2017 Volume 40 Issue 4 Pages 465-472
2017 Volume 40 Issue 4 Pages 465-472
Alzheimer’s disease (AD) is a most serious age-related neurodegenerative disorder accompanied with significant memory impairments in this world. Recently, microRNAs (miRNAs) have been reported to be invlolved in the pathophysiology of AD. Previous studies have shown that miRNA-206 (miR-206) is implicated in the pathogenesis of AD via suppressing the expression of brain-derived neurotrophic factor (BDNF) in the brain. Here, we examined the miR-206-3p and miR-206-5p expression in the hippocampus and cortex of Abeta precursor protein (APP)/presenilin-1 (PS1) transgenic mice treated with donepezil, a drug approved for treating AD in clinic. We found that the expression of miR-206-3p was significantly up-regulated in the hippocampus and cortex of APP/PS1 mice, while donepezil administration significantly reversed this dysfunction. In addition, enhancing the miR-206-3p level by the usage of AgomiR-206-3p significantly attenuated the anti-dementia effects of donepezil in APP/PS1 mice. Together, these results suggested that miR-206-3p is involved in the anti-dementia effects of donepezil, and could be a novel pharmacological target for treating AD.
Alzheimer’s disease (AD) is a serious neurodegenerative disorder in old people, and always accompanied with significant memory impairments, affecting mainly the hippocampus and frontal cortex.1,2) AD patients display relatively slow, chronic but progressive neurodegeneration and memory impairments, ultimately leading to full dementia.3,4) Recent survey found that AD is responsible for about 50–60% of all cases of dementia in people over age 65.3,4)
The etiology of AD remains unclear, though many pathophysiologic hallmarks of this disease have been disclosed and are currently well established. They involve a complex network of interconnected factors such as cholinergic dysfunction, aggregation and accumulation of β-amyloid (Aβ), intracellular formation of neurofibrillary tangles composed by tau, oxidative stress, neuronal loss, and so on.5–8) A lot of reports have shown that brain-derived neurotrophic factor (BDNF) is critical for the establishment and maintenance of memory, while the dysfunction of BDNF system leads to memory impairments.9,10) It is known that BDNF combines to tyrosine kinase receptor B (TrkB) in the cell membrane, and then inducing the activation of several intracellular signaling pathways.11–13) Accumulating evidences have indicated that BDNF is implicated in the pathophysiology of AD. Previous studies have already found the reduced expression of BDNF in several brain regions (especially the hippocampus) of postmortem AD samples.14,15) By using the immunohistochemical method, Connor et al. found a significant reduction in not only intensity, but also the number of BDNF-immunoreactive cell in the hippocampus and cortex of AD samples.16) In addition, it was also thought that the Aβ-induced neurotoxicity and dendrite atrophy may due to a consequence of BDNF deficiency.17,18)
Donepezil is one of the only four drugs currently approved for treatment of Alzheimer’s dementia. Although thought as a selective acetylcholinesterase inhibitor, the precise anti-dementia mechanism of donepezil in patients with AD is not fully understood.19,20) Leyhe et al. and Alvarez et al. have shown that donepezil increase the serum concentration of BDNF in AD patients.21,22) Autio et al. also revealed that donepezil administration led to rapid phosphorylation of TrkB in the hippocampus.23)
MicroRNAs (miRNAs) are single-stranded small non-coding RNA molecules which interact with the 3′-untranslated region (UTR) of specific target mRNAs to modulate gene expression.24) MiRNAs regulate a lot of biological processes, and are also involved in many diseases. MiRNA-206 (miR-206) is initially known to be abundant in muscle tissue and closely involved in muscle development.25) Recently, more and more studies have been demonstrating the role of miR-206 in the brain. Previous reports have already shown that AD patients and AD models all have high level of miR-206 in the brain, which contributes to memory impairments by suppressing the BDNF expression in the brain.26–28) Thus it is possible that donepezil produces anti-dementia actions by regulating the level of miR-206 in the brain. Here, we studied this speculation using APP/PS1 transgenic mice, behavioral test, and quantitative real-time reverse transcription PCR method.
The Abeta precursor protein (APP)/presenilin-1 (PS1) transgenic mice used in this study were male, ten-month-old, and developed in a C57BL/6J background. All the APP/PS1 mice and littermate wild-type (WT) mice were provided by the Model Animal Research Center of Nanjing University (China). Before experiments started, all the mice were housed 5 per cage for one week under standard conditions (free access to food and water; lights from 07 : 00 to 19:00; room temperature 24±1°C), as we previously described.29–32) All the behavioral experiments were performed during the day. All the procedures involving mice were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Drugs and TreatmentsDonepezil was provided by Tocris (Bristol, U.K.) and dissolved in 0.9% saline. AgomiR-206-3p was provided by RiboBio (Guangzhou, China). The dosage of donepezil (5, 10 mg/kg) and AgomiR-206-3p (100 nmol/kg) were chosen based on previous reports.29,33,34) Donepezil was administered intraperitoneally (i.p.) in a volume of 10 mL/kg, while AgomiR-206-3p was tail-vein injected.
Morris Water MazeThe Morris water maze was conducted as we previously did.29,35)
Western Blotting AnalysisThe Western blotting method was also performed as we previously did.29–32)
Quantitative Real-Time Reverse Transcription PCR (qRT-PCR) DectectionThe miR-206-3p, miR-206-5p, and BDNF mRNA levels were determined using qRT-PCR method.28,36–38) For the detection of miR-206, total miRNAs were isolated from the hippocampus and cortex using the mirVana miRNA isolation kit (Ambion, CA, U.S.A.). After the measurement of RNA quantity, the mirVana qRT-PCR miRNA kit (Ambion) was further used per the kit instructions. U6 was selected as the internal control. For the detection of BDNF mRNA, total RNA was isolated from the hippocampus and cortex using the TRIzol® reagent (Thermo Fisher, DE, U.S.A.), and the RT system (Promega, Madison, WI, U.S.A.) and SYBR® fast qPCR master mix (TaKaRa, Japan) were further used following the kit protocols. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was selected as the internal control. Each sample was assayed in triplicate. The primers for miR-206-3p (RT primer: 5′-GTC TGT ATG GTT GTT CTG CTC TCT GTC TCA TCC CTA TCT ACA ACC ATA CAG ACC CAC ACA CAT-3′; PCR forward primer: 5′-CTG CCG TGG AAT GTA AGG AA-3′; PCR reverse primer: 5′-TAT GGT TGT TCT GCT CTC TGT CTC-3′), miR-206-5p (RT primer: 5′-GTC TGT ATG GTT GTT CAC GAC TCC TTC ACA TCC CTA TCC AAC CAT ACA GAC TAT GAG GAT A-3′; PCR forward primer: 5′-GCT GCT CAG ACA TGC TTC TTT ATA-3′; PCR reverse primer: 5′-TAT GGT TGT TCA CGA CTC CTT CAC-3′), and U6 (RT primer: 5′-GTC GTA TCC AGT GCA GGG TCG AGG TGC ACT GGA TAC GAC AAA ATA TGG-3′; PCR forward primer: 5′-ATT GGA ACG ATA CAG AGA AGA TT-3′; PCR reverse primer: 5′-GGA ACG CTT CAC GAA TTT G-3′) were designed by GenePharma (Shanghai, China). The primers for BDNF mRNA (PCR forward primer: 5′-GAC AAG GCA ACT TGG CCT AC-3′; PCR reverse primer: 5′-CCT GTC ACA CAC GCT CAG CTC-3′) and GAPDH (PCR forward primer: 5′-ACA TTG TTG CCA TCA ACG AC-3′; PCR reverse primer: 5′-ACG CCA GTA GAC TCC ACG AC-3′) were designed by Sangon Biotech (Shanghai, China). The threshold cycle (CT) was defined as the fractional cycle number at which the fluorescence passed the fixed threshold. The relative gene expression levels of miR-206-3p, miR-206-5p, and BDNF mRNA were normalized to their internal controls, and calculated using the △△CT method.
Statistical AnalysisAll the data analysis was performed using two-way ANOVA followed by post-hoc Bonferroni’s test (SPSS 13.0 software). p<0.05 level was thought statistically significant in this study.
As a first step of this study, both the APP/PS1 and WT mice were administrated with donepezil or vehicle for 10 d, with the Morris water maze performed during the last five days. Mice were injected with donepezil/vehicle 30 min before the first learning trial of each training day. The behavioral data was summarized in Fig. 1. It was found that the vehicle-treated APP/PS1 mice displayed significantly longer escape latency than vehicle-treated WT mice in the 3rd and 4th training day (Fig. 1A, n=10, p<0.01 vs. WT+Vehicle), proving the effectiveness and reliability of our mice model of AD. It was also found that 5 mg/kg donepezil treatment reduced the escape latency of APP/PS1 mice starting from the 2nd training day, while 10 mg/kg donepezil-treated APP/PS1 mice displayed almost the same level of escape latency to vehicle-treated WT mice during all the 4 training days (Fig. 1A, n=10, p<0.01 vs. APP/PS1+Vehicle). Similarly, APP/PS1 mice exhibited significantly memory impairments in the probe test (Fig. 1B, n=10, p<0.01 vs. WT+Vehicle), while 10 mg/kg donepezil administration fully reversed this dysfunction (Fig. 1B, n=10, p<0.01 vs. APP/PS1+Vehicle). Moreover, donepezil administration did not affect the memory ability of WT control mice in the Morris water maze (n=10).
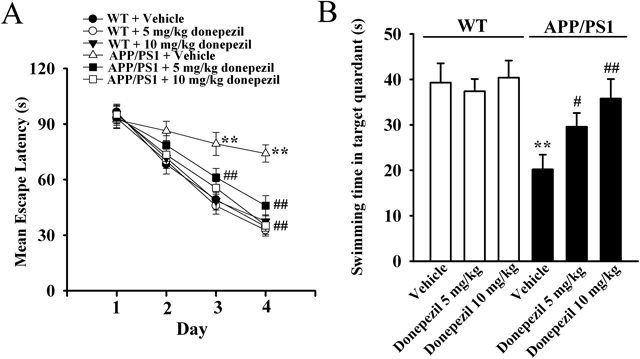
(A) Comparison of the mean escape latency during the training trials for vehicle-treated WT mice, 5 mg/kg donepezil-treated WT mice, 10 mg/kg donepezil-treated WT mice, vehicle-treated APP/PS1 mice, 5 mg/kg donepezil-treated APP/PS1 mice, and 10 mg/kg donepezil-treated APP/PS1 mice. (B) Comparison of the swimming time spent in the target quadrant during the probe test for each group. Data are expressed as means±S.E.M. (n=10); ** p<0.01 vs. WT+Vehicle; # p<0.05, ## p<0.01 vs. APP/PS1+Vehicle.
Next, Western blotting experiments were performed to detect the BDNF expression in the hippocampus and cortex, with β-actin used as the internal control. As shown in Fig. 2, the hippocampal and cortical BDNF expression in APP/PS1 mice were significantly less than that in WT control mice (n=5, p<0.01 vs. WT+Vehicle), consistent with previous findings.28,39,40) In line with the behavioral data, chronic donepezil administration significantly enhanced the expression of hippocampal and cortical BDNF in APP/PS1 mice (n=5, p<0.01 vs. APP/PS1+Vehicle). Donepezil treatment did not affect the BDNF expression in WT control mice (n=5).

(A) APP/PS1 mice displayed significantly less BDNF protein expression in the hippocampus than WT control mice, while this dysfunction was fully reversed by donepezil administration. (B) Similar to hippocampus, donepezil treatment also restored the decreased BDNF protein expression in the cortex of APP/PS1 mice. Data are expressed as means±S.E.M. (n=5); ** p<0.01 vs. WT+Vehicle; # p<0.05, ## p<0.01 vs. APP/PS1+Vehicle.
Furthermore, qRT-PCR experiments were done to examine the levels of miR-206-3p and miR-206-5p in the hippocampus and cortex of vehicle-treated WT mice, donepezil-treated WT mice, vehicle-treated APP/PS1 mice, and donepezil-treated APP/PS1 mice. As shown in Fig. 3A, APP/PS1 mice displayed significantly higher miR-206-3p level in both the hippocampus and cortex than WT control mice (n=5, p<0.01 vs. WT+Vehicle), consistent with previous finding.27,28) Detailed analysis revealed that the hippocampal miR-206-3p level in APP/PS1+vehicle mice was about 257.3+17.3% higher than that in WT control+vehicle mice, while the cortical miR-206-3p level in APP/PS1 mice was about 267.6+21.4% higher than that in WT control+vehicle mice. Interestingly, the usage of donepezil fully down-regulated the miR-206-3p levels in APP/PS1 mice (n=5, p<0.01 vs. APP/PS1+Vehicle). Detailed analysis also indicated that the hippocampal miR-206-3p level in APP/PS1+10 mg/kg donepezil mice was about 60.6+11.2% less than that in APP/PS1+vehicle mice, while the cortical miR-206-3p level in APP/PS1+10 mg/kg donepezil mice was about 51.7+10.4% less than that in APP/PS1+vehicle mice. Figure 3B illustrated the miR-206-5p data, and however, it was found that there was no significant change in the miR-206-5p level among all the groups (n=5). In addition, donepezil treatment did not influence the miR-206-3p and miR-206-5p levels in WT control mice (n=5). These data suggest that donepezil may produce anti-dementia effects through down-regulating the hippocampal and cortical miR-206-3p.

(A) APP/PS1 mice displayed significantly higher miR-206-3p level in the hippocampus and cortex than WT control mice, while donepezil treatment fully restored this dysfunction. (B) There were no significant changes in the hippocampal and cortical miR-206-5p level between all the groups. (C) In contrast to miR-206-3p, APP/PS1 mice had significantly less BDNF mRNA level in the hippocampus and cortex than WT control mice, while donepezil treatment also reversed this dysfunction. Data are expressed as means±S.E.M. (n=5); ** p<0.01 vs. WT+Vehicle; # p<0.05, ## p<0.01 vs. APP/PS1+Vehicle.
Correspondingly, the BDNF mRNA level in each group was also examined, and the data was shown in Fig. 3C. The change for BDNF mRNA in each group was in line with that for BDNF protein, while in contrast to that for miR-206-3p. It was found that APP/PS1 mice had significantly less BDNF mRNA in the hippocampus and cortex than WT control mice (n=5, p<0.01 vs. WT+Vehicle), while chronic donepezil treatment fully reversed this dysfunction (n=5, p<0.01 vs. APP/PS1+Vehicle).
The Anti-dementia Effects of Donepezil in APP/PS1 Mice Were Attenuated by miR-206-3p EnhancingTo further explore the relationship between miR-206-3p and the anti-dementia effects of donepezil, AgomiR-206-3p was used to enhance the level of miR-206-3p in the brain. APP/PS1 mice were co-administrated with AgomiR-206-3p (30 min before donepezil treatment) and donepezil for 10 d, with the Morris water maze performed during the last five days. The behavioral data are summarized in Figs. 4A and B, and we can see that compared to vehicle-treated APP/PS1 mice, the usage of AgomiR-206-3p further exacerbated the memory impairments in APP/PS1 mice (n=10, p<0.01 vs. APP/PS1+Vehicle). Importantly, AgomiR-206-3p pretreatment significantly attenuated the anti-dementia effects of donepezil in the tests (n=10).

(A) Comparison of the mean escape latency during the training trials for vehicle-treated WT mice, vehicle-treated APP/PS1 mice, AgomiR-206-3p-treated APP/PS1 mice, 10 mg/kg donepezil-treated APP/PS1 mice, and AgomiR-206-3p+10 mg/kg donepezil co-treated APP/PS1 mice. (B) Comparison of the swimming time spent in the target quadrant during the probe test for each group. Data are expressed as means±S.E.M. (n=10); ** p<0.01 vs. WT+Vehicle; # p<0.05, ## p<0.01 vs. APP/PS1+Vehicle.
After the behavioral tests, qRT-PCR and Western blotting experiments were also performed. The qRT-PCR results showed that AgomiR-206-3p+donepezil co-treated APP/PS1 mice displayed significantly higher miR-206-3p level and lower BDNF mRNA level in the hippocampus and cortex than donepezil-treated APP/PS1 mice (Figs. 5A, B, n=5). The Western blotting results revealed that AgomiR-206-3p+donepezil co-treated APP/PS1 mice displayed significantly lower BDNF protein expression in the hippocampus and cortex than donepezil-treated APP/PS1 mice (Figs. 6A, B, n=5). Collectively, these results suggest that the anti-dementia effects of donepezil were mediated through down-regulating the miR-206-3p level in the brain.

(A) qRT-PCR results showed that AgomiR-206-3p pretreatment significantly prevented the donepezil-induced decrease of hippocampal and cortical miR-206-3p in APP/PS1 mice. (B) qRT-PCR results showed that AgomiR-206-3p pretreatment also significantly prevented the donepezil-induced increase of hippocampal and cortical BDNF mRNA in APP/PS1 mice. Data are expressed as means±S.E.M. (n=5); ** p<0.01 vs. WT+Vehicle; # p<0.05, ## p<0.01 vs. APP/PS1+Vehicle.

(A) Western blotting results showed that AgomiR-206-3p pretreatment significantly prevented the donepezil-induced increase of hippocampal BDNF expression in APP/PS1 mice. (B) Western blotting results revealed that AgomiR-206-3p pretreatment also significantly prevented the donepezil-induced increase of cortical BDNF expression in APP/PS1 mice. Data are expressed as means±S.E.M. (n=5); ** p<0.01 vs. WT+Vehicle; # p<0.05, ## p<0.01 vs. APP/PS1+Vehicle.
Here, we reported that chronic donepezil administration not only improved the memory abilities and BDNF dysfunction in APP/PS1 mice, but also down-regulated the miR-206-3p expression in the hippocampus and cortex.
The anti-dementia effects of donepezil in AD animal models have already been demonstrated. In this study, we used the classic behavior test, Morris water maze, to assess the effects of donepezil in APP/PS1 mice model of AD. Consistent with previous reports,41–43) donepezil produced significant beneficial effects on the memory ability of APP/PS1 mice, suggesting the effectiveness of AD model and donepezil used in this study.
Although thought as a centrally acting reversible acetylcholinesterase inhibitor, more and more pharmacological effects of donepezil are being explored. For example, Ye et al. reported that donepezil reduced the Aβ accumulation in mitochondria.44) Guo et al. found that donepezil improved the cognitive deficits in APP/PS1 mice by inhibiting microglial activation and the release of pro-inflammatory cytokines.42) Solntseva et al. also showed that activation of sigma-1 receptor was involved in the protective effects of donepezil against the Aβ-induced hippocampal long-term potentiation (LTP) impairments.45) In our study, the finding that donepezil administration reversed the BDNF dysfunction in APP/PS1 mice may extend the clinical application of donepezil, as BDNF is also involved in some other neurological disorders.
How does donepezil reverse the BDNF dysfunction in APP/PS1 mice? MiRNAs are endogenous small noncoding RNAs which play important roles in diverse biological and pathological processes, like cell differentiation, apoptosis, heart disease, neurological disorders, cancer, and so on.46) MiR-206 is one of the known miRNAs and belongs to the miR-1 family.47) Recently, more and more physiological effects of miR-206 are being reported. In humans there is only one mature sequence for miR-206 (has-miR-206), while in mice there are two distinct mature sequences for miR-206 (mmu-miR-206-3p, mmu-miR-206-5p). Mmu-miR-206-3p is homological to has-miR-206, and also the major form of miR-206 in mice. Although there are numerous kinds of miRNAs in the brain, in our study, the most important reason for considering miR-206 comes from previous findings which have shown that miR-206 contributes to AD by suppressing the BDNF expression in the brain.26–28) Here, the finding that donepezil treatment decreased the hippocampal and cortical miR-206-3p expression is very interesting, extending the understanding of the pharmacological effects of donepezil, and suggests that miR-206 could be a novel pharmacological target for developing anti-dementia drugs. The miR-206-5p results are also interesting. As a minor form of miR-206 in mice, maybe the physiological role of miR-206-5p is not as wide as miR-206-3p.
AgomiRs are molecularly-modified oligonucleotides widely used in studies of miRNAs. Here, though donepezil administration decreased the hippocampal and cortical miR-206-3p expression in APP/PS1 mice, it is not enough to conclude that the anti-dementia effects of donepezil require miR-206-3p, and so we used AgomiR-206-3p. The results that AgomiR-206-3p further exacerbated the memory impairments and BDNF dysfunction in APP/PS1 mice suggest the effectiveness of AgomiR-206-3p used in this study. Importantly and as expected, AgomiR-206-3p usage blocked the anti-dementia effects of donepezil, confirming our speculation.
In addition to AD, miR-206 is reported to be involved in many other disorders: miR-206 is up-regulated in a rat model of myocardial infarction, and also a potential biomarker candidate for amyotrophic lateral sclerosis, and also involved in various types of cancer by regulating cell proliferation and migration, and so on.48–51) Since donepezil treatment down-regulates the miR-206-3p expression, these reports may also extend the clinical application of donepezil, which needs more study. Moreover, in addition to miR-206-3p, the pathophysiology of AD involves many other miRNAs, like miR-106b, miR-132, miR-98, and so on.52–54) It is possible that the anti-dementia effects of donepezil may also involve these miRNAs, and this needs further study.
This work was supported by the Grant from the National Natural Science Foundation of China to Dr. Bo Jiang (No. 81401116) and Dr. Yu Chen (No. 81403127), the grants from the Provincial Natural Science Foundation of Jiangsu Province (China) to Dr. Bo Jiang (No. 14KJB310013; No. BK20161284).
The authors declare no conflict of interest.