2017 Volume 40 Issue 4 Pages 444-450
2017 Volume 40 Issue 4 Pages 444-450
We attempted to design a combination ointment containing solid tranilast nanoparticles and dissolved sericin as a wound-healing drug (TS-combination ointment), and evaluated its usefulness as therapy for wound-healing deficits in streptozotocin-induced diabetic rat (STZ rat) using kinetic analyses as an index. Solid tranilast nanoparticles were prepared by bead mill methods with low-substituted methylcellulose; the mean particle size of the tranilast nanoparticles was 70 nm. The ointment was designed to contain the tranilast nanoparticles plus sericin powder and/or Carbopol® 934. Skin wound healing in STZ rats begins significantly later than in normal rats. Although the skin wound healing rate in STZ rats treated with an ointment containing tranilast nanoparticles was lower than in STZ rats treated with vehicle, the ointment was effective in reducing redness. An ointment containing sericin enhanced the skin-healing rate, but the preventive effect on redness was weak. On the other hand, the combination of tranilast and sericin increased both the skin healing rate and reduction in redness. In conclusion, we have adapted kinetic analyses to skin wound healing in rats, and found these analyses to be useful as an index of wound healing ability by a wound-healing drug. In addition, we show that treatment with the TS-combination ointment enhances the skin wound healing rate and reduces redness. These findings provide information significant to the search for new wound-healing therapies and for the design of wound-healing drugs.
Diabetes mellitus is characterized by a dysfunction in glucose metabolism associated with the subsequent negative effects on lipid and protein metabolism. The most common dermatological complications in diabetic patients are wound-healing deficits.1) Wound healing is a preprogrammed process consisting of four continuous phases: (1) bleeding controlled by hemostasis, (2) inflammatory response, (3) cellular and collagen proliferation, and (4) remodeling.2–5) Following injury, the inflammatory response takes place from 0–3 d, and cellular proliferation occurs at 3–12 d. During the inflammatory response, monocytes and macrophages release various cytokines, and the number of fibroblasts increases. The fibroblasts induce collagen synthesis, and collagen replenishes the skin deficit area. Thus, collagen synthesis via cytokine release (inflammatory response) is important in the process of skin wound healing. On the other hand, wound healing via abnormal collagen synthesis and formulation during a prolonged inflammatory response is the cause of redness and keloid formation in the skin. A decrease in collagen synthesis results in delayed skin healing.1–5)
It is known that prolongations and interruptions that occur during the collagen synthesis response can interrupt the wound healing process and lead to wound-healing deficits in diabetic patients.3) The most common complications of delayed wound healing in diabetics are: a reduction in the level of substance P (SP),6) decreased endothelial nitric oxide synthase activity,7) decreased vasculogenesis,8) and a reduction in the chemotactic and phagocytic activities of neutrophils.9) The underlying biochemical mechanisms in the disruption of the healing process involve mainly disturbances in collagen production, which result in delayed replenishment and re-epithelialization of wounds, and in the impaired migration and proliferation of keratinocytes and fibroblasts.10) In addition, wound-healing deficits in diabetic patients lead to skin redness and the development of keloids. Different treatments have been tested to address this complex clinical problem, but only a few have been proven to be effective. Therefore, the development of effective treatments for the treatment of wounds in diabetic patients is a challenging problem in clinical practice.
Tranilast, N-(3′,4′-dimethoxycinnamoyl) anthranilic acid, is a useful drug for improving allergic rhinitis, atopic dermatitis and bronchial asthma. Several reports have shown that tranilast can prevent or act to improve keloids and hypertrophic scars.11,12) Tranilast inhibits the abnormal collagen synthesis seen in human keloid tissues transplanted into the backs of mice, and by carrageenin-induced granulation-tissues in rats.13–15) Tranilast specifically suppresses collagen synthesis rather than cell proliferation in cultured fibroblasts derived from human keloid tissues.14) Recently, we prepared an ointment containing solid tranilast nanoparticles (nano-TL ointment), and showed that the application of this ointment to the skin supplied tranilast into the skin tissue, and maintained high tranilast concentrations in the local tissue.16) It is possible that an ointment containing tranilast nanoparticles could prevent the development of redness and keloids in the skin, and provide an effective therapy for wound-healing deficits in diabetic patients
Silk sericin is biocompatible, biodegradable, hot water soluble, and, as a byproduct of the silk industry,17) readily available at a low cost. Approximately 30% of the amino acid content of sericin is serine, the chief moistening factor in natural human skin.18) A moist environment accelerates the dynamic process of wound healing.19) Besides acting as a natural moisturizing agent, sericin also possesses anticoagulating, antioxidant, antiwrinkle, and antibacterial activities, and enhances the proliferation of mammalian cells in serum-free media.20–22) In addition, sericin can induce collagen production23,24) and promote fibroblast proliferation23) without the activation of pro-inflammatory cytokines.25)
In this study, we undertook kinetic analyses of skin wound healing in normal rats and diabetic model rats, streptozotocin-induced diabetic rats (STZ rat). In addition, we evaluated whether an ointment containing a combination of solid tranilast nanoparticles and dissolved sericin can provide a useful therapy for wound-healing deficits in STZ rats using kinetic analyses as an index.
Tranilast microparticles were kindly donated by Kissei Pharmaceutical Co., Ltd. (Nagano, Japan). Pure Sericin™ was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Low-substituted methylcellulose SM-4 (MC) was provided by Shin-Etsu Chemical Co., Ltd. (Tokyo, Japan). Carboxypolymethylene (Carbopol® 934) was purchased from Serva (Heidelberg, Germany). All other chemicals used were of the highest purity commercially available.
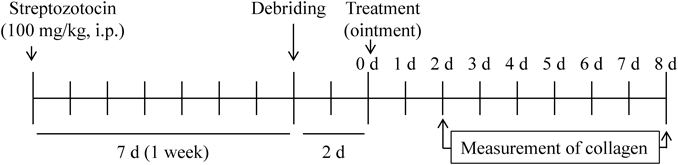
AnimalsSeven-week-old Wistar rats (male, normal rat) were obtained from Kiwa Laboratory Animals Co., Ltd. (Wakayama, Japan), and used in this study. Diabetes was induced in 7-week old Wistar rats by injecting streptozotocin (100 mg/kg, intraperitoneally (i.p.)), and waiting for 1 week (STZ rat). Figure 1 shows an experimental scheme. The experiments were performed in accordance with the Kindai University Faculty of Pharmacy Committee for the Care and Use of Laboratory Animals.
Carbopol® 934 was added to distilled water, allowed to swell for 1 h at room temperature, and neutralized with 5% ammonia water (gel base). Solid tranilast nanoparticles were prepared using zirconia beads and Bead Smash 12 (a bead mill, Wakenyaku Co., Ltd., Kyoto, Japan) as described in a previous report.16) Briefly, tranilast powder and 0.5% MC were mixed with zirconia beads (diameter: 2 mm), and the mixture was crushed with the Bead Smash 12 for 30 s (3000 rpm, 4°C). The mixture was then diluted in saline, and crushed with 0.1 mm zirconia beads (5500 rpm, 30 s×15 times, 4°C) using the Bead Smash 12. The particle size of milled tranilast was determined with a nanoparticle size analyzer SALD-7100 (Shimadzu Corp., Kyoto, Japan; refractive index 1.60–0.10i), to reveal a particle size of 70 nm (tranilast nanoparticles). Sericin solutions were prepared by dissolving sericin and 0.5% MC in saline (pH 6.5–7.5). The tranilast nanoparticles and/or sericin solution were added to the Carbopol® 934 gel base, and stirred to uniformity. In this study, we define the ointments used as follows: ointment containing 0.75% tranilast nanoparticles (nano-TL ointment), ointment containing 10% sericin (sericin ointment), combination ointment containing 0.75% tranilast nanoparticles and 10% sericin (TS-combination ointment). An ointment containing 0.75% tranilast microparticles was prepared by adding tranilast microparticles and MC to the Carbopol® 934 gel base (micro-TL ointment). The Carbopol® 934 gel base containing 0.5% MC was used as the control (vehicle). The drug concentrations in the ointments containing tranilast and/or sericin were determined as described previously.16,26,27)
Image Analysis of Skin Wound Healing in Rats Treated with OintmentOn the day before the start of the experiment, the hair on the middorsal area of normal and STZ rats was carefully removed with an electric clipper and electric razor. The following day, the rats were anesthetized by isoflurane (2.5%) using BS-400T (Brain Science Idea Co., Ltd., Osaka, Japan), and six wounds (6 mm in diameter) in the middorsal skin were made by debriding the epidermis and dermis. The skin-debrided rats rested for 2 d for hemostasis and stabilization, and then used in the following experiments. 0.3 g of ointment was fixed on the middorsal skin with an adhesive and immediately occluded with adhesive tape. The ointment were applied to the skin of the rats once a day for 8 d, and the size of each wound and redness was measured daily with an image J. The initial areas (0 d in this study) were as follows: non-treated Wistar rats, 23.4±1.7; vehicle-treated Wistar rats, 24.1±1.7; nano-TL ointment-treated Wistar rats, 23.1±2.1; sericin ointment-treated Wistar rats, 23.6±2.2; TS-combination ointment-treated Wistar rats, 23.3±1.7, non-treated STZ rats, 27.7±1.6; vehicle-treated STZ rats, 29.0±2.3; nano-TL ointment-treated STZ rats, 28.7±2.0; sericin ointment-treated STZ rats, 28.6±1.8; and TS-combination ointment-treated STZ rats, 28.0±1.9 (mm2; means±standard error of mean (S.E.) of 8 independent rats). The remaining skin wound areas were calculated, and the values (%) were determined as the ratio to the initial area of the respective wound.
The rate of skin wound healing is represented by the skin wound healing rate constant (kH, α and β, d−1). The skin wound healing rate constant was calculated from the following equations (Eqs. 1 and 2) and the iterative nonlinear least-squares regression procedure MULTI.28)
 | (1) |
 | (2) |
Rats were fasted for 12 h, after which blood was drawn from a tail vein at 9:00 a.m. without anesthesia for the measurement of the plasma glucose and insulin levels.26) Glucose were measured using an Accutrend GCT (Roche Diagnostics, Mannheim, Germany) meter, and insulin levels were measured using an enzyme-linked immunosorbent assay (ELISA) Insulin Kit according to the manufacturer’s instructions (Morinaga Institute of Biological Science Inc., Kanagawa, Japan). Briefly, monoclonal antibodies specific for rat insulin were pre-coated onto microplates, standards and samples were pipetted into the wells, and the microplates were incubated at 4°C for 2 h. After washing to remove unbound materials, rat insulin antibodies were added to the wells at room temperature and the plates were incubated for 30 min. After washing, the substrates were added. The enzyme reactions yielded blue products that turned yellow when the stop solutions were added. The absorbance was measured with a microplate reader (BIO-RAD, CA, U.S.A.) at 450 nm.
Measurement of Collagen Content in Rat SkinSkin samples for the measurement of collagen were obtained from rats 2 and 8 d after the start of treatment with the ointments. The collagen levels in the samples was measured using a total collagen assay kit according to the manufacturer’s instructions (QuickZyme Total Collagen Assay, Cosmo Bio., Ltd., Tokyo, Japan). Briefly, the skin tissue (sample) was homogenized in 4 M HCl, and incubated with assay buffer for 20 min in the wells of microplates at 22°C. Then, the detection reagent was added, and the plates were incubated for 60 min at 60°C in an oven. The absorbance was measured with a microplate reader at 570 nm.
Statistical AnalysisThe data were analyzed using the unpaired Student’s and Aspin–Welch’s t-tests and ANOVA followed by Tukey test’s multiple comparison; p values less than 0.05 were considered significant.
Figure 2A shows the plasma glucose levels in normal and STZ rats, and Figs. 2B and C show the wound area in skin-debrided normal and STZ rats. The plasma glucose levels in rats was significantly enhanced by the injection of STZ (100 mg/kg, i.p.), and the plasma glucose value in STZ rats was 4.6-fold higher than in normal rats 1 week after STZ injection (Fig. 2A). Moreover, the insulin levels in the blood of STZ rats (13.5±3.4 ng/dL, n=8) were significantly lower than in normal rats (218.9±18.2 ng/dL, n=8). A delay in wound healing in STZ rats was observed in comparison with normal rats (Figs. 2B, C). Table 1 shows the kinetic parameters calculated from the data in Fig. 2B using Eqs. 1 and 2. The Akaike’s Information Criterion (AIC) value of Eq. 2 was higher than that of Eq. 1 in both normal and STZ rats. Based on the AIC results, Eq. 1 was used for the analysis of the skin wound healing constant (kH). Although, the W0 values in normal and STZ rats were similar, the kH in STZ rats was significantly lower than in normal rats (Table 1).

A: Plasma glucose levels in normal and STZ rats. B: Changes in skin wound area in normal and STZ rats. C: Images of skin wounds in normal and STZ rats. Open symbols, normal rat; closed symbols, STZ rat. The data are presented as means±S.E. of 8 independent rats. * p<0.05, vs. normal for each category. Statistical comparisons are performed using the unpaired Student’s t-tests. The skin wound healing of STZ rats was significantly delayed as compared to normal rats.
| Eq. 1 | Eq. 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| W0 (%) | kH (×10−3, d−1) | AIC | A (%) | B (%) | α (×10−3, d−1) | β (×10−3, d−1) | AIC | |
| Normal rat | 93.2±3.5 | 42.7±4.1 | 65.9±6.1 | 95.1±3.5 | 4.9±3.5 | 4.7±0.7 | 4.3±4.4 | 68.8±6.6 |
| STZ rat | 100.5±2.7 | 22.8±2.2a) | 76.1±2.3 | 92.9±5.5 | 7.5±4.3 | 3.3±0.4a) | 1.2±0.6a) | 77.5±2.1 |
a) p<0.05 vs. normal rats for each category. The data are presented as means±S.E. of 8 independent rats. Statistical comparisons are performed using the unpaired Student’s and Aspin–Welch’s t-tests.
Figures 3–5 show images (Fig. 3), wound areas (Fig. 4) and redness (Fig. 5) during the skin wound healing process in normal and STZ rats treated with nano-TL ointment, sericin ointment or TS-combination ointment. Table 2 shows the W0 and kH values in normal and STZ rats treated with nano-TL ointment, sericin ointment or TS-combination ointment. Although, the skin wound healing rate in STZ rats treated with nano-TL ointment was slower than that of the vehicle-treated rats (Fig. 4, Table 2), the nano-TL ointment reduced redness in both normal and STZ rats (Figs. 3, 5). On the other hand, treatment with the sericin ointment enhanced the skin healing rate in both normal and STZ rats, and increased the kH values of normal and STZ rats by approximately 2.1- and 1.3-fold over those of vehicle treated rats, respectively (Fig. 4, Table 2). The redness area in rat treated with sericin was lower than that in vehicle, however, the preventive effect of sericin on redness was less than that in nano-TL ointment (Figs. 3, 5). The TS-combination ointment significantly enhanced the kH values in comparison with vehicle, and the kH values were similar to those of the sericin ointment (Fig. 4, Table 2). In addition, the TS-combination ointment also reduced redness (Figs. 3, 5). Figure 6 shows the collagen contents in skin-debrided normal and STZ rats treated with nano-TL ointment, sericin ointment or TS-combination ointment. At 2 d after the start of treatment, the collagen contents in the STZ rats were higher than in normal rats. However, the collagen content in STZ rats was significantly lower than that in normal rats after 8 d of treatment. The nano-TL ointment decreased the collagen content while the sericin ointment increased the collagen content in both normal and STZ rats.


Open symbols, normal rats; closed symbols, STZ rats. Circles (vehicle), rats treated with vehicle; rhombuses (nano-TL), rats treated with nano-TL ointment; triangles (sericin), rats treated with sericin; squares (TS-combination), rats treated with TS-combination ointment. The data are presented as means±S.E. of 8 independent rats. * p<0.05, vs. vehicle for each category. Statistical comparisons are performed using the Tukey test’s multiple comparison. Skin wound healing in STZ rats treated with the nano-TL ointment was delayed as compared with STZ rats treated with vehicle. On the other hand, ointments containing sericin (sericin ointment and TS-combination ointment) increased the skin wound healing rate in both normal and STZ rats.

Open columns, normal rats; closed columns, STZ rats. None, non-treated rats; Vehicle, rats treated with vehicle; nano-TL, rats treated with nano-TL ointment; sericin, rats treated with sericin ointment; TS-combination, rats treated with TS-combination ointment. The data are presented as means±S.E. of 8 independent rats. *1 p<0.05, vs. normal rats for each category. *2 p<0.05, vs. none for each category. *3 p<0.05, vs. sericin for each category. Statistical comparisons are performed using the Tukey test’s multiple comparison. The ointments containing tranilast (nano-TL ointment and TS-combination ointment) prevented redness.
| Vehicle | Nano-TL ointment | Sericin ointment | TS-Combination ointment | ||
|---|---|---|---|---|---|
| Normal rat | W0 (%) | 96.3±0.8 | 97.3±2.1 | 98.7±2.4 | 98.4±2.6 |
| kH (×10−3, d−1) | 38.3±2.6c) | 37.9±2.1c) | 80.6±10.1a, b) | 75.8±9.7a, b) | |
| STZ rat | W0 (%) | 101.5±3.0 | 100.7±2.6 | 99.4±2.2 | 102.1±2.0 |
| kH (×10−3, d−1) | 23.7±2.7c) | 21.6±2.5c) | 30.6±3.5a, b) | 29.9±3.1a, b) |
The contribution ratios (W0) and rate constants of skin wound healing (kH) were calculated using Eq. 1. The data are presented as means±S.E. of 8 independent rats. a) p<0.05 vs. vehicle for each category. b) p<0.05 vs. nano-TL ointment for each category. c) p<0.05 vs. sericin ointment for each category. Statistical comparisons are performed using Tukey test’s multiple comparison.

Open columns, normal rats; closed columns, STZ rats. None, non-treated rats; Vehicle, rats treated with vehicle; nano-TL, rats treated with nano-TL ointment; sericin, rats treated with sericin ointment; TS-combination, rats treated with TS-combination ointment. The data are presented as means±S.E. of 8 independent rats. *1 p<0.05, vs. normal rats for each category. *2 p<0.05, vs. none for each category. Statistical comparisons are performed using the Tukey test’s multiple comparison. Treatment with the nano-TL ointment decreased the collagen content in rats. On the other hand, the ointment containing sericin enhanced the collagen content in rats.
In the present study, we show that a combination ointment containing tranilast nanoparticles and sericin enhances the skin wound healing rate and prevents redness. In addition, we found that kinetic analysis of skin wound healing using Eq. 1 provides a useful index for studies involving the evaluation and development of wound-healing drugs.
Four continuous phases (hemostasis, inflammatory response, cellular proliferation and remodeling) comprise the skin wound healing process.2–5) Hemostasis begins immediately after the injury with the formation of fibrin clots accompanied by vasoconstriction. Proinflammatory cytokines and growth factors, including transforming growth factor (TGF-β), platelet-derived growth factor (PDGF), epidermal growth factor (EGF) and fibroblast growth factor (FGF), are expressed from the clot and the surrounding tissue after hemostasis. Inflammatory cells migrate to the inner side of the wound after hemostasis and start the inflammatory phase during which lymphocytes, macrophages and neutrophils migrate to the tissue.3) The proliferative phase follows and overlaps with the inflammatory phase. The proliferative phase is characterized by the proliferation of epithelial cells and the migration of these cells to the temporary matrix in the wound (re-epithelization).3) The main processes comprising the proliferative phase are angio-neogenesis, collagen deposition, granulation tissue formation, epithelization, and wound contraction. During angio-neogenesis, new blood vessels are formed from endothelial cells.29) Fibroblasts and endothelial cells are the main cells involved in the processes of capillary growth, collagen formation, and granulation tissue formation in the wound area in reparative dermis. The inflammatory response (0–3 d) and cellular and collagen proliferation (3–12 d) are present after injury, and any prolongation or interruptions that occur during these phases result in an interruption of the wound healing process and lead to wound healing deficits such as are observed in diabetic patients.3)
First, we investigated differences in skin wound healing between normal and STZ rats, and found that skin wound healing in STZ rats is delayed in comparison with normal rats, and that pronounced skin redness is a feature observed in STZ rats (Figs. 2B, C). Using kinetic analyses, the behavior of wound healing in normal and STZ rats was observed as a single phase since the AIC value of Eq. 1 was lower than that of Eq. 2 for both normal and STZ rats (Table 1). This result shows that both the inflammatory response and cellular proliferation are related to the deficits in skin wound healing observed in the STZ rats. In addition, we measured the changes in collagen content in the wounds of normal and STZ rats (Fig. 6). Although the collagen content in skin-debrided STZ rats receiving no ointment treatment (non-treated STZ rat) was higher than that in normal rats in the early stages of the wound healing process, the collagen level in the non-treated STZ rats was 60% of that in the skin-debrided normal rats after 8 d. This result suggests abnormal collagen proliferation and formulation in the early stages of the wound healing process, and these deficits in collagen production may lead to the delay in wound healing and skin redness.
Next, we examined the therapeutic effect of ointments containing tranilast and/or sericin on skin wound healing in STZ rats using kinetic analyses. In a previous study, we developed a new ointment system that includes tranilast nanoparticles prepared using Bead Smash 12 and additives (nano-TL ointment).16) The percutaneous penetration of tranilast from the ointment and the therapeutic effect on inflammation of the nano-TL ointment were both significantly higher than those of a micro-TL ointment. Thus our findings suggested that a transdermal therapeutic system using nanoparticles could enable a medication to be applied without high-systemic levels, providing efficient and effective therapy that spares patients from unwanted side effects. In addition, it is possible to prepare the nano-TL ointment without solvents or additives that can cause irritation, such as dimethyl sulfoxide and surface-active agents, and the toxicity of the nano-TL ointment is lower than that of conventional formulations.16) Therefore, in this study, we evaluated the therapeutic effect on wound-healing of the nano-TL ointment. Treatment with the nano-TL ointment reduced the skin redness in rats (Figs. 3, 5), this preventive effect on redness was higher than that of a micro-TL ointment (data not shown). On the other hand, the skin healing rate of rats treated with the nano-TL ointment was lower than that of vehicle-treated STZ rats (Fig. 4, Table 2). Tranilast is known to inhibit collagen synthesis by fibroblasts via the down-regulation of cytokine release from monocytes and macrophages, and abnormal collagen synthesis and formulation via the resulting prolonged inflammatory response lead to skin redness and the formation of keloids.30,31) In addition, tranilast specifically suppresses collagen synthesis rather than cell proliferation by cultured fibroblasts derived from human keloid tissues.14) The need for collagen in process of skin wound healing, and the suppression of collagen synthesis cause a delay in wound healing.1–5) In this study, we found that treatment with the nano-TL ointment decreased the collagen levels in both normal and STZ rats at 8 d after the start of treatment (Fig. 6B). Thus, we believe that the prevention of collagen synthesis may induce the decrease in the skin healing rate, while the suppression of abnormal collagen formulation by fibroblasts via the inflammatory response results in the reduced skin redness observed in rats treated with the nano-TL ointment.
When developing therapies for skin wound healing, it is important to increase skin wound healing rate without causing redness. It has been reported that sericin can induce collagen production23,24) and promote fibroblast proliferation without the activation of pro-inflammatory cytokines,23,25) and Aramwit and Sangcakul32) showed that the topical application of sericin enhances wound healing rate and produces a wound-size reduction in rats. We also reported that sericin has a potent effect in promoting the corneal wound healing rate in rats, probably by increasing cell movement and proliferation in corneal epithelial cells.26–28) Therefore, we examined the effects of a sericin ointment to enhance the skin wound healing rate via cell movement, cellular proliferation and collagen production, and found that this treatment increases the skin wound healing rate and collagen production in both normal and STZ rats (Figs. 4, 6, Table 2). On the other hand, the redness area in rat treated with sericin tended to decrease in comparison with that in vehicle, however, the preventive effect of sericin on redness was less than that in nano-TL ointment (Figs. 3, 5). The decrease in redness area in rat treated with sericin may be led by the high skin wound healing rate. Based on these results, we prepared an ointment containing tranilast nanoparticles and sericin (TS-combination ointment), and demonstrated the therapeutic effect of this combination ointment on skin wound in STZ rats. The TS-combination ointment resulted in less redness (Figs. 3, 5), and a significantly enhanced kH for wound healing in comparison with the vehicle; in addition, the collagen content was also higher than in the rats treated with the nano-TL ointment (Fig. 6, Table 2). These results suggest that the combination of tranilast and sericin is a useful development in the search for effective treatments for wounds in diabetic patients.
Further studies are needed to elucidate the precise mechanism and behavior of the skin wound healing process in patients with diabetic wounds using kinetic analyses. In addition, it is important to clarify the changes in factors related to inflammation and collagen production in STZ rats treated with tranilast and/or sericin. Therefore, we are now investigating the skin wound healing process using an immunological method in STZ rats.
In conclusion, we have adapted kinetic analyses to skin wound healing in normal and diabetic rats, and found that both the inflammatory response and collagen/cellular proliferation are related to the deficits in skin wound healing observed in STZ rats. In addition, we have shown that treatment with an ointment containing tranilast nanoparticles and sericin enhances the skin-healing rate and prevents redness. These findings provide information significant to the search for therapies for wound healing and the design of wound-healing drugs.
The authors declare no conflict of interest.