2017 Volume 40 Issue 4 Pages 540-545
2017 Volume 40 Issue 4 Pages 540-545
Glycosaminoglycans (GAGs) play important roles in various biological processes such as cell adhesion and signal transduction, as well as promote anti-inflammatory activity. We previously revealed that glycol-split heparin (HP)-aliphatic amine conjugates form self-assembled nanoparticles and suppress the production of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β in lipopolysaccharide (LPS)-stimulated macrophages much more strongly than native HP (J. Control. Release, 194, 2014, Babazada et al.). Considering that HP is not the only GAG to have anti-inflammatory activity, the present study was initiated to examine whether conjugation of GAGs with aliphatic amines is generally effective in their activity augmentation against LPS-stimulated macrophages. We newly synthesized the stearylamine conjugates of chondroitin sulfate (CS), hyaluronic acid (HA), and low-molecular-weight heparin (LH), and investigated the effect of the position and degree of sulfation and molecular weight of GAGs on their anti-inflammatory activity. All of the conjugates formed self-assembled nanoparticles in aqueous solution. The IC50 value for suppression of TNF-α production from the macrophages was the smallest with the derivative of LH, followed by HP, CS, and HA. The degree of sulfation appeared to be important in determining their anti-inflammatory activity, which would correspond to previous results using the derivatives of site-selectively desulfated HP. Comparison of HP and LH derivatives revealed that fractionated smaller heparin has greater anti-inflammatory activity.
Glycosaminoglycans (GAGs) are linear polysaccharides that play important roles in processes such as cell adhesion and signal transduction.1) Heparin (HP) is one of the highly sulfated GAGs that consist of repeats of alternating uronic acid and D-glucosamine.2) HP is a well-known anticoagulant that activates antithrombin, leading to anticoagulation3); moreover, it has a variety of additional biological effects such as anti-angiogenesis and anti-inflammation. Chondroitin sulfate (CS), consisting of repeats of alternating D-glucuronic acid and N-acetyl-D-galactosamine,2,4) is used clinically to reduce pain and improve articular function in patients with osteoporosis.5,6) Hyaluronic acid (HA) consists of repeats of alternating D-glucuronic acid and N-acetyl-D-glucosamine, but characteristically lacks sulfo groups.2,4) HA has been used to protect synovial membranes and heal injuries.7–9)
Although the biological activity varies for each GAG, a common feature is the presence of an anti-inflammatory effect. Several in vitro10–14) and in vivo15,16) studies have shown that HP, CS, and HA suppress the lipopolysaccharide (LPS)-induced production of inflammatory cytokines by inhibiting the activation of MyD88, p38 mitogen-activated protein kinase (MAPK), and nuclear factor-kappaB (NF-κB).
Previously, we reported that glycol-split HP conjugated with D-erythro-sphingosine or aliphatic amines forms self-assembled nano-sized particles and strongly inhibits the production of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β in LPS-stimulated macrophages.17) The anti-inflammatory activity of the HP conjugates was greater than that of native HP. Since HP conjugates prevented phosphorylation of IL-1 receptor-associated kinase-1 (IRAK-1) and degradation of inhibitor of NF-κB (IκBα), its anti-inflammatory effect was concluded to occur via inhibition of the Toll-like receptor 4 (TLR4)-mediated MyD88-dependent NF-κB pathway.17) A subsequent study demonstrated that the HP-D-erythro-sphingosine conjugate delayed the progression of arthritis in type II collagen-induced arthritis mouse models.18)
The present study was initiated to examine whether conjugation with hydrophobic amines is effective for not only HP but also other GAGs in augmentation of anti-inflammatory activity against LPS-stimulated macrophages. Our previous study17) revealed that desulfation of the 6-O-sulfo group of the HP conjugates remarkably lowers their anti-inflammatory effect, but desulfation at either 2-O- or N-sulfo groups does not. This prompted us to hypothesize that the structure of GAGs, including the position and degree of sulfation, greatly affects their anti-inflammatory effect. It is known that the degree of inhibition of LPS stimulation varies among GAGs.19,20) However, the effect of conjugation with hydrophobic amines against different GAGs remains to be elucidated. As a result, we newly synthesized the stearylamine conjugates of CS and HA, which differ from HP in the chain of alternating sugars and the position and degree of sulfation, and investigated the effect of the conjugates on anti-inflammatory activity against LPS-stimulated mouse peritoneal macrophages in vitro. Effect of molecular size of GAGs on the anti-inflammatory effect was also investigated by comparing the stearylamine conjugate of HP with that of fractionated low-molecular-weight heparin (LH).
Glycol-split GAGs were obtained by periodate-oxidation and borohydride-reduction of HP (average molecular weight (M.W.) of 15 kDa, Nacalai Tesque, Kyoto, Japan), LH (5 kDa, Kissei, Tokyo, Japan), CS (chondroitin 6-sulfate, 15 kDa, Nacalai Tesque, Kyoto, Japan), and HA (20 kDa, Lifecore, U.S.A.) as described previously.17) Next, 20 mg of each glycol-split GAG was dissolved in 1.5 mL formamide containing 10 mg 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and mixed with 5 mg of stearylamine (Wako Pure Chemical Industries, Ltd.) dissolved in 1 mL tetrahydrofran. The reaction mixture was stirred at room temperature for 24 h. The reaction mixtures were then precipitated in ethanol, centrifuged at 15000×g for 10 min, and decanted. This step was repeated three times to remove unreacted stearylamine and reaction solutions. Precipitates were dried and re-suspended in water, then dialyzed (M.W. cutoff 2 kDa) and lyophilized to yield self-assembling glycol-split HP-stearylamine (gs-HPST), CS-stearylamine (gs-CSST), LH-stearylamine (gs-LHST), and HA-stearylamine (gs-HAST) conjugates. Their chemical structures were shown in Fig. 1.

Size distribution and zeta potential of 0.1 mg/mL nanoparticles were measured using Zetasizer Nano ZS (Malvern Instruments, Worcestershire, U.K.).
Critical Micelle Concentration (c.m.c.) MeasurementsThe c.m.c. of conjugates were determined by fluorescence spectroscopy using pyrene as a probe, as described previously.21) Fluorescence spectra were measured using a Fluoremax-4 spectrofluorometer (Horiba Ltd., Kyoto, Japan) at 25°C. A solution of pyrene in water (7.5×10−8 M final concentration) was mixed with conjugates in concentrations varying from 6.25×10−4 to 1 mg/mL. The solutions were equilibrated 12 h before the measurements. Spectra were accumulated with an integration time of 1 nm/s. The excitation and emission wavelengths were 339 and 390 nm, respectively.
Cell Isolation and in Vitro Assay for CytokinesMouse peritoneal macrophages were isolated as described previously.17) All animal experiments were performed in accordance with the Principles of Laboratory Animal Care as directed by the U.S. National Institutes of Health and the Guidelines for Animal Experiments of Kyoto University. Macrophages were harvested with ice-cold RPMI 1640 from 5-week-old female ICR mice (SLC, Shizuoka, Japan) 4 d after intraperitoneal injection of 1 mL 2.9% thioglycolate medium (Nissui Pharmaceutical, Tokyo, Japan). Cells were washed three times and suspended in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), penicillin G (100 U/mL), and streptomycin (100 µg/mL), then plated onto 96-well plates (Falcon, Becton Dickinson, Lincoln Park, NJ, U.S.A.) at a density of 1.0×105 cells/well. After incubation for 2 h at 37°C in a humidified atmosphere of 5% CO2 in air, non-adherent cells were washed off. The remaining cells were incubated for 24 h. Next, the medium was replaced with Opti-MEM® I (Gibco®, Life Technologies) and cells were treated with GAG-stearylamine conjugates at a final concentration of 0.5 or 0.1 mg/mL plus 20 ng/mL LPS, a selective TLR4 ligand (Sigma-Aldrich), 100 ng/mL Pam2CSK4, a selective TLR2 ligand (InvivoGen), or 10 µg/mL polyinosinic-polycytidylic acid (poly(I:C)), a selective TLR3 ligand (InvivoGen), and incubated for 24 h. Supernatants were analyzed for TNF-α using an enzyme-linked immunosorbent assay (ELISA) kit (Peprotech, Rocky Hill, NJ, U.S.A.) according to the manufacturer’s protocol. Native or glycol-split GAGs (0.5 mg/mL) were used to compare the inhibitory effects. Their IC50 values were derived from the corresponding dose–response curves.
In Vitro Cytotoxicity AssayEffect of native GAGs, glycol-split GAGs, and their stearylamine conjugates on cell viability was determined using 2-(iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium salt (WST-1) (Roche Applied Biosystems) based colorimetric assay according to the manufacturer’s instructions.
StatisticsStatistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, Inc.). Statistical significance between multiple groups was determined by one-way ANOVA with Dunnett’s post hoc test. Data are presented as the mean±standard error of the mean (S.E.M.) unless otherwise stated.
Table 1 summarizes physicochemical properties of the nanoparticles comprised of glycol-split GAG-stearylamine conjugates. gs-HPST and gs-LHST nanoparticles were smaller in particle size (ca. 100 nm) compared to gs-CSST and gs-HAST nanoparticles (ca. 150 nm). Zeta potential of gs-CSST and gs-HAST nanoparticles had much lower negative charge compared to gs-HPST and gs-LHST nanoparticles. All conjugates had a c.m.c. between 0.014 and 0.018 mg/mL. Cytotoxicity of the GAG derivatives, along with their native GAGs and synthetic intermediates, was preliminarily confirmed in primary cultured mouse peritoneal macrophages, using the WST-1 assay (Supplementary Fig. 1). Although viability of the cells was decreased by extremely high concentrations of the GAG-stearylamine conjugates (>1 mg/mL), the derivatives were found to be reasonably safe.
| Particle size (nm) | PdIb) | Zeta potential (mV) | c.m.c.c) (mg/mL) | |
|---|---|---|---|---|
| gs-HPSTd) | 107.6±3.4 | 0.19±0.01 | −33.4±0.7 | 0.014 |
| gs-LHSTd) | 93.9±0.8 | 0.15±0.02 | −39.9±2.0 | 0.015 |
| gs-CSSTd) | 147.3±16.7 | 0.25±0.03 | −27.5±0.6 | 0.018 |
| gs-HASTd) | 153.4±24.0 | 0.31±0.03 | −20.7±1.0 | 0.014 |
a) Data represent mean±standard deviation (S.D.) b) Polydispersity index. c) Critical micelle concentration. d) Stearylamine conjugates of glycol-split glycosaminoglycans: heparin (gs-HPST), low-molecular-weight heparin (gs-LHST), chondroitin sulfate (gs-CSST), and hyaluronic acid (gs-HAST).
Anti-inflammatory effect of the glycol-split GAG-stearylamine conjugates was evaluated by measuring TNF-α production in LPS-stimulated peritoneal macrophages. As shown in Fig. 2, all GAGs except CS slightly reduced TNF-α production in their native forms, whereas LH and HA were relatively more effective. It is known that HA ranges in molecular weight from 4 to 8000 kDa and has size-dependent biological activities.4,22,23) High-molecular-weight HA has anti-inflammatory and anti-angiogenic properties,16,23) whereas low-molecular-weight HA stimulates expression of pro-inflammatory cytokines, chemokines, and growth factors.22,23) Because unfractionated HA was used in this study, we performed a preliminary experiment, demonstrating that the HA itself did not induce the expression of TNF-α form macrophage (Supplementary Fig. 2).

Isolated macrophages were seeded on a 96-well pate at a density of 1.0×105 cells/well. The cells were treated with 0.5 mg/mL native GAGs, glycol-split GAGs, or glycol-split GAG-stearylamine conjugates, together with 20 ng/mL LPS for 24 h. Concentration of TNF-α in the culture medium was measured by ELISA. Each value represents the mean±standard error of the mean (S.E.M.) (n=3). †p<0.01. N.D., not detected (<16 pg/mL).
Figure 2 also shows the anti-inflammatory effect of glycol-split and further stearylamine-conjugated GAGs. Glycol-splitting did not appear to influence the anti-inflammatory activity of GAGs; however, subsequent conjugation with stearylamine resulted in a marked increase in anti-inflammatory activity for all GAGs. Figure 3 illustrates concentration–response curves for the anti-inflammatory effect of glycol-split GAG-stearylamine conjugates. The calculated IC50 value was the smallest with gs-LHST (0.010 mg/mL), followed by gs-HPST (0.018 mg/mL), gs-CSST (0.033 mg/mL), and gs-HAST (0.113 mg/mL).
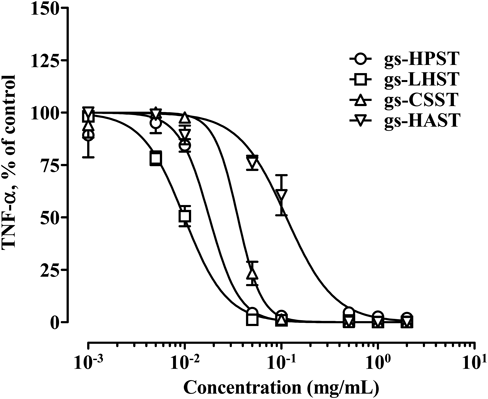
Isolated macrophages were seeded on a 96-well pate at a density of 1.0×105 cells/well. Cells were treated with varying concentrations of glycol-split heparin (gs-HPST), low-molecular-weight heparin (gs-LHST), chondroitin sulfate (gs-CSST), and hyaluronic acid (gs-HAAST) at the concentration of 0.001–2 mg/mL together with 20 ng/mL LPS for 24 h. Concentration of TNF-α in the culture medium was measured by ELISA. Each value represents the mean±S.E.M. (n=3) relative to the non-treated group.
Like HP, LH is known to suppress LPS-induced inflammation in vitro24) and in vivo.25) In addition, LH is much more likely to bind to vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF).26,27) Clinically, there is evidence that LH significantly improves 3 month survival compared to HP in patients with cancer.28) The present study demonstrated that stearylamine conjugate of glycol-split LH has a more profound anti-inflammatory effect than that for HP (Fig. 3). Therefore, LH might generally have higher biological activities.
HP is a highly sulfated GAG with an average sulfation per saccharide of 2–3, CS is moderately sulfated (0–2), and HA is not sulfated.2) The degree of sulfation of GAGs influences GAG-protein interaction. For example, when CS and HA were sulfated, interaction with transforming growth factor (TGF)-1β becomes stronger according to their degree of sulfation.29) Our analysis of the anti-inflammatory effect of GAG-stearylamine conjugates revealed a similar tendency. The position of sulfation in GAGs also appears to be important in determining their biological activities. In a previous study, we prepared glycol-split HP-sphingosine conjugates and their site-selectively desulfated derivatives, demonstrating that the 6-O-sulfo group of D-glucosamine residue is critical in suppressing TNF-α production in LPS-stimulated macrophages.17) The 6-O-sulfo group of HP has also been shown to be required for blocking L- and P-selectin.30) Of the GAGs-stearylamine conjugates we synthesized, gs-HAST derived from HA lacks the 6-O-sulfo group. This may be the reason why gs-HAST had significantly weaker TNF-α suppression activity than gs-HPST or gs-CSST.
The effect of the GAG derivatives on TLR2 and TLR3 activated macrophages was also investigated. The suppressive effect of HP-sphingosine conjugates on TNF-α production was observed in LPS-treated macrophages but not in Pam2CSK4- or poly(I:C)-treated macrophages.17) Similar experiments were carried out for all the synthesized GAGs conjugates (Fig. 4). None of the conjugates suppressed TNF-α production in macrophages treated with a TLR2 ligand Pam2CSK4. In contrast, all GAGs conjugates except gs-HAST exhibited anti-inflammatory effect in TLR3 ligand poly(I:C)-treated macrophages. However, considering that TNF-α production followed by poly(I:C) stimulation was not so extensive and that poly(I:C) treatment tended to reduce cell viability, it cannot be ruled out that the suppressive effect of the GAGs conjugates might be an artifact of cellular damages.
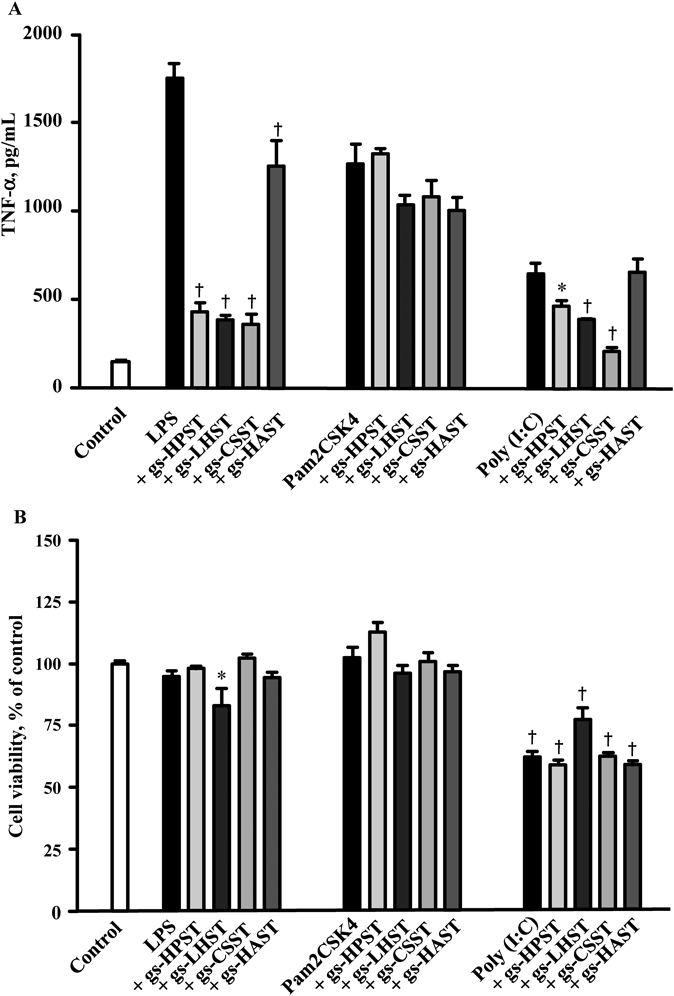
Isolated macrophages were seeded on a 96-well pate at a density of 1.0×105 cells/well. Cells were incubated with 20 ng/mL LPS, 20 ng/mL Pam2CSK4, or 10 µg/mL poly(I:C) in the absence or presence of glycol-split heparin (gs-HPST), low-molecular-weight heparin (gs-LHST), chondroitin sulfate (gs-CSST), and hyaluronic acid (gs-HAAST) at 0.1 mg/mL for 24 h. Each value represents the mean±S.E.M. (n=3). * p<0.05. † p<0.01.
In conclusion, the glycol-split GAG-stearylamine conjugates have much greater inhibitory effect against LPS-induced cytokine production as compared to native GAGs. Especially, gs-LHST derived from low molecular weight heparin was the most potent among the conjugates synthesized. Although conjugation with sterarylamine provides GAGs with a self-assembling property, it is unclear whether formation of nanoparticles is a direct cause of enhanced anti-inflammatory effect. It is conceivable that the some part of conjugate molecules exist as monomer and possess higher affinity for the receptors as a result of increased hydrophobicity. Further investigations would be required to uncover how the glycol-split GAG-stearylamine conjugates interact with the receptors.
This work was financially supported in part by Cosmetology Research Foundation (FY) and by JSPS KAKENHI under Grant numbers 15K08071(FY) and JP16H01861(MH). This work was also supported by the iCeMS, Kyoto University, supported by the World Premier International Research Center Initiative (WPI Program), Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.
Fig. S1. WST-1 cytotoxicity assay of native glycosaminoglycans (GAG), glycol-split GAGs, and glycol-split GAGs-stearylamine conjugates in mouse peritoneal macrophages.
Fig. S2. Effect of native hyaluronic acid on TNF-α production in mouse peritoneal macrophages.