2017 Volume 40 Issue 8 Pages 1255-1259
2017 Volume 40 Issue 8 Pages 1255-1259
The free fatty acid receptor 1 (GPR40/FFAR1) is activated by polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acids (DHA). This receptor has been the focus of many studies regarding physiological functions of the central nervous system. PUFAs are essential for neuronal development and maintenance of neuronal function; thus, the decrease of PUFAs in the brain is closely related to the induction of psychiatric diseases associated with emotional disorder, such as anxiety, depression, and schizophrenia. However, details of the mechanisms remain unclear. In this study, we investigated changes of maternal and/or emotional behavior caused by a deficiency of GPR40/FFAR1 signaling. GPR40/FFAR1 deficient (FFAR1−/−) female mice exhibited impaired maternal care such as retrieving behaviors and an increased rate of neglect and infanticide when compared to wild type (WT) female mice. Furthermore, FFAR1−/− female mice showed increased time spent in the open arms in an elevated plus maze test, reduction of locomotor activity and social interaction behavior, and decreased sucrose intake, when compared to WT female mice. In conclusion, these findings suggest that PUFAs-GPR40/FFAR1 signaling might function, at least in part, as a regulatory factor of emotional and maternal behavior in mice.
The dietary intake of n-3 fatty acids such as docosahexaenoic acid (DHA) has dramatically decreased with the westernization of dietary habits and food satiation.1) Although the influence of the change of these food habits on mental health has not been considered until now, the functional relationship between n-3 fatty acids and psychiatric or neurodegenerative disease has been the focus of many recent studies.2) It has become apparent that functional properties of fatty acids are modulated by factors such as the amount of individual fatty acid intake and their distribution among organs. Particularly, it is reported that, in patients with depression, n-3 fatty acid content in the brain is decreased, and an intake of n-3 fatty acids can relieve these psychiatry symptoms.3) Epidemiological studies of healthy people, and postpartum women, indicate a negative correlation between dietary n-3 fatty acid intake and mental illness, including serious mood disorders, aggression, depression, and bipolar disorder.4) Therefore, mental illness may be closely linked to the reduction of dietary n-3 fatty acid intake.
More recently, Bondi et al. have demonstrated that n-3 fatty acid deficiency caused impaired cognitive and motivated behavior in adolescent rats.5) In addition, Harauma and Moriguchi have shown that rats experiencing a n-3 fatty acid deficiency and social isolation stress show high levels of anxiety.6) Therefore, fatty acid signaling in the central nervous system may be related to nerve activity and modulation of synapse plasticity and cognitive function. And also, Hibbeln found that fish consumption is inversely related to the prevalence of depressive illnesses and postpartum depression.7,8) A recent study reported that the risk of developing postpartum depression during pregnancy increases with a low intake of seafood.8–10) This can disrupt early mother-infant interaction, and constitutes a risk factor for early child developmental problems. However, the detailed mechanisms of these disorders are not clear.
Free fatty acid receptor-1 (FFAR1) is a G-protein-coupled receptor (also known as GPR40) activated by polyunsaturated fatty acids (PUFAs) such as DHA.11) We previously demonstrated that the activation of brain GPR40/FFAR1 exerts antinociception, mediated by the modulation of the descending pain control system,12,13) and produces anti-depression-like effects in a forced swim test.14)
In this study, we investigated whether a deficiency of GPR40/FFAR1 signaling damages instinctive behavior that is associated with maternal care and emotional behavior, using GPR40/FFAR1 deficient (FFAR1−/−) female mice.
C57BL/6J wild type (WT) (8–12 weeks old, male and female) mice were obtained from Japan SLC (Hamamatsu, Japan). WT and FFAR1−/− mice (8–12 weeks old; male and female) were kept in a pathogen free facility at 23–24°C with a 12-h light–dark cycle (lights from 8 a.m. to 8 p.m.) and food and water provided ad libitum. FFAR1−/− mice were provided by Dr. Hirasawa15) and maintained on C57BL/6J background (n=10). Exon1 of the Ffar1 was substituted as previously described,15) FFAR1−/− mice were generated by breeding heterozygous pairs. Behavioral experiments were conducted during the light phase of the cycle, and mice (except pups) were at least 8 weeks old at the time of training. WT and FFAR1−/− female mice were used in this study. The present study was conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals, adopted by the Japanese Pharmacological Society. All experiments were approved by the Ethical Committee for Animal Experimentation of Kobe Gakuin University (approval number A16–22; Kobe, Japan).
The Rate of Birth, Infanticide, and Neglect in FFAR1−/− Female MiceBirth rate was determined as the number of live births relative to the number of females paired with males for breeding. Litter size was determined by counting live pups on postpartum day 0. We counted the number of pups in the nest at the end of the experiment and checked if infanticide occurred. Maternal neglect rate was determined by examining the number of live litters on postpartum days 1–5 relative to the number of live litters born.16)
Pup-Retrieval TestWT and FFAR1−/− virgin or multiparous female mice were isolated in a cage with wood-chip bedding at least one day prior to the experiment. These mice were introduced to unfamiliar pups (C57BL/6J background) for consecutive 3 d and parental behaviors were scored on day 3 when animals exhibited the highest level of parental behavior. The experiment was performed on day 4 (10:00–13:00). C57BL6J pups (1–5 d old), born from different C57BL/6J mothers, were placed in 3 corners of the cage (220×320×135 mm). Maternal behaviors were recorded for 30 min using a video camera. We measured two behaviors as maternal care: 1) Sniffing (time of first sniff pup), 2) 1st, 2nd and 3rd retrieving (time of bring back 3 pups into the nest).16)
Elevated Plus-Maze (EPM) TestThe apparatus was consisted of two open arms and two enclosed arms (both 25 cm length ×8 cm width) extending from a center platform (8 cm length ×8 cm width) elevated 50 cm above the floor. The illumination level on center of platform was approximately 360 lx and all arms were similar. Mice behavior was recorded for 5 min using a web camera. The ratio of open arms crossing was calculated from the number of open arms crossing divided all four arms crossing, Crossings (%)=(number of entries into open arms/number of entries into four arms)×100. The ratio of open arms time was quantified from time spent in open arms divided all four arms, Time (%)=(time spent in open arms/time spent in four arms)×100.17)
Open Field TestMice behavior was recorded in the open field (45×45×35 cm) for 60 min using web camera positioned 100 cm above the center of open field. The illumination level was set approximately 6 lx. Total travel distance for experimental time and time spent in center zone (27×27 cm) were assessed using a video tracking system (ANY-maze, BrainScience idea. Co., Ltd., Tokyo, Japan). The time spent in the center zone was calculated divided the time spent in the total area.17)
Social Interaction TestSocial behaviors were measured as described previously.17) Experimental mice explored in the open field (45×45 cm) with an empty plastic box with some holes (8×8 cm) located at one end for 2.5 min in the absence of unfamiliar gender and age-matched virgin mice (no target phase). After explored, mice spent in their home cage for 1 min, and unfamiliar mice were placed in the plastic box with some holes. Experimental mice were returned to the open field with unfamiliar mice for 2.5 min (target phase). Total distance moved and the time spent in the interaction zone surrounding the plastic box was recorded during both the no target and target phase using the web camera.
Sucrose PreferenceBottles (230 mL) with stoppers fitted with ball-point sipper tubes were filled with a 1% sucrose solution or tap water. Prior to the main test, virgin female mice spent in condition of drinking from two bottles were filled with tap water for 3 d. After 3 d, either one of the two bottles was changed 1% sucrose; mice were given a free choice of 1% sucrose solution or tap water. The positions of the two bottles were alternated throughout every 10–12 h. Each liquid intake was estimated from the bottles weight. The sucrose preference was calculated as a percentage of total liquid intake and was averaged over 3 d of testing.17)
Statistical AnalysisThe survival rate curve was obtained by Kaplan–Meier method. Analysis of survival rate was used a log-rank test. The data of social interaction test was analyzed by two-way ANOVA followed by Bonferroni’s post hoc test. Other behavioral test data were analyzed using unpaired Student’s t-test (for comparisons between two groups). Using GraphPad Prism software (version 6, GraphPad Software, San Diego, CA, U.S.A.). Values of p<0.05 were regarded as significant.
The total number of pups did not significantly differ between WT and FFAR1−/− female mice (Fig. 1A). The survival rate of FFAR1−/− mouse pups was decreased significantly after birth (Fig. 1B, p<0.01). At the end of weaning, survival rate in FFAR1−/− female mice showed 60% reduction compared to WT mice (Fig. 1C). The rate of neglect and infanticide was increased in FFAR1−/− female mice.

The number of pups found in the cage per mouse (A) is shown, as well as the number of surviving pups from birth to weaning (B), and the ratio of survival, neglect and the ratio of infanticide (C). WT female mice: n=9; FFAR1−/− female mice: n=9. W; WT female mice, F; FFAR1−/− female mice.
The latency to sniff the first pup and the latency to retrieve each of the three pups (1st, 2nd, and 3rd) did not show significant differences between virgin WT and virgin FFAR1−/− mice (Figs. 2A, B, p>0.05). The latency to retrieve the first pup (1st) showed tendency of increase in virgin or multiparous FFAR1−/− mice (Fig. 2A, p>0.05). The latency to retrieve 2nd and 3rd pup in multiparous FFAR1−/− mice were significantly delayed compared to multiparous WT mice (Fig. 2B, p<0.01), but not virgin female mice.

Pup retrieval behavior of WT and FFAR1−/− female mice. The latency to sniff the first pup (A). The latency to retrieve the first, second and third pup during the retrieving experiment (B). C57BL6J pups (1–5 d old), born from different C57BL6J mothers, were placed in 3 corners of the cage and maternal behavior of the test mouse was recorded for 30 min using a video camera. *p<0.05 vs. WT postpatum female mice. WT mice: n=9; FFAR1−/− n=9. W; WT female mice, F; FFAR1−/− female mice.
In the open field test, the total travel distances of FFAR1−/− female mice were significantly decreased compared to WT female mice (Fig. 3A, p<0.05). Furthermore, the time spent in the center zone did not show a significant difference between WT and FFAR1−/− female mice (Fig. 3B, p>0.05). In the EPM, the time spent in the open arms and total crossings was significantly greater for the FFAR1−/− female mice compared to WT female mice (Fig. 3D, p<0.01; Fig. 3E, p<0.05), but not the number of crossings (Fig. 3C). Furthermore, there was no significant difference between WT and FFAR1−/− mice in the total distance of the social interaction test (Fig. 3F, gene×target interaction: F(1, 12)=0.11, p>0.05, gene effect: F(1, 12)=0.29, p>0.05, target effect: F(1, 12)=18.25, p<0.01). The time spent in the interaction zone of WT female mice was significantly increased in the target session compared with the no target session (Fig. 3G, gene×target effect: F(1, 12)=0.78, p>0.05, gene effect: F(1, 12)=10.54, p<0.01, target effect: F(1, 12)=3.023, p>0.05). The time spent in the interaction zone in the target session was significantly decreased in FFAR1−/− female mice compared with WT female mice (Fig. 3G, p<0.01). Sucrose preference did not show a significant difference between WT and FFAR1−/− female mice (Fig. 3H). Total or sucrose intake by FFAR1−/− female mice was significantly decreased compared to WT female mice (Figs. 3I–L, p<0.01).
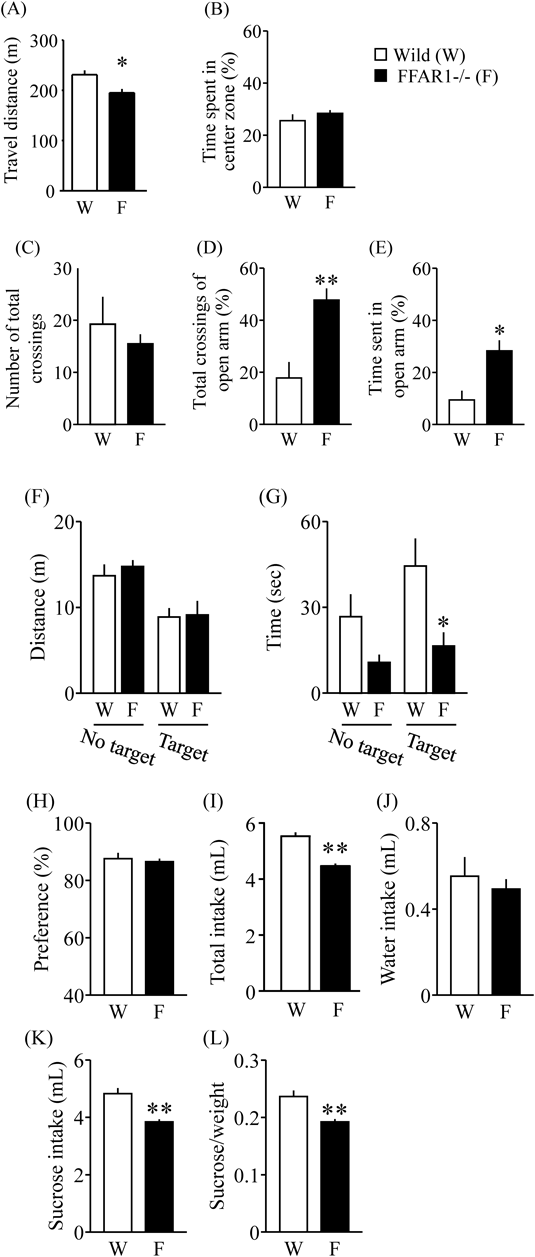
An emotional behavior in FFAR1−/− female mice (A–L). Performance of FFAR1−/− female mice in the open field test. The total travel distances (A), *p<0.05 vs. WT female mice. The time spent in the center zone (B). Performance of FFAR1−/− female mice in the elevated plus-maze test. The number of crossing (C), total crossing (D) and the time spent in the open (E). Performance of FFAR1−/− female mice in social interaction test. Travel distance (F), the time spent in the interaction zone in the no target or target session (G). *p<0.05 vs. WT female mice in Target. Performance of FFAR1−/− female mice in the sucrose preference test. Sucrose preference (H), total intake (I), water intake (J), sucrose intake (K) and the ratio of sucrose to weight (L). WT female mice: n=9; FFAR1−/− female mice: n=9. ** p<0.01 vs. WT female mice. W; WT female mice, F; FFAR1−/− female mice.
There is growing evidence that the dietary intake of n-3 fatty acids has a large enough impact on brain function to significantly affect mental health.1,18) In this study, we hypothesized that the decrease in n-3 fatty acids might induce a dysfunction of brain lipid signaling. First, we examined the maternal behavior of FFAR1−/− female mice to test whether this signaling is necessary to maternal care, which is known to be an instinctive behavior. As observed in nursing mothers after parturition, FFAR1−/− female mice showed significant impairment in maternal behavior compared to WT female mice, suggesting that the decrease of brain lipid signaling via GPR40/FFAR1 induces damage to maternal care. In the retrieval test, there was no significant difference between virgin WT and virgin FFAR1−/− female mice. Interestingly, FFAR1−/− multiparous female mice showed delay of retrieving time compared to WT multiparous female mice. Therefore, our results indicate that brain lipid signaling could be dramatically changed after parturition immediately, and might result in damage to maternal behavior.
We then conducted several behavioral tests on FFAR1−/− virgin female mice and WT female mice to test their emotional responses. In the EPM test, virgin FFAR1−/− female mice showed an increased time spent in the open arms compared to WT female. Generally, in an EPM experiment, an increase in the time a subject stays in the open arm, or an increase in the number of entries into it, are normally interpreted as a reduction of anxiety.14) Furthermore, it was considered that the increment of percent time spent in the open arms is a sign of impulsive-like behavior, as previously described.19) Our results suggest that FFAR1−/− female mice might show increased of impulsive-like behavior. In addition, the decrease of sucrose intake by FFAR1−/− female mice could be represented an abnormality of instinctive behavior phenotypes. From these results, we suggest that FFAR1−/− female mice, at least in part, are clearly different from WT female mice in several behavioral tasks, possibly related to an altered emotional state.
Current research questions focus on the why the decreases of PUFA in the brain, intriguingly, are associated with psychiatric disorders. Shimamoto et al. demonstrated that disturbances in brain-expressed free fatty acid binding protein (FABP) could represent an underlying disease mechanism in a proportion of schizophrenia and autism spectrum disorder sufferers.20) It is known that the reduction of FABP induces the decrease of PUFA in the tissue. However, the detailed mechanism thereof remains unclear. Therefore, we speculate that the pathological mechanism of psychiatric disorders might be mediated through the dysfunction of brain GPR40/FFAR1 signaling.
In summary, we found that FFAR1−/− female mice showed significant impairments in maternal behavior, and exhibited an emotionally altered behavior, when compared to WT mice. Our findings suggest that the dysfunction of brain PUFA-GPR40/FFAR1 signaling might induce damage to emotional and maternal behavior in female mice.
Part of this work was supported by the Takeda Science foundation, by Grants-in-Aid and Special Coordination Funds from the Kobe Gakuin University Joint Research (A) and a Grant-in-Aid for Scientific Research (C) (15K10566) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors declare no conflict of interest.