2017 Volume 40 Issue 8 Pages 1199-1206
2017 Volume 40 Issue 8 Pages 1199-1206
The aim of the work was the evaluation of selected biological and physicochemical, applicative properties of ethylated ascorbic acid (AAE) compared to ascorbic acid (AA). Thermogravimetry (TG), differential thermogravimetry (DTG), and differential thermal analysis (DTA) were conducted, followed by the evaluation of AAE decomposition by the UV-Vis spectroscopic method including the influence of temperature and pH. Scavenging, antimicrobial activity and cytotoxicity against L929 fibroblasts were also performed. The difference in mass loss between AA and AAE was 30% via TG. DTA revealed characteristic exothermic and endothermic effects. The AAE solution was more thermally stable than AA. The calculated zero-order rate constants of free-radical scavenging kinetics for AAE were in the range of 4.9×10−3–1.35×10−2 s−1. The activation energy for the process was 11,2281 kJ/mol. AAE was active against Staphylococcus (S.) aureus and Enterococcus (E.) faecalis and acted stronger against Candida (C.) albicans than AA. The concentrations of AA ≥2.5% were cytotoxic, whereas in the case of AAE, a 10% concentration was considered cytotoxic. DTG enables the detailed differentiation between AA and AAE. AAE in aqueous solution is more stable compared to AA. The antioxidant activity of AAE is significant. However, the reaction with 2,2-diphenyl-1-picrylhydrazyl (DPPH) indicates prolonged activity compared to AA. Variability in the antimicrobial activity of AAE may find practical application in the pharmaceutical and cosmetic industries. The potential for applicative aims may be supported by the relatively low in vitro toxicity of AAE.
Ascorbic acid (AA) plays an important role as a co-factor for several enzymes. It is active in various processes, such as the post-translational hydroxylation of collagen, carnitine biosynthesis, conversion of dopamine to norepinephrine, peptide amidation, and the metabolism of tyrosine. AA regulates gastrointestinal uptake of iron ions and stabilizes iron-binding proteins.1,2) Dried AA may be stored for several years. However, in the aqueous environment, it is very sensitive to increased temperature, light, oxygen, and pH changes, and it is easily decomposed, losing its biological properties. The dissociation rate exponent of AA is approximately 4.04. The oxidation process involves the elimination of two protons at pH 1–4 and one proton at pH values over 5. Up to 8.4, the dissociation rate remains almost constant.3,4) The composition of the product may influence AA oxidation as a result of limited exposure to atmospheric oxygen.5) Various authors have supported and evaluated the idea of antimicrobial activity of AA.6,7) Some researchers reported the beneficial activity of high doses of ascorbic acid in Escherichia coli endotoxin-induced hyporeactivity to acetylcholine in the human forearm,8) which may be connected to the effect of Escherichia coli endotoxin on AA transport in cells.9) AA is widely used in cosmetics because of its powerful antioxidant activity and ability to scavenge free radicals, linking it to prevention of the aging process. The wider use of AA is hindered mainly by its low stability. Among the new derivatives of AA with higher stability and lower tendency to degrade, there is an ethylated derivative of AA (AAE), and its structure, along with the AA structure, is presented in Fig. 1.
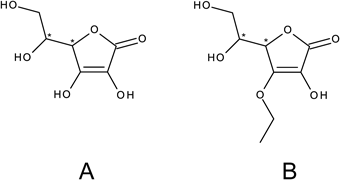
Based on the available literature, the lipophilic and hydrophilic groups of AAE present a stable structure10) and possess interesting biological properties, including an influence on skin aging, and may be considered for application in dermatological products.11) The aim of this study was to compare selected physical and chemical properties, including antioxidant properties, and to probe the cytotoxicity of AA and its derivative AAE.
The following reagents were used in the present study: ascorbic acid (AA, Pharma Cosmetic, Kraków, Poland) conforming to the European Pharmacopoeia, the AA derivative ethyl ascorbic acid (AAE, Protec Ingredia, Warsaw, Poland), the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH, Sigma-Aldrich, Poznan, Poland), and ethyl alcohol (Polmos, Warsaw, Poland). The pH of the solution was stabilized using Na2HPO4–citric×H2O buffer (pH range 3–8, Chempur, Piekary Śląskie, Poland) and phosphoric buffer (pH 6.7, POCH, Gliwice, Poland). The bacterial strains Staphylococcus (S.) aureus ATCC 6538, Pseudomonas (P.) aeruginosa ATCC 15442, Candida (C.) albicans ATCC 10231, and Enterococcus (E.) faecalis ATCC 29212 were used for evaluation of the antimicrobial activity of AA and AAE. Murine immortalized L929 fibroblasts (ATCC CCL-1) were used for assessment of the cytotoxicity of AA and AAE.
The thermobalance (TG 209 F1 Libra, Netzsch, Selb, Germany) with an automatic sample changer (ASC, Netzsch, Selb, Germany) was applied. Micronized in mortar samples of 5.85 mg AA and 5.82 mg AAE were assessed in an inert nitrogen atmosphere, with flow of 25 mL of N2 per min. The heating rate of 20 K per min was applied in this research. Standard thermogravimetric (TG) plots, differential thermogravimetry (DTG) plots, and differential thermal analysis (DTA) plots were generated for further interpretation. The applied reference substance was Al2O3.
Thermal Stability of Solutions of AA and AAE at Increasing TemperaturesThe stability and antioxidant properties of AA and AAE solutions were determined over time at increasing temperatures between 25 and 55°C in UV-Vis spectrometer (Halo DB-20S Dynamica, Newport Pagnell, United Kingdom) and recorded on a connected PC. The decomposition kinetics of AA and AAE in water were measured at an initial solution concentration of 1.17×10−4 mol·L−1. The maxima of absorbance were determined using the recorded spectra. The absorbance was recorded for 11 min, from the start of the reaction and at every two seconds, both for the AA solution and AAE solution.
The Influence of pH on the Stability of AAEThe effect of pH was assessed only for the AAE, as there is available bibliography dealing with the influence of pH on AA decomposition.12) The stability of the substance at pH values between 3-8 was evaluated via measurement of the absorbance of the solution immediately after preparation and after 24 h. The measurements were carried out in five repetitions.
Antioxidant Properties via IC50The antioxidant properties were evaluated via determination of the IC50 value. Sets of test tubes with DPPH solution (3.99×10−5 mol·L−1), AA with DPPH solution (3.77×10−5–4.04×10−6 mol·L−1 of AA and 3.99×10−5 mol·L−1 of DPPH), and AAE with DPPH solution (1.26×10−4 to 5.04×10−5 mol·L−1 of AAE and 3.99×10−5 mol·L−1 of DPPH) were incubated for 10 min directly after preparation. The absorbance at the initial stage and after incubation was measured at 517 nm. The DPPH free radical scavenging activity was calculated according to the formula:
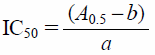 | (1) |
The method was based on the spectrophotometric evaluation of the reaction of DPPH radicals with AA or AAE as in the available literature.13–15) The measurements were performed in quartz cuvettes with a path length of 1.0 cm at 25–55°C in a total volume of 3.0 mL. Increasing quantities (30, 40, 50, 60, 70 or 80 µL) of freshly prepared aqueous solutions of AA or AAE at a concentration of 2×10−2 mol×dm−3 were added to 3.0 mL of a freshly prepared ethanolic solution of DPPH at a concentration of 8×10−5 mol×dm−3, and the absorption of DPPH was measured at 517 nm. The relative concentration of DPPH, expressed in mol×l−1 (CDPPH), was calculated from the respective calibration curve, and it was found to be CDPPH=6.9802×10−5×ADPPH (r2=0.9948), where A is the absorbance of the DPPH solution at wavelength λ=517 nm. The first-order rate constant (k1) and the second-order rate constant (k2) were determined with the antioxidant concentration [AN] in large excess as compared with the radical concentration [FR] following the equation:
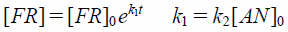 | (2) |
The activation energy was calculated on the basis of the Arrhenius equation, where k is the rate constant for the reaction, z is a proportionality constant, Ea is the activation energy for the reaction, R is the ideal gas constant, and T is the temperature:
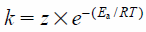 | (3) |
A total of 100 µL of the microbial solutions with a density of 1×105 cfu×mL−1 was placed into wells of a test plate, together with the respective evaluated entity AA or AAE in 12 concentrations between 0.15% and 10.00 mg×mL−1. The absorbance of the solutions was measured immediately using specialized spectrophotometer (Thermo Scientific Multiskan GO, Waltham, U.S.A.) at 580 nm. The plate was incubated for 24 h at 37°C in a shaker, and the absorbance measurement was repeated. All experiments were completed in triplicate. The control samples were solutions of AA or AAE, solutions of microbes, sterile medium, and sterile medium with AA or AAE. The relative number of microbial cells (RNM) was calculated on the basis of the equation:
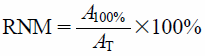 | (4) |
A total of 100 µL of 106 cells per 1 mL of L929 fibroblasts was cultured in wells of the test plate and incubated in minimal essential medium (MEM) for 24 h at 37°C in 5% CO2. After MEM was removed, AA and AAE solutions were introduced for 24 h of contact time and followed by replacement with fresh MEM. A cytotoxicity assay was performed using 0.2 mL of 0.5 mg×mL−1 (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT); thiazolyl blue) solution. The plate was incubated for 3 h, and 0.1 mL of dimethyl sulfoxide (DMSO) was introduced into the wells. The absorbance was measured using a wavelength of 530 nm. L9292 cells incubated in medium were used as a control. The RNM was calculated using the equation above. The concentration of AA or AAE that led to a decrease in the cell number of greater than 25% was considered cytotoxic. All experiments were repeated 6 times.
In this study, samples of AA and AAE powders were assessed using dynamic thermogravimetry, and respective thermogravimetric (TG) plots were obtained. They are shown in Fig. 2. Shown in the recorded TG curves, dry solid AA and its derivative AAE are thermal stable, and their decomposition started at 185.8°C for AA and at 162.2°C for AAE.
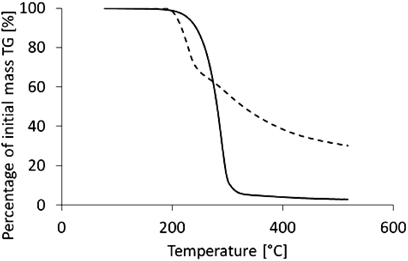
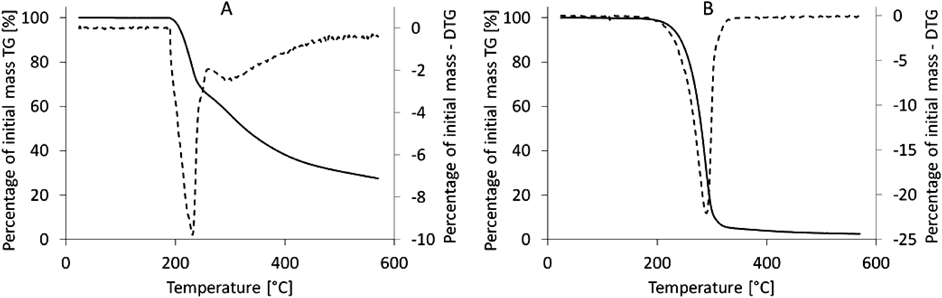
As a result of the differential thermogravimetric analysis (DTG), plots of the first derivative of mass loss vs. time were obtained, as shown in Fig. 3A for AA and Fig. 3B for AAE. The total mass loss of the sample is represented by the peak area in these plots. For the AA sample, the weight loss was found to be 68.35%, and for AAE, it was 97.02%.
Figures 4A and B present the TG-DTA plots. In the case of the AAE TG-DTA plot, the first peak was endothermic and observed at 146.6°C. During melting, which started at 185.8°C, a carbonaceous residue was formed, and on the TG-DTA plot, an endothermic peak at 192.0°C was observed. The results indicate that AA began to decompose. The TG-DTG plot showed an initial mass loss upon melting at 185.8°C. At the much higher temperature of 240.1°C, an exothermic peak occurred.
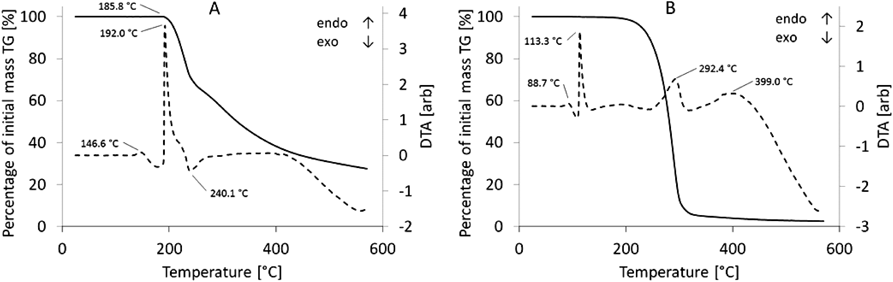
In the case of AAE, four endothermic peaks at temperatures of 88.7, 113.3, 292.4 and 399.0°C were observed, whereas two exothermic peaks were present at temperatures of 134.1 and 317.8°C.
Stability of AA and AAE in Aqueous SolutionsThe results of the spectrophotometric measurements of AA solutions performed at 25, 35, 45 and 55°C are shown in Figs. 5A–C. According to Fig. 5A, the maximum absorbance, assessed at a fixed wavelength, decreased with time. The decrease was very fast in the case of the AA solution. Initially, the absorbance decreased rapidly, while after a longer time, it entered into the plateau phase. The plateau phase increased at 25°C after approximately 3 h, whereas in the case of 55°C, the plateau phase was observed after less than 20 min.
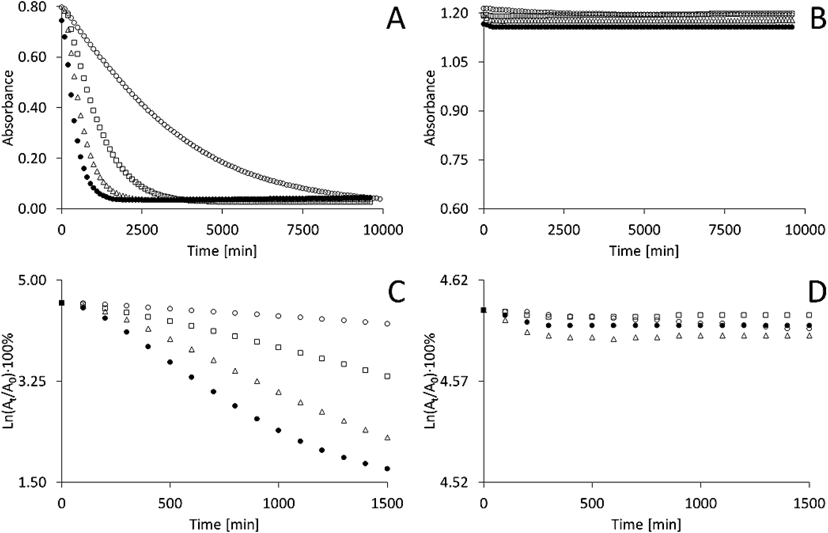
For A–C: 25°C—○, 35°C—□, 45°C—△, and 55°C—●.
The results fit to first-order kinetics and are presented in Fig. 5C. The calculated values of the decomposition rate constants are 2.6×10−4 (±2.5×10−6) s−1 at 25°C, 9.2×10−4 (±1.5×10−5) s−1 at 35°C, 1.6×10−3 (±3.0×10−5) s−1 at 45°C, and 1.9×10−3 (±9.5×10−5) s−1 at 55°C. The slopes of the straight lines reflect the variability of the degradation process, which increases with increased temperature. According to Figs. 5B and D, AAE was stable over time, through 3 h, at 25–55°C in the aqueous solution, and thus 3-O-methyl ascorbic acid exhibits a substantially greater thermal stability, stability to light than AAs.
Effect of pH on the Stability of AAEThe influence of pH on the AAE solution absorbance assessed in various buffers is shown in Fig. 6.
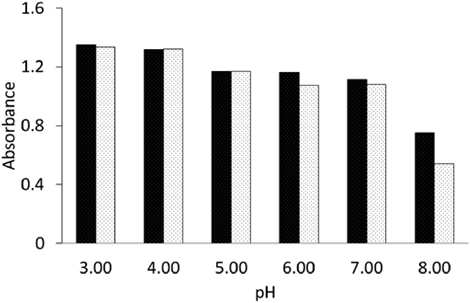
The results obtained in the first and second series of evaluations were compared using Student’s t-test for two groups. Taking into account the accuracy of the spectrophotometric device (0.002), the data indicate a statistically significant absorbance decrease at pH values 6, 7 and 8, with errors of 2×10−8, 2×10−7, and 4×10−10, respectively.
IC50 of AA and AAEThe respective IC50 values are presented in Fig. 7.
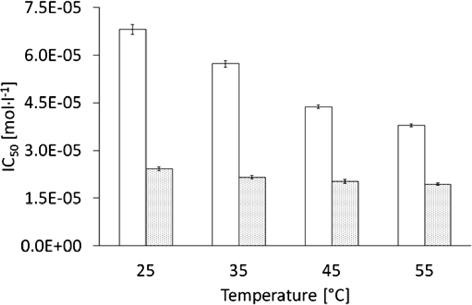
The aqueous solution of AAE at 25°C showed a prominent IC50 value of 6.805×10−5 mol×L−1, which was approximately three times higher than AA, with an IC50 value of 2.421×10−5 mol×L−1. The error bars indicate the standard deviations, which were between 4.49×10−7 and 1.59×10−6 in the case of AAE. For the case of AA the standard deviations were between 3.85×10−7 and 6.18×10−7.
Kinetics of Free Radical Scavenging CapabilityKinetic studies of DPPH scavenging in the presence of AAE were performed at various temperatures between 25 and 55°C. The reaction of AA proceeded very rapidly, and it was not possible to observe the time dependence of the decrease in the amount of radical DPPH. The calculated rate constants for AAE are presented in Table 1. According to the Arrhenius equation, the activation energy, 11 kJ×mol−1, of the reaction was calculated.
| T [°C] | VAAE [µL] | k1 [s−1] | S.D. | r2 | S.D. | k2 [mol−1·s−1] | S.D. | r2 | S.D. | Ea [kJ·mol−1] |
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 30 | 4.90·10−3 | 1.40·10−4 | 0.9914 | 9.40·10−3 | 3.4330 | 0.1029 | 0.9000 | 0.0005 | 11.2281 |
| 40 | 6.30·10−3 | 3.50·10−4 | 0.9676 | 2.33·10−2 | ||||||
| 50 | 7.00·10−3 | 3.20·10−4 | 0.9781 | 2.14·10−2 | ||||||
| 60 | 7.50·10−3 | 2.00·10−4 | 0.9920 | 1.36·10−2 | ||||||
| 70 | 9.10·10−3 | 3.00·10−4 | 0.9880 | 2.04·10−2 | ||||||
| 80 | 9.60·10−3 | 3.50·10−4 | 0.9877 | 2.38·10−2 | ||||||
| 35 | 30 | 5.50·10−3 | 1.60·10−4 | 0.9920 | 1.04·10−2 | 22.0250 | 0.9945 | 0.7504 | 0.0009 | |
| 40 | 7.00·10−3 | 2.40·10−4 | 0.9891 | 1.54·10−2 | ||||||
| 50 | 7.40·10−3 | 3.10·10−4 | 0.9839 | 1.98·10−2 | ||||||
| 60 | 9.00·10−3 | 4.70·10−4 | 0.9762 | 2.94·10−2 | ||||||
| 70 | 9.60·10−3 | 4.80·10−4 | 0.9782 | 3.03·10−2 | ||||||
| 80 | 1.04·10−2 | 4.90·10−4 | 0.9877 | 3.09·10−2 | ||||||
| 45 | 30 | 7.10·10−3 | 2.50·10−3 | 0.9891 | 1.57·10−2 | 28.6980 | 1.4331 | 0.9877 | 0.0013 | |
| 30 | 9.00·10−3 | 2.10·10−4 | 0.9324 | 2.00·10−2 | ||||||
| 40 | 1.07·10−2 | 5.00·10−4 | 0.9808 | 3.15·10−2 | ||||||
| 50 | 1.13·10−2 | 5.90·10−4 | 0.9815 | 2.73·10−2 | ||||||
| 60 | 1.25·10−2 | 3.00·10−4 | 0.9053 | 5.34·10−2 | ||||||
| 70 | 1.33·10−2 | 8.70·10−4 | 0.9713 | 4.02·10−2 | ||||||
| 80 | 8.00·10−3 | 3.30·10−4 | 0.9850 | 2.06·10−2 | ||||||
| 55 | 30 | 8.50·10−3 | 3.20·10−4 | 0.9162 | 3.12·10−2 | 28.9260 | 1.5570 | 0.9857 | 0.0014 | |
| 40 | 8.70·10−2 | 3.70·10−4 | 0.9854 | 2.04·10−2 | ||||||
| 50 | 1.25·10−3 | 9.60·10−4 | 0.9705 | 6.06·10−2 | ||||||
| 60 | 1.30·10−2 | 5.00·10−4 | 0.8934 | 2.85·10−2 | ||||||
| 70 | 1.35·10−2 | 6.00·10−4 | 0.9495 | 2.32·10−2 | ||||||
| 80 | 4.90·10−3 | 1.40·10−4 | 0.9914 | 9.40·10−3 |
T—temperature, VAAE—volume of applied solution of AAE, S.D.—standard deviation, r2—Pearson’s coefficient.
The results obtained in this study show that AA inhibits the growth of the tested microorganisms. AA was the most efficient against S. aureus. Application of the low tested concentrations of AA, namely 0.15 and 0.31%, correlated with growth decreases of 90%, whereas the higher concentrations diminished the number of bacteria by 95%. Similar results were obtained in the case of E. faecalis. Concentrations between 0.15 and 0.31% diminished the number of cells by 84%, whereas higher concentrations did so up to 91%. In case of one of the leading opportunistic pathogens, P. aeruginosa, application of AA concentrations below 0.31% was non-bacteriostatic. However, the 0.31% concentration was a breakpoint, and it corresponded with an 86% decrease in growth. The higher concentrations of AA, 0.625–10%, led to a 95% decrease of P. aeruginosa growth. AA caused relatively weak bacteriostatic activity against C. albicans, and the 0.16% concentration led to only a 20% decrease in growth. The concentration in the range of 0.3125–0.625% resulted in a 45–50% decrease in growth. Even at the higher concentrations between 1.25 and 10%, up to 25% of the microorganisms were able to survive.
AAE was weaker than AA with regard to S. aureus and E. faecalis. In the case of S. aureus, AAE concentrations of 7.5–10% displayed an effect that was similar to AA. The difference between number of cells observed in the medium without AA or AAE and number of cells in the solution with AA or AAE may be presented as percentage of growth inhibition. Surprisingly, AAE acted stronger against C. albicans than AA and inhibited its growth at a level of 35–69%-text in bold. For P. aeruginosa, a 1.25% concentration of AAE was needed to strongly inhibit the growth of this bacterium by 76%. A 2.5% concentration of AAE caused a 70% decrease in the bacterial cell number, whereas 5–10% concentrations impeded growth by 90% (Table 2).
| AA or AAE concentration | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CS | 0.15625 | 0.3125 | 0.625 | 1.25 | 2.5 | 3.0 | 3.5 | 4.0 | 4.5 | 5.0 | 7.5 | 10.0 | |
| Strain | Staphylococcus aureus ATCC 6538 | ||||||||||||
| RNM (AA) | 100.00 | 9.42 | 10.65 | 5.58 | 5.03 | 4.94 | 4.82 | 4.97 | 5.10 | 5.06 | 5.07 | 5.71 | 4.86 |
| RNM (AAE) | 100.00 | 86.57 | 70.22 | 59.87 | 47.00 | 34.97 | 24.90 | 21.85 | 18.73 | 15.45 | 13.48 | 5.70 | 5.80 |
| Strain | Enterococcus faecalis ATCC 29212 | ||||||||||||
| RNM (AA) | 100.00 | 15.82 | 15.58 | 8.97 | 8.34 | 8.17 | 8.18 | 8.40 | 8.25 | 8.31 | 8.45 | 8.60 | 8.75 |
| RNM (AAE) | 100.00 | 65.13 | 76.89 | 70.37 | 45.22 | 49.34 | 34.11 | 17.77 | 22.91 | 14.74 | 11.24 | 9.27 | 10.21 |
| Strain | Candida albicans ATCC 10231 | ||||||||||||
| RNM (AA) | 100.00 | 78.11 | 47.83 | 44.13 | 25.67 | 27.44 | 24.42 | 23.77 | 24.62 | 24.12 | 24.10 | 25.66 | 26.24 |
| RNM (AAE) | 100.00 | 50.31 | 38.81 | 52.49 | 52.75 | 69.03 | 51.94 | 35.90 | 35.08 | 35.39 | 58.21 | 50.76 | 37.95 |
| Strain | Pseudomonas aeruginosa ATCC 15442 | ||||||||||||
| RNM (AA) | 100.00 | 102.84 | 13.97 | 5.87 | 5.45 | 5.39 | 5.64 | 5.61 | 5.66 | 6.05 | 5.37 | 5.42 | 5.15 |
| RNM (AAE) | 100.00 | 97.04 | 96.31 | 96.09 | 76.40 | 30.96 | 7.49 | 7.07 | 5.93 | 5.87 | 6.67 | 6.09 | 6.61 |
Interestingly, as shown in Fig. 8, AA was more cytotoxic to fibroblasts than AAE. The ≥2.5% concentrations of AA were cytotoxic, whereas in the case of AAE, a 10% concentration could be considered cytotoxic. An interesting phenomenon was observed for AAE. The use of 0.25–2.5% concentrations led to an increase in the fibroblast number, as compared to the control samples K (>100%). In the case of the 0.5% concentration, this result was statistically significant (Kruskal–Wallis test, p<0.05) (Fig. 8).
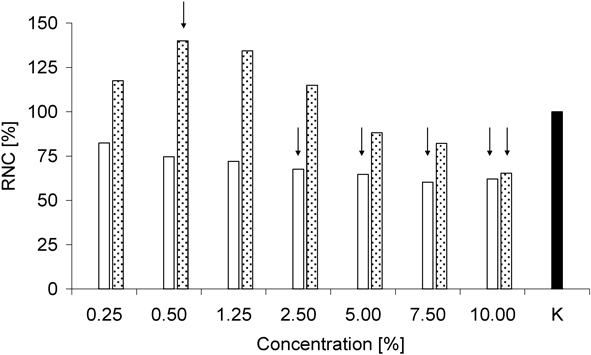
 ) against L929 Fibroblasts
) against L929 FibroblastsThe concentrations with significant influence are marked with black arrows. The Kruskal–Wallis statistical analysis revealed that the presence of AAE at 0.5% led to a statistically significant increase in viable L929 cells as compared to the control sample, i.e., L9292 cells incubated in medium without AA or AAE (black column, K).
Thermogravimetric study enables the recording of changes in the mass of the test substance depending on a temperature increase during phase transitions, such as evaporation and sublimation, as well as combustion and oxidation. We also performed DTG in parallel to improve the readability of the TG assay. According to the TG and DTG data, there is an almost 30% difference in mass loss between AA and AAE. The DTA plots enabled a more detailed insight into the chemical processes in evaluated samples because of temperature increases and revealed that there are characteristic peaks varying between AA and AAE. Observed endothermic effects may be associated with dehydration/water evaporation and dehydroxylation because of the presence of OH groups. The exothermic effects may be ascribed to the combustion or oxidation of organic entities.
The absorbance value of the solution of AAE measured at a wavelength of 244 nm at increasing temperatures between 25 and 55°C was almost the same over 3 h, and this implies that AAE in aqueous solution is rather stable over time. AAE exhibits substantially greater thermal stability than AA. As shown in the kinetic plots, AA degraded remarkably over time, and the temperature influenced this process. AA decomposed in water over time, and its stability decreased with an increase in temperature. According to available bibliographic data, at pH range 1.0–4.4 the degradation of AA increases, whereas at pH 5.4–7.2 is reduced; than the decomposition rate remains almost constant for the further increase up-to the pH 8.8.12) Due to our research AAE seems to be stable at pH values in the range of 3.0–5.0.
The DPPH antioxidant assay is widely applied, as the DPPH free radical is stable and turns colourless in the presence of antioxidants. The antioxidant properties of both compounds were evaluated in the reaction with DPPH radical using the IC50 parameter. The results for AAE are rather diffuse, whereas in the case of AA, the results are close to each other, as the reaction of AA with the radical DPPH runs faster compared to the reaction with AAE. Due to the Fig. 7 IC50 parameter decreases with increasing temperature for both reaction, however for the AAE, which seems to be less thermosensitive, the slope is definitively low. Consequently the Fig. 7 is more demonstrative in the case of AA: the reaction with DPPH needs higher concentrations of AAE for the performance of the antioxidant acitivity. The ability of AA and AAE to neutralize free radicals was widely assessed using the decrease in absorbance of DPPH radicals at 515 nm, applying kinetic models.16,17) The increased initial concentration of AAE resulted in an increased rate constant k1. Additionally, the increase in the temperature from 25 to 55°C positively influenced the rate constant k1. The calculated rate constant k2 was stable at unique temperatures, and it increased with increasing temperature.
Several authors have already described the bacteriostatic activity of AA against Staphylococcus aureus,18,19) as well as its synergy with some antibiotics.20,21) Interesting results were obtained for Pseudomonas aeruginosa22,23) alone and in the presence of an antibiotic24) and for Candida albicans.25–27) Escherichia coli seems to be inhibited in some conditions in the presence of AA.28,29) Thus, the results obtained in the present study are in line with results obtained by the researchers mentioned above. Presently, no data explaining what was observed in this study or differences between the bacteriostatic activity of AA compared with AAE are available. At the moment, the metabolism of AA in microbial biochemical pathways was described sufficiently for Escherichia coli30,31) but not for other clinically important microorganisms. Therefore, the importance of AA ethylation remains an open question. In the case of Enterococcus faecalis, we obtained different results than Mehmeti et al., who observed the ability of this bacterium to grow in the presence of 0.22015% AA.32) The reason behind this discrepancy might be strain-specific.
The ability of AA to induce collagen expression in fibroblast cells was observed by Tiedtke et al.33) This phenomenon may be the reason for the higher absorbance observed in the cytotoxicity assay when a 0.5% concentration of AAE was applied. The overexpression of collagen forming extracellular matrix may lead to increased adherence of fibroblasts to the bottom of the well of the plate. Subsequently, a decreased level of de-attachment is observed, which occurs during washing and rinsing procedures required for the cytotoxicity assay. Therefore, further experiments are required. They should be performed to directly evaluate collagen expression in fibroblasts subjected to incubation with AAE.
Differential thermogravimetry studies enabled the detailed differentiation between AA and AAE because of the presence of characteristic peaks, resulting from respective endothermic and exothermic processes in the samples. The stability of AAE in aqueous solution is remarkably higher compared to the stability of AA, and both are temperature dependent. The stability of the aqueous solution of AAE depends on pH, and at pH values over 5, it decreases. The antioxidant activity of AAE is significant. However, the reaction with DPPH indicates rather prolonged activity compared to the antioxidant activity of AA. Interesting variability in the antimicrobial activity of AAE is an issue that should be further investigated and may have practical application in the pharmaceutical and cosmetic industries. Its future applications are supported by the relatively low in vitro toxicity of AAE.
Spectrophotometric and thermogravimetric measurements were performed in the Laboratory of Elemental Analysis and Structural Research of the Pharmaceutical Faculty of Wroclaw Medical University. AAE was obtained from Protec Ingredia, Poland. The work was supported by grant ST-847 of Wroclaw Medical University.
The authors declare no conflict of interest.