2017 Volume 40 Issue 5 Pages 548-552
2017 Volume 40 Issue 5 Pages 548-552
DJ-1, encoded in a causative gene of familial Parkinson’s disease (PARK7), has multiple functions: it works as an antioxidant, in transcriptional regulation, as a molecular chaperone and in protein degradation. Three types of pathogenic mutants of DJ-1 (M26I, D149A and L166P) have been reported to disrupt proper structures and lead to a loss of function. DJ-1 receives oxidation at the cysteine residue, and the degree of oxidation at the C106 residue determines DJ-1 activity. In this decade, DJ-1 has been reported to suppress the progression of various neurodegenerative disorders in animal models. The administration of recombinant wild-type DJ-1 protein suppresses the neuronal loss associated with both Parkinson’s disease and ischemic stroke in rats. Furthermore, in studies focused on DJ-1 as the therapeutic target, compounds that have the capacity of binding to DJ-1 at the C106 residue have been reported to exert therapeutic effects on various neurodegenerative disorders such as Parkinson’s disease, Alzheimer’s disease and ischemic stroke. DJ-1 and DJ-1-targeting molecules/compounds will be useful therapeutic targets for various neurodegenerative disorders due to their various functions such as antioxidant capacity, chaperone function and as a proteolytic pathway.
Parkinson’s disease (PD) is a progressive neurodegenerative disorder accompanied by tremors, bradykinesia, hypokinesia and rigidity. Pathologic features of PD include the selective loss of dopaminergic neurons in the substantia nigra (SN) and deposition of α-synuclein (α-syn) in dopaminergic neurons. Sporadic PD accounted for about 90% of all cases of PD; familial PD, with a monogenic mutation, accounts for fewer than 10% of all cases of PD. Familial PD is classified into 18 types of monogenic mutants.1,2) DJ-1 is one of the causative genes of familial PD (PARK7).3,4) Human DJ-1 consists of 189 amino acids and normally formed homodimers. Three types of pathogenic DJ-1 mutations—methionine 26 to isoleucine (M26I), aspartic acid 149 to alanine (D149A) and leucine 166 to proline (L166P)—have been identified and are estimated to cause the abnormal conformation and functional loss.5,6) DJ-1 has three cysteine residues in its amino acid sequence at residues 46, 53 and 106 (Fig. 1A). Oxidation and nitrosylation of a cysteine residue determines the function of DJ-17–10) (Fig. 1B). Especially, the C106 residue in DJ-1 is the site of cysteine residue most sensitive to hydrogen peroxide (H2O2)-mediated oxidation.11) Recently, accumulating lines of evidence have reported on the association of DJ-1 and neurodegenerative disorders. Here we discuss the role of DJ-1 in pathogenic conditions, as well as the therapeutic potency of DJ-1 and DJ-1-targeting molecules/compounds in several neurodegenerative disorders.
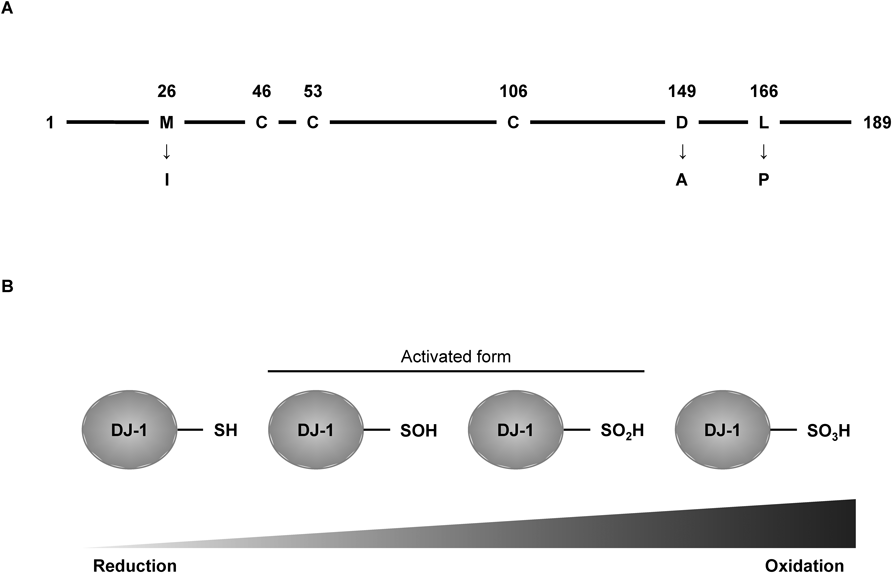
A) DJ-1 is constituted of 189 amino acids. Pathogenic mutants of DJ-1 (M26I, D149A and L166P) were identified from PD patients. DJ-1 has three cysteine residues that work as sensors against oxidative stress. B) Oxidation of C106 residue decides DJ-1 activity. Sulfonated form (–SO3H) is irreversible.
In 2004, Taira et al. focused on the efficacy of DJ-1 as an antioxidant, since oxidative stress is strongly associated with PD pathogenesis. Mouse embryonic fibroblast NIH3T3 cells, harboring pathogenic mutants of DJ-1, were more vulnerable to H2O2-induced cytotoxicity than cells with wild-type (WT) DJ-1.12) Furthermore, treatment of recombinant WT DJ-1, but not the L166P mutant of DJ-1, reduced the H2O2-induced fluorescence of 2′,7′-dichlorodihydrofluorescein, a reactive oxygen species (ROS) specific indicator, in rat primary mesencephalic cultures and human neuroblastoma SH-SY5Y cells.13,14) Deficiency of DJ-1 in neurons differentiated from embryonic stem cells and SH-SY5Y cells showed vulnerability to oxidative stress.13,15) Another point of view is that DJ-1 activates an antioxidant pathway, such as the nuclear factor erythroid 2-related factor 2 (Nrf2)–antioxidant responsive element (ARE) pathway. The Nrf2–ARE pathway is known to be a common antioxidant pathway for the production of antioxidants (e.g. glutathione, thioredoxin and peroxiredoxin). Im et al. reported that DJ-1 was associated with the production of thioredoxin1 by the activation of the Nrf2–ARE pathway without direct interaction with Nrf2 and Kelch-like ECH-associated protein 1. By contrast, pathogenic mutants of DJ-1 (M26I and L166P) and a missense mutant of DJ-1 at cysteine 106 residue to serine (C106S) did not increase thioredoxin1 expression.16,17)
2.2. Transcriptional RegulationDJ-1 was reported to be a transcriptional regulator in several pathways, including the Nrf2–ARE pathway mentioned above. DJ-1, but not pathogenic mutants of DJ-1, positively regulated transcriptional activation and suppressed polypyrimidine tract-binding protein-associated splicing factor-induced apoptosis in SH-SY5Y cells.18) On the other hand, DJ-1 also acts as a negative regulator of transcription. Fan et al. revealed that DJ-1 interacted with p53 and inhibited p53-mediated Bax expression and the subsequent apoptosis pathway in mouse neuroblastoma neuro2a cells and human embryo kidney 293 cells.19) Moreover, DJ-1 regulated transcription factors such as androgen receptors and sterol regulatory element-binding protein.20–22) Transcriptional regulation by DJ-1 was also associated with the expression of tyrosine hydroxylase (TH), the rate-limiting enzyme of dopamine synthesis.23)
2.3. Chaperone ActivityAbnormal protein aggregations, such as α-syn, amyloid β (Aβ), tau, and huntingtin, are observed as characteristic features in neurodegenerative disorders. In pathogenic conditions, these proteins form abnormal folding and aggregation. Shendelman et al. revealed that WT DJ-1, but not the L166P mutant of DJ-1, prevented the aggregation of α-syn in cell-free assay and reduced the α-syn content in a detergent-insoluble fraction from murine neuroblastoma Cath.a-differentiated (CAD) cells.24) Huntington’s disease is characterized by a mutant huntingtin (mHTT) protein with polyglutamine repeats. Sajjad et al. showed the chaperone activity of DJ-1 in resolving the abnormal aggregation of mHTT.25)
2.4. Protein DegradationDJ-1 has been reported to be involved in proteolytic pathways, such as the ubiquitin proteasome system and autophagy lysosome system. Especially, DJ-1 seems to be associated with the degradation of α-syn. α-Syn is known to be taken into neurons, astrocytes and microglia and to receive clearance in these cells.26,27) Parkin is known as the causative gene of familial PD (PARK2), and functions as a ubiquitin E3 ligase.28) Xiong et al. reported that the complex of Parkin, phosphatase and tensin homolog deleted from chromosome 10 (PTEN)-induced putative kinase 1 (PINK1) and DJ-1 worked as a ubiquitin E3 ligase complex, and promoted proteolysis.29) Furthermore, DJ-1 is also associated with the autophagy lysosome system. In the PD brain, autophagy failure, induced by the production of ROS and reactive nitrogen species, is observed.30) Autophagy is known as the self-degradation of cellular organelles and protein aggregates. Recent studies have revealed the autophagy pathway involved in the degradation of α-syn, implying that autophagy is strongly associated with PD pathogenesis.31–33) DJ-1 deficiency exhibited an impairment of autophagy in mouse embryonic fibroblasts and in M17 human dopaminergic neuroblastoma cells.34,35)
In 2006, Inden et al. investigated the efficacy of the recombinant WT DJ-1 protein for 6-hydroxydopamine (6-OHDA)-injected hemiparkinsonian rats. Co-injection of recombinant WT DJ-1 protein with 6-OHDA dramatically reduced the loss of TH-positive dopaminergic neurons in the SN, and increased the dopamine content in the striatum. In contrast, the L166P mutant of DJ-1 did not exert a protective effect against 6-OHDA-induced neurotoxicity.14) Sun et al. also verified the therapeutic potency of the recombinant WT DJ-1 protein against 6-OHDA-injected PD model rats and MG-132-injected PD model rats.36) Gao et al. injected adeno-associated virus (AAV) vector encoding DJ-1 into the SN. Overexpression of DJ-1 in the SN suppressed the loss of TH-positive dopaminergic neurons induced by rotenone injection into the SN.37) Not only full length DJ-1, but also a short peptide segment of DJ-1 containing 13 amino acids had neuroprotective effects against the PD mouse model. ND-13, a small peptide consisting of YGRKKRRKGAEEMETVIPVD containing 13 amino acids of DJ-1 sequences (lysine 12 to aspartic acid 24), rescued TH-positive dopaminergic neurons in 6-OHDA-injected hemiparkinsonian mice and in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice.38) DJ-1 has three steps of oxidized forms at the C106 residue (Fig. 1B). Especially, its sulfenated form (–SOH) and sulfinated form (–SO2H) have been estimated to exert potent antioxidant efficacy. Miyazaki et al. obtained some small molecules binding to the C106 residue of DJ-1 from 30000 compounds by in silico virtual screening. Compound A (UCP0045037) can bind to the reduced form of DJ-1, and compound B (UCP0045038) can bind to the oxidized form of DJ-1 at C106 as an SO2H form. These compounds reduced the sulfonated form (–SO3H) and kept the reduced form of DJ-1 in H2O2-treated SH-SY5Y cells.39) Co-injection of DJ-1-binding compounds (compounds A, B and 23) with 6-OHDA inhibited the loss of TH-positive dopaminergic neurons.39–41) These compounds were confirmed by in vitro assay to have anti-oxidative effects.39–41) Furthermore, peripheral administration of DJ-1-binding compounds suppressed the loss of dopaminergic neurons and motor dysfunction induced by MPTP or rotenone treatment.40–42)
3.2. Alzheimer’s DiseaseMore recently, we expected the efficacy of DJ-1 to act as an antioxidant in Alzheimer’s disease (AD) model mice. Repeated intraperitoneal administration (50–56 d) of DJ-1-binding compound B improved the spatial memory of mice expressing a chimeric mouse/human amyloid precursor protein (APP) in Swedish mutation and in human presenilin 1 (PS1) Δexon 9 mutation (APP/PS1 mice). In addition, compound B treatment decreased the deposition of Aβ plaques in the hippocampus.43)
3.3. Ischemic StrokeOxidative stress followed by ischemic/reperfusion injury is known to be involved in the progression of brain tissue damage. Experimentally, the rodent model of ischemic stroke is induced by middle cerebral artery occlusion (MCAO). Yanagisawa et al. conducted the intrastrial microinjection of recombinant WT DJ-1 10 min before reperfusion (110 min after the onset of MCAO). Injection of recombinant WT DJ-1 clearly decreased infarct volume and mitigated neurological dysfunctions.13) In the same way as in PD, DJ-1-binding compounds were investigated to prevent neuronal death after ischemic reperfusion injury. Compound A, compound B and compound 23 decreased infarct volume in MCAO mice.41,44,45)
DJ-1 is widely expressed in mammalian body and forms a homodimer. In pathogenic conditions, monogenic mutants (M26I, D149A and L166P) and chemical modification of cysteine residues disrupt the three-dimensional structures and functions of DJ-1. Especially, DJ-1 is suggested to be associated with neurodegenerative disorders. In this decade, many reports have shown the therapeutic potency of DJ-1 and DJ-1-targeting molecules/compounds in treating several neurodegenerative disorders (Table 1). Although detailed mechanisms of this therapeutic effect remain to be demonstrated in further studies, we already know that DJ-1 and DJ-1-targeting compounds/molecules exert therapeutic effects in neurodegenerative disorders, such as PD, AD and ischemic stroke, by ROS scavenging, protein refolding and degradation of abnormal protein aggregates (Fig. 2). Overall, DJ-1 is a promising therapeutic target for exerting a therapeutic effect on various neurodegenerative disorders due to its multiple functions, such as antioxidant capacity, chaperone function and proteolytic pathway.
| Disease | Animal models of disease | DJ-1 or DJ-1-targeting molecules/compounds | Therapeutic effects | References | |
|---|---|---|---|---|---|
| Parkinson’s disease | 6-OHDA injection into SN | Rat | Recombinant DJ-1 protein | Protection of TH+neurons | Inden et al., 200614) |
| Sun et al., 201236) | |||||
| Compound A/UCP0054277 | Miyazaki et al., 200839) | ||||
| Compound B/UCP0054278 | Miyazaki et al., 200839) | ||||
| Inden et al., 201140) | |||||
| Compound 23 | Kitamura et al., 201141) | ||||
| Mouse | ND-13 | Lev et al., 201538) | |||
| Rotenone injection into SN | Rat | AAV-DJ-1 | Gao et al., 201237) | ||
| MG-132 injection into SN | Rat | Recombinant DJ-1 protein | Sun et al., 201236) | ||
| MPTP treatment (i.p.) | Mouse | Compound 23 | Takahashi-Niki et al., 201542) | ||
| ND-13 | Lev et al., 201538) | ||||
| Rotenone treatment (i.p.) | Mouse | Compound B/UCP0054278 | Inden et al., 201140) | ||
| Compound 23 | Suppression of motor dysfunction | Kitamura et al., 201141) | |||
| Alzheimer’s disease | APP/PS1 | Mouse | Compound B/UCP0054278 | Recovery of cognitive function Inhibition of Aβ deposition | Kitamura et al., 201743) |
| Ischemic stroke | Transient middle cerebral artery occlusion | Rat | Recombinant DJ-1 protein | Reduction of infarct volume | Yanagisawa et al., 200813) |
| Compound A/UCP0054277 | Yamane et al., 200944) | ||||
| Compound B/UCP0054278 | Yanagida et al., 200945) | ||||
| Compound 23 | Kitamura et al., 201141) | ||||
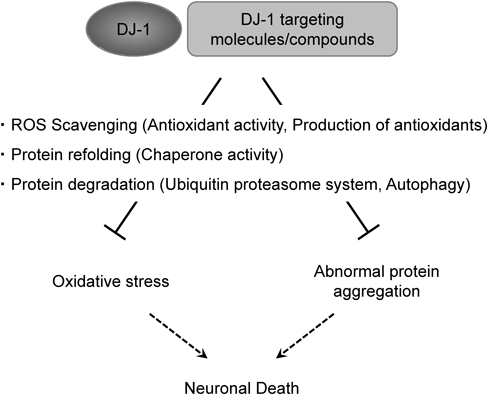
DJ-1 and DJ-1-targeting molecules/compounds suppress neuronal death by reducing oxidative stress and inhibiting abnormal protein aggregation. Detailed mechanisms have been estimated to include ROS scavenging, protein refolding and protein degradation.
This work was supported by JSPS KAKENHI, MEXT, Japan, the Smoking Research Foundation (SRF), and Ritsumeikan University in Japan.
The authors declare no conflict of interest.