the barrier layer of the skin, the stratum corneum, and delivered the antigen to the epidermis. Therefore, we have proposed that the DMs would be a better device to deliver the allergen through the skin. In this report, a proof of concept study on use of DMs as an alternative skin allergy test was performed in rats, using poly-L-arginine as a model antigen.
Poly-L-arginine, polyethylene glycol (PEG) 1000 and dextran (mean molecular weight 40 kDa) were obtained from Nacalai Tesque Inc. (Kyoto, Japan). Brilliant blue (BB) and cellulose acetate were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Hydroxypropyl cellulose was obtained from Nippon Soda Co., Ltd. (Tokyo, Japan). Wistar Hannover rats used in the study and standard solid-meal commercial food were obtained from Japan SLC Inc. (Hamamatsu, Japan). All other materials were of reagent grade.
Preparation of DM Array ChipsDextran glue was prepared by mixing 20 µL of distilled water into a mixture of 1.2 or 5.5 mg poly-L-arginine, and 5.7 or 5.9 mg dextran. After the glue was degassed under reduced pressure, it was dispensed into a 1.0 cm2 mold containing 300 inverted cone-shaped wells. The top of each 500 µm deep well was 300 µm in diameter. The mold was covered with a 300 g steel plate, and the glue was filled into the wells and dried. A chip of 2.0 mm width and 17 mm diameter was made of a mixture of cellulose acetate and hydroxypropyl cellulose (10 : 1) using a tableting machine, Handtab-100 (Ichihashi Seiki, Kyoto, Japan). After the cover was removed, glue consisting of 15 mg of chondroitin sulfate and 25 mL of distilled water was painted over the chip and the chip was placed on the mold. After drying under the pressure of a stainless steel plate for 3 h, the chip was removed and DMs were obtained as arrays on a chip.
Preparation of Ointment for Prick TestA mixture of 1.4 mg poly-L-arginine, 1.0 g PEG 1000, and 1.0 mL distilled water was kneaded well together to obtain the ointment.
Solution for Intradermal InjectionDifferent amounts of poly-L-arginine: 0.2, 0.7 or 1.4 mg, was dissolved in 1.0 mL of saline to obtain the injectable solution.
Microscopic Observation of DMsA DM array chip, its acral portion stained with BB, was observed using a digital video microscope (VH-5500; Keyence Co., Osaka, Japan) under normal light.
Determination of Poly-L-arginine Content in DMsA DM array chip was dissolved in 1 mL distilled water, centrifuged at 12000 rpm for 15 min and the supernatant was used for the assay. The amount of poly-L-arginine was quantified on the basis of the Bradford method using 10% Coomassie reagent in phosphate buffer (pH 7.4). Ten microliters of the supernatant was transferred to each well on a microplate, 300 µL of 10% Coomassie reagent added, and the absorbance at 595 nm measured. One milligram of poly-L-arginine was dissolved in 1 mL of distilled water and then diluted for use as standard. A standard curve representing the linear relationship between the concentration of poly-L-arginine (range 10–100 µg/mL) and absorbance was plotted.
In Vivo Pharmacological Evaluation of DM Preparations and Intradermal Injections in RatsMale Wistar Hannover rats, weighing 324±21 g, were anesthetized by inhalation of 4% isoflurane and divided into three groups. Each group consisted of 4–5 rats. The dorsal skin of the rats was shaved and they were placed in an acryl cage for 30 min. In one group, the low-, medium- or high-dose DMs were administered by pressing the chip into the skin with fingers and keeping it there for 3.0 min by covering with an adhesive tape. Thereafter, the DM array chip was removed from the skin and the scratching behavior was recorded for 30 min. Another group of rats, was injected intradermally at the shaved dorsal area with a solution containing 0, 10, 35, and 70 mg poly-L-arginine per rat. After injection, the scratching behavior was recorded for 30 min. The skin prick test was performed on the last group of rats, and the scratching behavior was determined after either, 1, 2, 3, 5, or 10 pin pricks, subsequent to application of poly-L-arginine ointment. All animal protocols were approved by the Institutional Animal Care and Use Committee. Experiments were conducted in accordance with the Guidelines for Animal Experimentation, Kyoto Pharmaceutical University.
StatisticsAll values are expressed as the mean±standard error (S.E.). Statistically significant differences were assumed to be significant when p<0.05 or 0.01 (Student’s unpaired t-test).
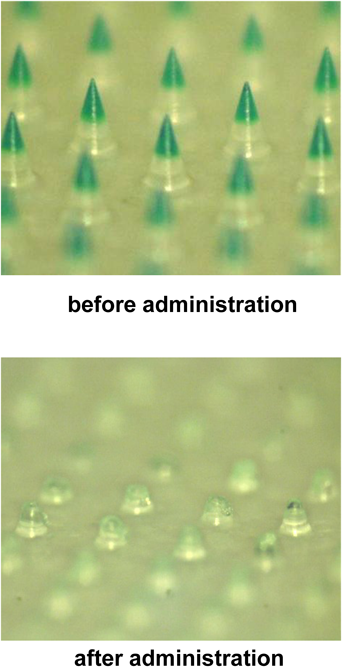
Three kinds of DMs with different doses of poly-L-arginine—17.1±0.5 µg (low-dose DM), 42.2±0.8 µg (medium-dose DM), and 87.4±1.1 µg (high-dose DM)—and 300 DMs per chip were prepared in this study. As shown in Table 1, for the low-, medium-, and high-dose DMs, the mean length was 489±3, 485±3, and 492±1 µm and the mean diameter of their base was 301±2, 299±1, and 299±2 µm, respectively. After administration of these DMs to rat skin, the DM array chips were recovered and their lengths were recorded. The upper panel of Fig. 1 shows the low-dose DMs before administration, where the tip of the DM was stained with BB because poly-L-arginine was transparent. After administration to rat skin, the tip of the DM disappeared because of dissolution in the skin, as shown in the lower panel. The mean length of the recovered low-dose DMs was 281±2 µm. By subtracting this value from the mean length before administration, the mean inserted length of DMs was calculated to be 208 µm. Similarly, the mean inserted length for the medium- and high-dose DMs were 216 and 232 µm, respectively. Table 2 shows the formulated amount of poly-L-arginine in the three DMs. Before administration to the skin, the poly-L-arginine contents in the low-, medium-, and high-dose DMs were 17.1±0.5, 42.2±0.8, and 87.4±1.1 µg, respectively. In contrast, the recovered poly-L-arginine content in the low-, medium-, and high-dose DMs was 7.8±0.9, 11.1±1.3, and 25.5±4.7 µg, respectively. By subtracting the recovered amount from the amount of poly-L-arginine formulated in the DMs, the administered doses of poly-L-arginine were estimated to be 9.3±1.9, 31.1±1.3, and 61.9±4.7 µg for the low-, medium-, and high-dose DMs, respectively. As a positive control, poly-L-arginine solution was injected intradermally at three different doses: 10, 35, and 70 µg per rat.
| DMs | Height (µm) | Diameter of the basement (µm) | |
|---|---|---|---|
| Before administration | After administration | ||
| High dose | 492±1 | 260±2 | 299±2 |
| Middle dose | 485±3 | 269±2 | 299±1 |
| Low dose | 489±3 | 281±2 | 301±2 |
Each value represents the mean±S.E. (n=5–10).
| Formulation | Poly-L-arginine (µg) | Recovered poly-L-arginine (µg) | Administered dose (µg) | |
|---|---|---|---|---|
| DMs | High | 87.4±1.1 | 25.5±4.7 | 61.9±4.7 |
| Middle | 42.2±0.8 | 11.1±1.3 | 31.1±1.3 | |
| Low | 17.1±0.5 | 7.8±0.9 | 9.3±1.9 | |
| Intradermal injection | High | — | — | 70* |
| Middle | — | — | 35* | |
| Low | — | — | 10* | |
Each value represents the mean±S.E. (n=4–5). * Injected dose.
Figure 2 shows the results of the pharmacological study where the number of scratching behavior per 30 min were compared in rats that had been subjected to the following treatments: DMs, intradermal injections, and prick tests. Doses of intradermally injected poly-L-arginine were 10, 35, and 70 µg per rat. Doses of poly-L-arginine administered by DMs were 9.3±1.9, 31.1±1.3, and 61.9±4.7 µg. The scratching behavior of rats injected with a low dose (10 µg) of poly-L-arginine was 0.4±0.4 times, which was not significantly different from that seen in response to the placebo DM. When the poly-L-arginine dose increased to 35 and 70 µg per rat, scratching behavior increased to 5.0±2.7 and 20.5±3.7 times, respectively. In contrast, DMs resulted in greater scratching behavior: 9.8±3.4 times for low dose, 60.4±8.3 times for medium dose, and 95.7±10.6 times for high dose. In the high-dose experiment, the pharmacological response obtained with DMs was approximately 5-fold that obtained with intradermal injection of a high dose of poly-L-arginine. As the poly-L-arginine with DMs was delivered to the epidermis where the Langerhans cells were densely distributed, the higher immunoreactivity was obtained than that with the intradermal injection. This is the reason why the differences in the immunoreactivity between DMs and the intradermal injection were occurred. To ascertain the superiority of DMs against the conventional prick test, an ointment with 1.4 mg/mL poly-L-arginine was used as an additional positive control. When one prick was made on the rat skin, no pharmacological response was observed. However, when the number of pricks was increased to 1, 2, 3, 5, and 10, the scratching behavior increased as shown in Fig. 2. In the prick test, the amount of poly-L-arginine delivered into the skin was not estimated. However, DMs elicited a stronger pharmacological response than the prick test.
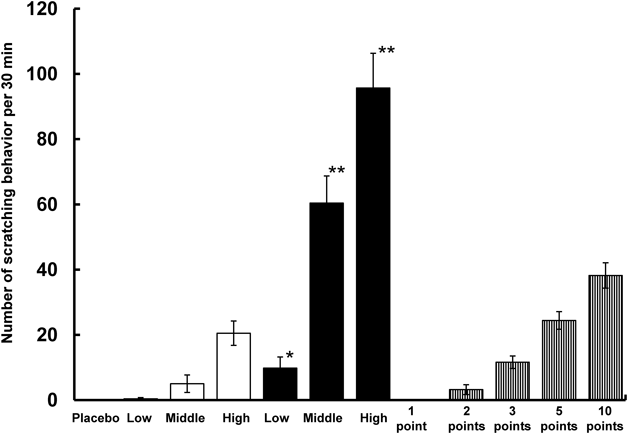
Blank bar shows intradermal injection group, solid bar shows DM group and vertical-striped bar shows Prick test group. Each point represents the mean±S.E. of 4–6 experiments. * and ** show significant difference from the intradermal injection (p<0.05, 0.01).
The amount of poly-L-arginine in the DMs ranged between 9.3 and 61.9 µg. However, in the prick test, 0.3–0.6 g of poly-L-arginine ointment and 2–10 pricks to the skin were required to elicit scratching behavior. As the ointment was applied with, which the concentration of antigen in the ointment was 1.4 mg/mL, it is equivalent to 420–840 µg of antigen. Thus, the prick test required approximately 10 times the antigen dose used in the DMs. Therefore, DMs are also economically superior to the conventional prick test.
This study was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities, 2008–2013.
The authors declare no conflict of interest.