2017 Volume 40 Issue 6 Pages 824-829
2017 Volume 40 Issue 6 Pages 824-829
In general, the risk of adverse drug reactions (ADRs) is higher in elderly patients than in younger patients. In this study, we performed a comprehensive assessment of the risks of possible drug–ADR combinations in elderly patients using the Japanese Adverse Drug Event Report (JADER) database of the Pharmaceutical and Medical Devices Agency (PMDA, Japan) using the reporting odds ratio (ROR) as an index. Data recorded from April 2004 to September 2015 in the JADER database were downloaded from the PMDA website. The patients were classified into younger (≤69 years old) and elderly (≥70 years old) groups. The ROR and 95% confidence interval (CI) were calculated for all combinations of drugs and ADRs for which there were three or more reports in the database, focusing particularly on the combinations where more than 100 cases had been reported in elderly and younger patients. The most frequently reported drug–ADR combination was methotrexate with interstitial lung disease (646 cases). The combination with the highest ROR was methotrexate with lymphoproliferative disorder (ROR: 484.6, 95% CI: 334.1–702.9). In total, 27 drug–ADR combinations were found to have high risk in elderly patients. In conclusion, the findings of this comprehensive assessment of drug–ADR combinations using the JADER database will be valuable for updating the ADR risks for elderly patients in clinical setting.
In general, the risk of adverse drug reactions (ADRs) is higher in elderly patients compared with younger patients,1,2) and this is a common clinical problem irrespective of the drug type. Previous studies showed that ADRs is high in ≥65 years old.3–5) One possible reason is the physiological change elderly patients have undergone, often including a reduction of physiological functions such as renal or hepatic clearance, which can affect the pharmacokinetics and pharmacodynamics of many drugs.6) In addition, risks of ADRs in elderly patients increase because of the issue of polypharmacy which is commonly seen in elderly and one of the causes for increasing ADRs.7) It is reported that elderly patients taking 5 to 8 drugs at a time were at greater risk of ADRs than those taking 0 to 4 drugs.8) Elderly patients often have chronic disease comorbidity requiring many drugs.9,10) For these reasons, elderly patients generally have higher risks of ADRs.
Drugs that potentially increase ADRs in elderly patients are listed in Beers Criteria11) and in Screening Tool of Older People’s potentially inappropriate Prescriptions (STOPP).12) Moreover, the Japan Geriatrics Society has published Guidelines for Medical Treatment and Its Safety in the Elderly 2015.13) Many countries have developed databases of ADR information; these can allow research into inappropriate drug usage that can cause ADRs of the clinical use of a drug in advance. In April 2004, Japan’s Pharmaceuticals and Medical Device Agency (PMDA) established a spontaneous reporting system (SRS), the Japanese Adverse Drug Event Report (JADER) database, a unitized large-scale database used to assess the risk of ADRs.14) Similarly, the United States Food and Drug Administration (FDA) has the FDA Adverse Event Reporting System (FAERS), and the United Kingdom’s Medicines and Healthcare Products Regulatory Agency (MHRA) has its Adverse Event Reporting System.
The reporting odds ratio (ROR)15) is an index often used for pharmacovigilance analysis to assess the risk of ADRs, especially by the PMDA in Japan. Similar indices include the empirical Bayes geometric mean (EBGM),16) often used by the FDA, and the proportional reporting ratio (PRR)17) is often used by MHRA. Previous studies have reported RORs for some drugs and AEs based on information from JADER.18,19) Umetsu et al.19) reported RORs for hypoglycemia from a number of hypoglycemic agents (sulfonylurea, meglitinides, biguanides, thiazolidine, alpha-glucosidase inhibitors, and dipeptidyl peptidase-4 inhibitors). Sasaoka et al.20) reported RORs for the association of hand–foot syndrome with anticancer drugs. However, there has been little comprehensive ROR analysis of ADRs for elderly patients based on JADER.
In this study, we performed a comprehensive analysis of the risks for ADRs in elderly patients, using the JADER database to detect developing drugs that pose a high risk for the elderly. RORs for every drug–ADR combination reported in JADER were assessed and compared between elderly and younger patients.
Data recorded from April 2004 to September 2015 in the JADER database were downloaded from the PMDA website (http://www.pmda.go.jp/). The database consists of four data tables: patient demographic information (demo), drug information (drug), adverse events (reac), and primary disease (hist). In “Demo” file, the demographic information table stored age in 10-year intervals, such as 60–69 years. In this study, we defined “younger patients” to be those assigned to “Under 10s,” “10s,” “20s,” “30s,” “40s,” “50s,” “60s,” and “elderly patients” to be those assigned to “70s,” “80s,” “90s,” “100s.” There were other codes for age classification such as “first trimester,” “second trimester,” “third trimester,” “newborn,” “infant,” “pediatric,” “youth,” “adult,” “elderly,” “unknown,” and the data from those classes were excluded from the analysis because the actual age ranges were unclear. We could not separate the patients’ age groups at 65 years old, although it is a general definition, because of the limitation of the available data. The patients’ data of 65–69 years old were included in the younger group because our objective was to find the possible risks of drug–ADR combinations in elderly patients and we thought it conservative to exclude the data of 60s from the elderly group as our objective was to find possible risks of drug–ADR combinations in elderly patients.
Adverse events in “Reac” file are coded according to the terminology in the Medical Dictionary for Regulatory Activities (MedDRA).21) In “Drug” file, the drug information table assigned the following role codes to each drug: suspected (higiyaku in Japanese), interacting (sougosayou), or concomitant (heiyouyaku). Drug names were changed from the Japanese Accepted Name to the United States Adopted Name based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) drug database (http://www.genome.jp/kegg/).22) Any adverse events and drug names that were not defined in MedDRA or KEGG were translated by us. We extracted all suspected drugs and ADRs for the elderly and younger patient groups. RORs were calculated with 95% confidence intervals (CIs) from the following equations, with a, b, c, and d defined by the 2×2 table as; a: number of cases which occurred interested AE after using the suspected drug(s), b: number of cases which occurred all other AEs after using the suspected drug(s), c: number of cases which occurred interested AE after using all other drugs, d: number of cases which occurred all other AEs after using all other drugs.
 | (1) |
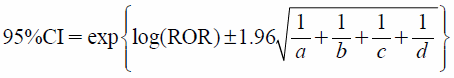 | (2) |
RORs were calculated for the cases where the number of combinations of all suspected drugs and ADRs was reported more than three times in the elderly patients than in the younger patients. We also defined an index for the difference between elderly and younger (DEY) patients as the difference between the lower limit of the 95% CI in the elderly patients and the upper limit of the 95% CI in the younger patients. We focused, in particular, on the drug–ADR combinations that satisfied the following two conditions: (1) more than 100 cases were reported in the elderly patients and (2) the value of DEY was greater than 1.0. All data analyses were performed using R (version 3.23; downloaded from http://www.r-project.org).23)
In total, 361403 reports were obtained from the JADER database, 124771 for elderly patients, 214641 for younger patients, and 21991 reports that were excluded because the age classifications were unclear. The total number of suspected drugs was 3341 and of ADRs was 7301, with a total of 24392641 combinations of suspected drugs and ADRs. Of these, 21359 combinations were reported more than three times in elderly patients. There were 301168 and 589730 combinations of suspected drugs and ADRs for the elderly and younger patients, respectively.
In some cases, the names of ADRs or drugs were not provided in the Standardized MedDRA queries or the KEGG drug databases, and the names were reported in Japanese. The English expressions for these names are, human red blood cell solution, radiation exposure, hypoglycemia, transfusion-related acute lung injury, pneumocystis pneumonia, pneumatosis intestinalis and infusion-related reaction, and these data were included in the analysis.
Table 1 lists 20 combinations of suspected drugs and ADRs in decreasing order of reported cases in elderly patients. The most frequently reported combination was methotrexate with interstitial lung disease (646 cases). Other ADRs associated with methotrexate were pancytopenia (483 cases) and pneumocystis pneumonia (366 cases). Interstitial lung disease was also associated with other drugs such as gefitinib (515 cases), gemcitabine hydrochloride (414 cases), docetaxel hydrate (358 cases), erlotinib hydrochloride (323 cases), and amiodarone hydrochloride (315 cases). The ROR values are not necessarily higher in elderly patients than younger patients, suggesting that the risks for some drug–ADR combinations are not always high in elderly patients.
| Suspected drug | Adverse event | Elderly patients (≥70 years) | Younger patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | ROR | 95% CI | a | b | c | d | ROR | 95% CI | |||
| 1 | Methotrexate | Interstitial lung disease | 646 | 5159 | 12355 | 283008 | 2.9 | 2.6–3.1 | 754 | 8669 | 12427 | 567880 | 4.0 | 3.7–4.3 |
| 2 | Glimepiride | Hypoglycemia | 596 | 802 | 3200 | 296570 | 68.9 | 61.6–77 | 190 | 658 | 2144 | 586738 | 79.0 | 66.9–93.4 |
| 3 | Gefitinib | Interstitial lung disease | 515 | 849 | 12486 | 287318 | 14.0 | 12.5–15.6 | 493 | 830 | 12688 | 575719 | 27.0 | 24.1–30.2 |
| 4 | Methotrexate | Pancytopenia | 483 | 5322 | 2377 | 292986 | 11.2 | 10.1–12.4 | 289 | 9134 | 3650 | 576657 | 5.0 | 4.4–5.6 |
| 5 | Valaciclovir hydrochloride | Acute kidney injury | 459 | 2766 | 2978 | 294965 | 16.4 | 14.8–18.3 | 101 | 1345 | 4155 | 584129 | 10.6 | 8.6–13 |
| 6 | Gemcitabine hydrochloride | Interstitial lung disease | 414 | 1408 | 12587 | 286759 | 6.7 | 6–7.5 | 408 | 2297 | 12773 | 574252 | 8.0 | 7.2–8.9 |
| 7 | Oxaliplatin | Neutropenia | 398 | 2862 | 2375 | 295533 | 17.3 | 15.5–19.4 | 1132 | 5988 | 5480 | 577130 | 19.9 | 18.6–21.3 |
| 8 | Human red blood cell solution (radiation exposure) | Transfusion-related acute lung injury | 390 | 2109 | 384 | 298285 | 143.6 | 124–166.4 | 266 | 1463 | 627 | 587374 | 170.3 | 146.3–198.4 |
| 9 | Zoledronic acid hydrate | Osteonecrosis of jaw | 383 | 944 | 808 | 299033 | 150.2 | 130.9–172.3 | 488 | 1166 | 614 | 587462 | 400.4 | 350.9–457 |
| 10 | Heparin sodium | Heparin-induced thrombocytopenia | 368 | 966 | 133 | 299701 | 858.4 | 697.1–1057 | 510 | 1286 | 124 | 587810 | 1879.9 | 1533.4–2304.8 |
| 11 | Methotrexate | Pneumocystis pneumonia | 366 | 5439 | 1063 | 294300 | 18.6 | 16.5–21 | 444 | 8979 | 2108 | 578199 | 13.6 | 12.2–15.1 |
| 12 | Alteplase | Haemorrhagic cerebral infarction | 364 | 699 | 337 | 299768 | 463.2 | 392.5–546.7 | 205 | 375 | 223 | 588927 | 1443.7 | 1164.4–1790 |
| 13 | Ribavirin | Anaemia | 360 | 2235 | 3724 | 294849 | 12.8 | 11.4–14.3 | 1540 | 12545 | 7167 | 568478 | 9.7 | 9.2–10.3 |
| 14 | Docetaxel hydrate | Interstitial lung disease | 358 | 1677 | 12643 | 286490 | 4.8 | 4.3–5.4 | 367 | 2950 | 12814 | 573599 | 5.6 | 5–6.2 |
| 15 | Tegafur, Gimeracil, Oteracil Potassium | Diarrhoea | 348 | 3662 | 2512 | 294646 | 11.1 | 9.9–12.5 | 423 | 4424 | 5378 | 579505 | 10.3 | 9.3–11.4 |
| 16 | Iopamidol | Anaphylactic shock | 346 | 938 | 3561 | 296323 | 30.7 | 27–34.9 | 442 | 1340 | 8867 | 579081 | 21.5 | 19.3–24 |
| 17 | Human red blood cell solution (radiation exposure) | Blood pressure decreased | 344 | 2155 | 2560 | 296109 | 18.5 | 16.4–20.8 | 244 | 1485 | 3552 | 584449 | 27.0 | 23.5–31.1 |
| 18 | Erlotinib hydrochloride | Interstitial lung disease | 323 | 980 | 12678 | 287187 | 7.5 | 6.6–8.5 | 429 | 1184 | 12752 | 575365 | 16.3 | 14.6–18.3 |
| 19 | Alendronate sodium hydrate | Osteonecrosis of jaw | 319 | 1281 | 872 | 298696 | 85.3 | 74.2–98.1 | 118 | 748 | 984 | 587880 | 94.2 | 76.9–115.6 |
| 20 | Amiodarone hydrochloride | Interstitial lung disease | 315 | 590 | 12686 | 287577 | 12.1 | 10.5–13.9 | 188 | 697 | 12993 | 575852 | 12.0 | 10.2–14.1 |
* These combinations are listed in the order of the reported values for a for the elderly patients (a, b, c, and d are defined in text). CI, confidence interval; ROR: reporting odds ratio.
Total number of drug–ADR combinations with DEY greater than 1.0 was 341, and the combinations reported more than 50, 100 or 200 cases in elderly patients were 53, 27, and 9, respectively. Table 2 lists the 27 combinations of suspected drugs and ADRs reported in more than 100 cases in elderly patients for which the DEY index was greater than 1.0 in the descending order of the cases, indicating a higher risk of the ADRs in elderly patients compared with younger patients for these combinations. The combination with the highest DEY was methotrexate with lymphoproliferative disorder (ROR: 484.6, 95% CI: 334.1–702.9, DEY: 158.0). Methotrexate also showed high DEY values with some other ADRs, as shown in Table 2.
| Suspected drug | Adverse event | Elderly patients (≥70 years) | Younger patients | DEY | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | ROR | 95% CI | a | b | c | d | ROR | 95% CI | ||||
| 1 | Methotrexate | Pancytopenia | 483 | 5322 | 2377 | 292986 | 11.2 | 10.1–12.4 | 289 | 9134 | 3650 | 576657 | 5.0 | 4.4–5.6 | 4.5 |
| 2 | Valaciclovir hydrochloride | Acute kidney injury | 459 | 2766 | 2978 | 294965 | 16.4 | 14.8–18.3 | 101 | 1345 | 4155 | 584129 | 10.6 | 8.6–13.0 | 1.8 |
| 3 | Methotrexate | Pneumocystis pneumonia | 366 | 5439 | 1063 | 294300 | 18.6 | 16.5–21.0 | 444 | 8979 | 2108 | 578199 | 13.6 | 12.2–15.1 | 1.4 |
| 4 | Ribavirin | Anaemia | 360 | 2235 | 3724 | 294849 | 12.8 | 11.4–14.3 | 1540 | 12545 | 7167 | 568478 | 9.7 | 9.2–10.3 | 1.0 |
| 5 | Iopamidol | Anaphylactic shock | 346 | 938 | 3561 | 296323 | 30.7 | 27–34.9 | 442 | 1340 | 8867 | 579081 | 21.5 | 19.3–24 | 3.0 |
| 6 | Methotrexate | Lymph proliferative disorder | 281 | 5524 | 31 | 295332 | 484.6 | 334.1–702.9 | 341 | 9082 | 150 | 580157 | 145.2 | 119.7–176.2 | 158.0 |
| 7 | Prednisolone | Pneumocystis pneumonia | 257 | 4931 | 1172 | 294808 | 13.1 | 11.4–15.0 | 391 | 11437 | 2161 | 575741 | 9.1 | 8.2–10.2 | 1.3 |
| 8 | Ribavirin | Haemoglobin decreased | 251 | 2344 | 1768 | 296805 | 18.0 | 15.7–20.6 | 1123 | 12962 | 4115 | 571530 | 12.0 | 11.2–12.9 | 2.8 |
| 9 | Methotrexate | Bone marrow failure | 241 | 5564 | 1092 | 294271 | 11.7 | 10.1–13.5 | 216 | 9207 | 2612 | 577695 | 5.2 | 4.5–6.0 | 4.2 |
| 10 | Irinotecan hydrochloride hydrate | Leukopenia | 187 | 2746 | 1478 | 296757 | 13.7 | 11.7–16.0 | 313 | 4582 | 4200 | 580635 | 9.4 | 8.4–10.6 | 1.1 |
| 11 | Allopurinol | Drug reaction with eosinophilia and systemic symptoms | 185 | 2192 | 716 | 298075 | 35.1 | 29.7–41.5 | 229 | 2281 | 3985 | 583235 | 14.7 | 12.8–16.9 | 12.8 |
| 12 | Valaciclovir hydrochloride | Encephalopathy | 179 | 3046 | 171 | 297772 | 102.3 | 82.7–126.6 | 79 | 1367 | 832 | 587452 | 40.8 | 32.2–51.7 | 31.0 |
| 13 | Allopurinol | Stevens-Johnson syndrome | 158 | 2219 | 1946 | 296845 | 10.9 | 9.2–12.8 | 137 | 2373 | 5452 | 581768 | 6.2 | 5.2–7.3 | 1.9 |
| 14 | Carbamazepine | Drug reaction with eosinophilia and systemic symptoms | 146 | 1487 | 755 | 298780 | 38.9 | 32.3–46.7 | 780 | 5314 | 3434 | 580202 | 24.8 | 22.8–26.9 | 5.4 |
| 15 | Methotrexate | Lymphoma | 137 | 5668 | 163 | 295200 | 43.8 | 34.8–55.0 | 234 | 9189 | 520 | 579787 | 28.4 | 24.3–33.2 | 1.7 |
| 16 | Cetuximab | Infusion related reaction | 127 | 871 | 118 | 300052 | 370.8 | 286.1–480.5 | 147 | 1256 | 396 | 587931 | 173.8 | 142.7–211.6 | 74.4 |
| 17 | Infliximab | Pneumocystis pneumonia | 127 | 969 | 1302 | 298770 | 30.1 | 24.8–36.5 | 196 | 3504 | 2356 | 583674 | 13.9 | 11.9–16.1 | 8.7 |
| 18 | Levofloxacin hydrate | Hypoglycemia | 126 | 1455 | 3670 | 295917 | 7.0 | 5.8–8.4 | 25 | 2512 | 2309 | 584884 | 2.5 | 1.7–3.7 | 2.1 |
| 19 | Peginterferon Alfa-2a (Genetical Recombination) | Anaemia | 118 | 986 | 3966 | 296098 | 8.9 | 7.4–10.8 | 240 | 3371 | 8467 | 577652 | 4.9 | 4.3–5.5 | 1.8 |
| 20 | Telaprevir | Haemoglobin decreased | 117 | 570 | 1902 | 298579 | 32.2 | 26.3–39.5 | 777 | 5435 | 4461 | 579057 | 18.6 | 17.1–20.1 | 6.2 |
| 21 | Voglibose | Pneumatosis intestinalis | 114 | 470 | 186 | 300398 | 391.7 | 305.1–503 | 28 | 490 | 251 | 588961 | 134.1 | 89.8–200.1 | 104.9 |
| 22 | Imatinib mesilate | Pleural effusion | 113 | 2112 | 778 | 298165 | 20.5 | 16.8–25.1 | 71 | 3551 | 1119 | 584989 | 10.5 | 8.2–13.3 | 3.4 |
| 23 | Methotrexate | Diffuse large B-cell lymphoma | 111 | 5694 | 62 | 295301 | 92.8 | 68–126.8 | 171 | 9252 | 233 | 580074 | 46 | 37.7–56.1 | 11.9 |
| 24 | Celecoxib | Erythema multiforme | 111 | 1480 | 1336 | 298241 | 16.7 | 13.7–20.5 | 96 | 1191 | 4583 | 583860 | 10.3 | 8.3–12.7 | 1.0 |
| 25 | Human red blood cell solution (radiation exposure) | Pyrexia | 106 | 1666 | 3468 | 295928 | 5.4 | 4.4–6.6 | 115 | 2859 | 9949 | 576807 | 2.3 | 1.9–2.8 | 1.6 |
| 26 | Paroxetine hydrochloride hydrate | Inappropriate antidiuretic hormone secretion | 101 | 1210 | 649 | 299208 | 38.5 | 31–47.8 | 45 | 3247 | 685 | 585753 | 11.9 | 8.7–16.1 | 14.9 |
| 27 | Tacrolimus hydrate | Pneumonia | 101 | 1287 | 4447 | 295333 | 5.2 | 4.2–6.4 | 227 | 8671 | 6353 | 574479 | 2.4 | 2.1–2.7 | 1.5 |
* These combinations were those reported more than 100 times in elderly patients, with a difference greater than 1.0 between the lower limit of the reporting odds ratio (ROR) 95% confidence interval (CI) for the elderly patients and the upper limit of the ROR 95% CI for the younger.
In this study, we analyzed the risks of ADRs using the JADER database, focusing especially on risks for elderly patients. We found 27 combinations of drugs and ADRs with a higher risk for elderly patients over 70 years old than for younger patients, of which the greatest difference in risk was found in the combination of methotrexate and lymphoproliferative disorder. This association has previously been reported as a methotrexate associated lymphoproliferative (MTX-associated LPD).24) Although, the mechanism and possible risk factors of MTX-associated LPD remain unclear, some studies have reported its occurrence in elderly patients.25,26) Further risks with higher RORs associated with methotrexate included diffuse large B-cell lymphoma (111 cases, ROR: 92.8, 95% CI: 68.0–126.8, DEY: 11.9), pancytopenia (483 cases, ROR: 11.2, 95% CI: 10.1–12.4, DEY: 4.5), bone marrow failure (241 cases, ROR: 11.7, 95% CI: 10.1–13.5, DEY: 4.2), lymphoma (137 cases, ROR: 43.8, 95% CI: 34.8–55.0, DEY: 1.7), and pneumocystis pneumonia (366 cases, ROR: 18.6, 95% CI: 16.5–21.0, DEY: 1.4). Methotrexate is a drug that requires careful administration for elderly patients. In total, 3951 reports contained methotrexate as a suspected drug. In most cases, the methotrexate was used to treat rheumatoid arthritis (3387 cases).
Pneumocystis pneumonia was reported as an ADR associated with suspected drugs such as infliximab (127 cases, ROR: 30.1, 95% CI: 24.8–36.5, DEY: 8.7), methotrexate (366 cases, ROR: 18.6, 95% CI: 16.5–21.0, DEY: 1.4), and prednisolone (257 cases, ROR: 13.1, 95% CI: 11.4–15.0, DEY: 1.3). Pneumocystis pneumonia is a type of opportunistic infection resulting from a weakened immune system. In general, elderly patients have defects in T-cell immunity and thus an increased risk of opportunistic infection,27) and care should be taken when using these drugs for elderly patients.
Recently, various analytical methods have been applied to SRS database analyses.28–30) For example, Fujiwara et al.28) used association analysis to report ADRs for antidepressant drugs such as selective serotonin reuptake inhibitors (SSRI), serotonin–noradrenaline reuptake inhibitors (SNRI) and noradrenergic and specific serotonergic antidepressants (NaSSA) using the association analysis. Narushima et al.29) used a mixed effects logistic model to report ADRs for DPP-4 inhibitors and GLP-1 receptor agonists using a mixed effects logistic model. These analyses may be more sophisticated for data mining; however, in the present study, we used ROR analysis because the objective was a comprehensive evaluation of the risks for all possible drug–ADR combinations. We considered a simpler analysis better for performing the analysis and for understanding the results to meet this objective.
In this study, we have presented a list of drugs posing a high risk for ADRs in elderly patients, but further epidemiological studies are needed to verify the real risk for these ADRs. A further limitation is that the SRS for the JADER database has various biases, such as the lack of a denominator that indicates the total number of patients who received the drugs of interest, as well as missing data and confounding factors.31) Moreover, the ROR does not provide a robust indication of signal strength.15) Despite these limitations, signal analysis using SRS is useful for gaining early and comprehensive information about ADRs.
In conclusion, we undertook a comprehensive assessment of the risk of ADRs in the elderly using the JADER database, and our results have provided a list of possible high-risk combinations of drugs and ADRs. These findings can be used to update information used for prescriptions for elderly patients.
The authors declare no conflict of interest.