2018 Volume 41 Issue 1 Pages 36-46
2018 Volume 41 Issue 1 Pages 36-46
Chelerythrine (CHE) is a type of benzophenanthridine alkaloid found in many herbs and is also the main alkaloid constituent of Toddalia asiatica (L.) LAM. It has been proven to have various activities including antitumor, antifungal, anti-inflammatory and anti-parasitic effects. We have previously demonstrated that CHE can inhibit proliferation and promote apoptosis in human hepatocellular carcinoma (HCC) cells. However, the effect of CHE on the metastasis of HCC and its related molecular mechanisms have yet to be validated. In this study, we investigated the effects of CHE on the migration and invasion of the HCC cell line Hep3B. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), wounding healing, transwell migration and invasion assays and cytoskeleton staining demonstrated that CHE could inhibit the migration and invasion of Hep3B cells in a dose-dependent manner with change of cell structure. RNA interference studies made a knockdown of matrix metalloproteinase (MMP)-2/9 respectively in Hep3B cells. And the results of wounding healing and transwell invasion assay with the treatment of small interfering RNA (siRNA) investigated that MMP-2/9 are positively associated with Hep3B cell metastasis. The results of enzyme-linked immunosorbent assay (ELISA), Western blotting and quantitative RT-PCR showed that CHE suppressed the expression of MMP-2/9 at both mRNA and protein levels. CHE also exhibited an inhibitory effect on the phosphorylation of Focal adhesion kinase (FAK), phosphatidylinositol 3-kinase (PI3K), Akt, mammalian target of rapamycin (mTOR), c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinases (ERK) and p38. In summary, on Hep3B cells, CHE could change the cell cytoskeletal structures through reducing the expression of p-FAK and inhibit the metastasis of Hep3B cells by downregulating the expression of MMP-2/9 mainly through PI3K/Akt/mTOR signaling pathway.
Despite the groundbreaking progress in the diagnosis and treatment of malignant tumors, cancer remains the greatest health concern in the world due to high morbidity and mortality.1) Hepatocellular carcinoma (HCC) is one of the most common types of cancer and the second leading cause of cancer-related death with the 5th highest morbidity rate according to recent studies.2,3) Currently, there are a few treatment options of HCC, including surgical resection and liver transplantation. However, most of the patients who undergo the treatments mentioned above experience new tumors in the residual liver, and their five-year survival rate is no more than 10%.4–6) Metastasis and recurrence are the two main reasons for poor prognosis in patients with HCC.7) Therefore, key efforts should be focused on blocking invasion and migration of cancer cells.
Natural products have been used to treat cancer for over 40 years. Approximately 60% of the clinically used anti-cancer drugs are natural products or derivatives.8) The traditional Chinese medicinal herb Toddalia asiatica LAM. is a kind of traditional folk medicine commonly used in southwest China. It can disperse pathogenic wind and relieve pain, remove blood stasis to stop bleeding, clear away toxic materials and alleviate edema, as stated in the Chinese theory of medicine. We extracted total alkaloids from Toddalia asiatica LAM. and observed obvious anti-inflammatory, analgesic and anti-tumor effects.9,10) 1,2-Dimethoxy-N-methyl[1,3]benzodioxolo[5,6-c]phenanthridinium, commonly known as Chelerythrine (CHE), is a kind of benzophenanthridine alkaloid and the main alkaloid constituent of Toddalia asiatica.10,11) CHE has a wide range of biological activities including antimicrobial, antifungal, anti-inflammatory, anti-parasitic and anti-tumor effects.11–14) Regarding cancer therapy, we have reported that CHE can inhibit proliferation and promote apoptosis in HepG2 cells.15) However, the effect of CHE on the metastasis of HCC and its related molecular mechanisms are still undefined.
Studies have shown that tumor metastasis involves a serious of complex processes. The primary tumor cells proliferate, invade the basement membrane (BM) and enter the blood and lymphatic circulation. Finally, metastases are formed at distal sites, resulting in secondary tumors.16,17) In the process described above, the degradation of the extracellular matrix (ECM) is key to tumor migration and invasion. Many protein kinases play important roles in the degradation of the ECM, including matrix metalloproteinases (MMPs).18) Among the family of MMPs, the overexpression of MMP-2 and MMP-9 is associated with tumor metastasis.19) The phosphatidylinositol 3-kinase (PI3K)/Akt and mitogen-activated protein kinase (MAPK) signal transduction pathways are both closely related to the metastasis of HCC. In addition, a large number of studies have shown that the two signaling pathways are involved in the regulation of MMP-2 and MMP-9 activity.20–24)
This study aims to investigate the anti-metastatic effect of CHE on Hep3B cells and its related molecular mechanisms.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to measure the viability of Hep3B cells after treatment with CHE. The results demonstrated the anti-proliferation effect of CHE at different concentrations (0.625–20 µM) on HCC cells Hep3B. CHE showed an anti-proliferation effect on Hep3B cells in a dose-dependent manner at both 24 and 48 h (Fig. 1). At concentrations of 10 and 20 µM, CHE significantly inhibited cell proliferation compared to the vehicle. However, below 5 µM, such effect was not observed. We finally chose a concentration range of CHE from 0.625 to 5 µM for subsequent experiments.
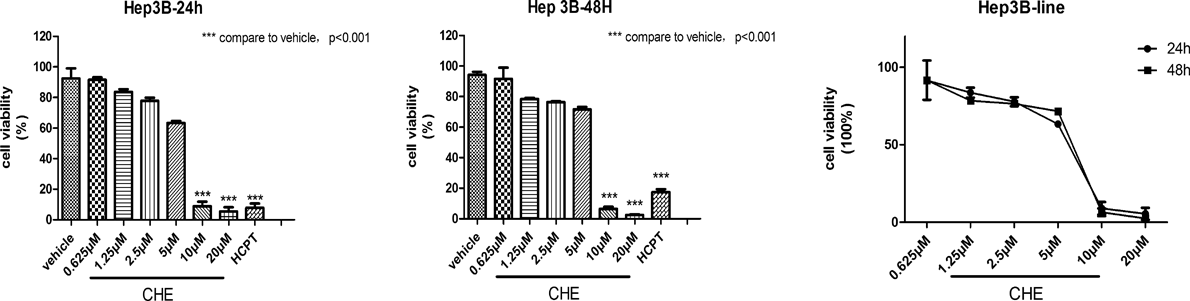
The viability of Hep3B cells was measured using the MTT assay. The data are shown as the means±S.D. of three independent experiments. Vehicle: DMSO-treated cells.
The effect of CHE on the migration of HCC Hep3B cells was investigated by wound healing assay. The results shown in Fig. 2 indicate that the wound width was smaller in the control group than in the CHE-treated group. Such inhibition was observed when the cells were incubated with CHE at different concentrations (0.625–20 µM) at 24 and 48 h. These results demonstrated that CHE inhibited cell migration in a dose- and time-dependent manner (Fig. 2).
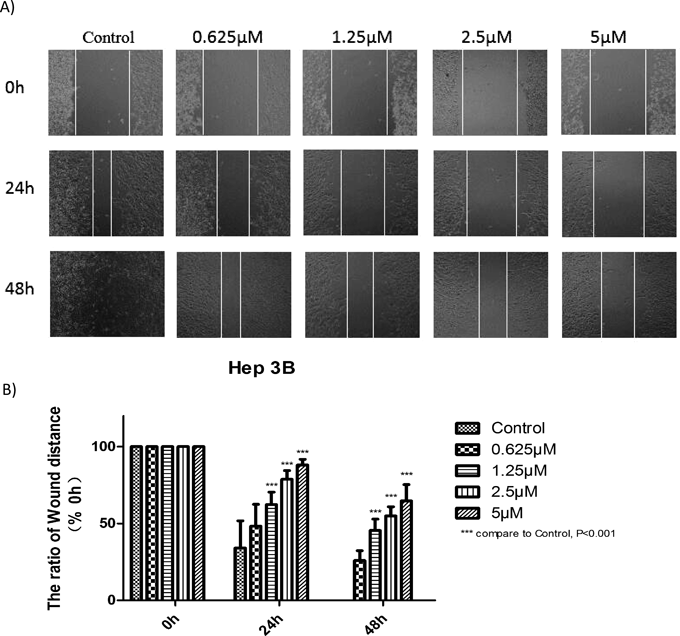
Cells were exposed to several concentrations (0 (Control), 0.625, 1.25, 2.5 and 5 µM) of CHE for 24 and 48 h, and then the distance migrated by the cells was evaluated by wound healing assay. The ratio of the wound width at the measure times relative to the initial wound width is shown in B.
Transwell migration and Matrigel invasion assays were performed to study the effects of CHE on the cell migratory abilities. The results indicated that CHE efficiently suppressed the migration and invasion of Hep3B cells (Fig. 3) in a dose-dependent manner. After treatment with 1.25, 2.5 and 5 µM of CHE, the migration/invasion rates of Hep3B cells were 75.49/44.45, 22.91/14.18 and 1.64/0.61% at 24 h and 67.74/51.75, 33.34/2.80 and 1.23/0.56% at 48 h, respectively. The data demonstrated that CHE inhibited the migration and invasion of the Hep3B cells in vitro.
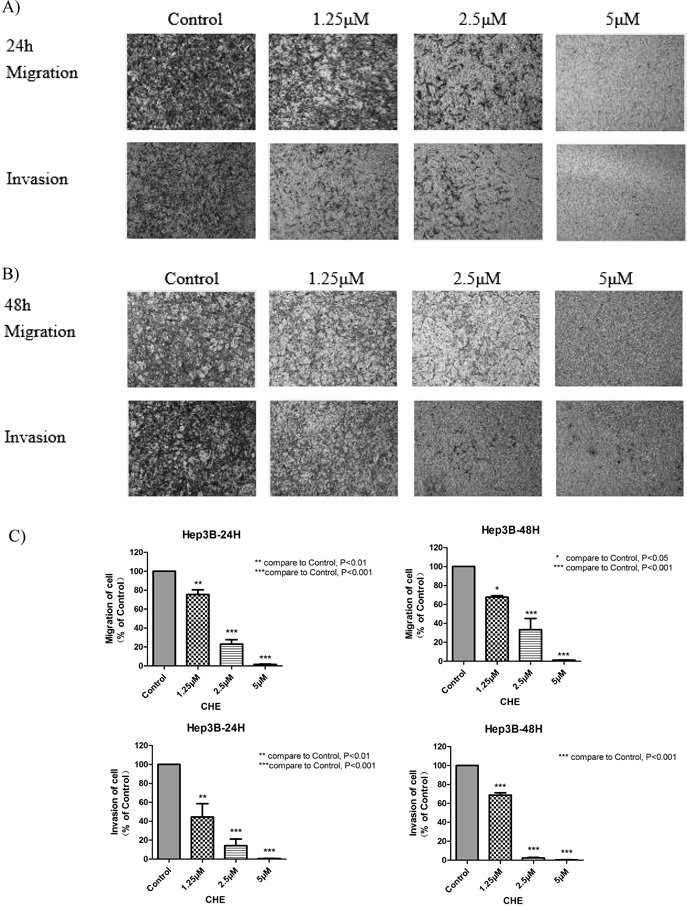
Cells were treated with 0, 1.25, 2.5 and 5 µM of CHE for 24 h (A) and 48 h (B). Cellular migration or invasion was measured by transwell or Matrigel-coated transwell assays. The summary of the data for the transwell migration and invasion assays are presented as the means±S.D. (C).
The cell cytoskeleton is involved in the maintenance of cellular morphology and movement in eukaryotic cells.25) The cytoskeleton consists of microtubules, microfilaments and intermediate filaments. As shown in Fig. 4, we used a fluorochrome specific to F-actin to stain the cells. In the control group, the microfilaments were filamentous, spindly and clearly and regularly diffused. With the increase in the concentration of CHE, we observed the contraction of the pseudopodia, changes in microfilamentous structures and aggregation of F-actin. In the group treated with 5 µM of CHE, after 48 h of treatment, the microfilaments were seen to disappear and a large number of fluorescent granular structures appeared. The results indicated that CHE can induce changes in cell cytoskeletal structures, which are based on F-actin.
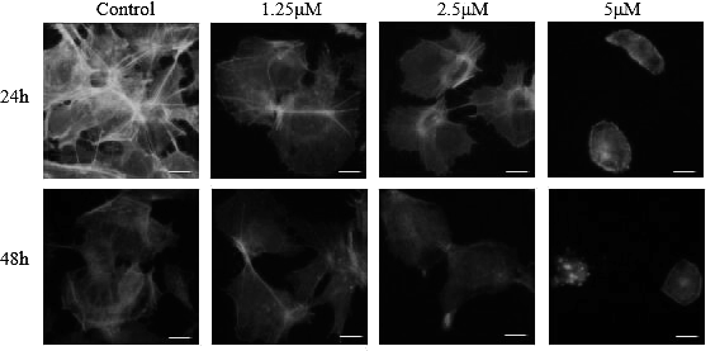
Fluorescence image of the actin cytoskeleton stained with FITC-phalloidin. Treatment of Hep3B cells with various concentrations of CHE for 24 and 48 h led to the aggregation of F-actin and changes in microfilament structure and cell shape. Scale bar=50 µM.
MMP-2 and MMP-9 are two important members of the MMP family. They are highly expressed in many malignant tumors, and they participate in the degradation and destruction of the ECM and BM and promote tumor metastasis.26,27) To clarify whether these two MMPs are involved in the inhibitory effect of CHE on the metastasis of Hep3B cells, enzyme-linked immunosorbent assay (ELISA), Western blot and qRT-PCR were used to detect the expression of MMP-2 and MMP-9 both at mRNA and protein levels. The results demonstrated that CHE reduced the expression levels of MMP-2 and MMP-9 at both 24 and 48 h in a dose-dependent manner (Fig. 5).
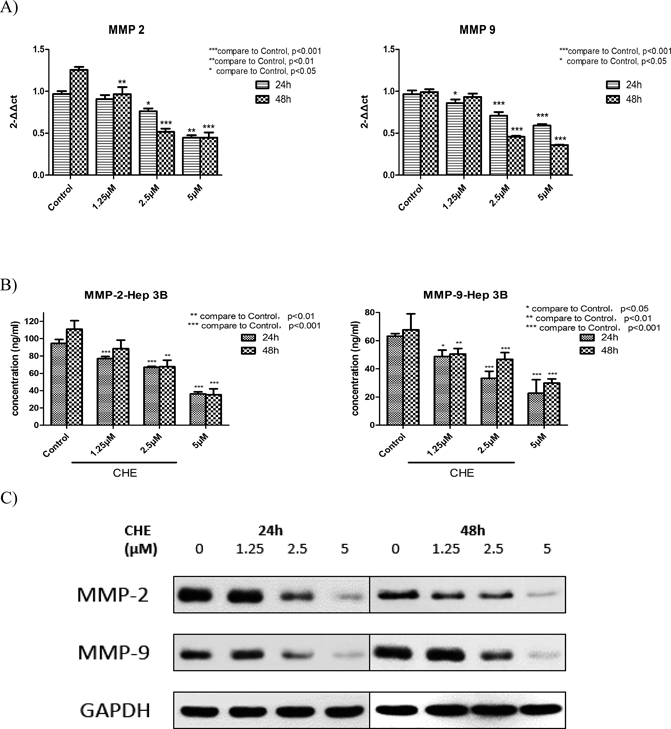
Cells were treated with several concentrations of Chelerythrine (0, 1.25, 2.5 and 5 µM) for 24 or 48 h. The mRNA levels of MMP-2 and MMP-9 were determined using qRT-PCR (A). The protein levels of MMP-2 and MMP-9 were determined using ELISA and Western blot analysis (B, C). The expression levels of MMP-2 and MMP-9 were decreased in a dose-dependent manner at 24 and 48 h both at mRNA and protein levels. GAPDH was used as the internal control.
Many studies have shown that, a higher expression of MMP-2 and MMP-9 has existed in those invasive and highly tumorigenic cancers like HCC, breast cancer, brain metastases and colorectal tumors.28) We have used siRNA to knockdown MMP-2 and MMP-9 respectively and investigated whether knockdown of MMP-2 and MMP-9 could influence the migration and invasion ability of Hep3B cells. In this study, qRT-PCR and Western blot were used to detect the mRNA and protein expression of MMP-2 and MMP-9 in Hep3B cells. The results showed that MMP-2 and MMP-9 siRNA caused a reduction in MMP-2 and MMP-9 expression both in gene and protein levels respectively, while control groups showed no change (Fig. 6(A)). The wound healing assay and transwell invasion assay were used to expound the roles of MMP-2 and MMP-9 in cell metastasis. We have observed that the wound width of control groups was much smaller than which of siRNA treatment groups after 48 h. And treatment with MMP-2 siRNA and MMP-9 siRNA both caused a significant decrease in the invasion rate compared to control groups after 24h (Fig. 6(B, C)). All the data above indicated that MMP-2 and MMP-9 are positively associated with Hep3B cell metastasis.
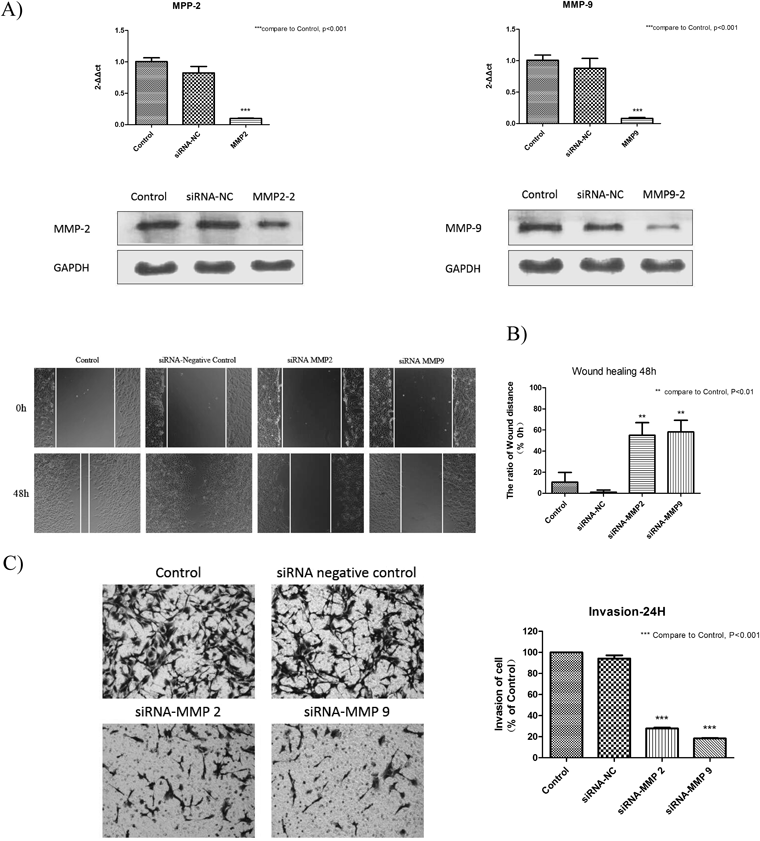
After treatment with MMP2 and MMP9 siRNA in Hep3B cells respectively, the mRNA and protein expression of MMP-2 and MMP-9 were detected by qRT-PCR and Western blot (A). Wound healing assay and transwell invasion assay were carried out to measure the influence of MMP2 and MMP9 siRNA in cell metastasis (B, C). The summary of the data for the wound healing and transwell invasion assays are presented as the means±S.D.
Western blot analysis was used to investigate the effects of CHE on the FAK/PI3K/Akt/mTOR signaling pathway. The results demonstrated that CHE had no effect on the protein levels of FAK, PI3K, Akt, mTOR at 24 and 48 h. However, the expression of the activated form of these proteins, namely, the phosphorylated form, was decreased (Fig. 7).
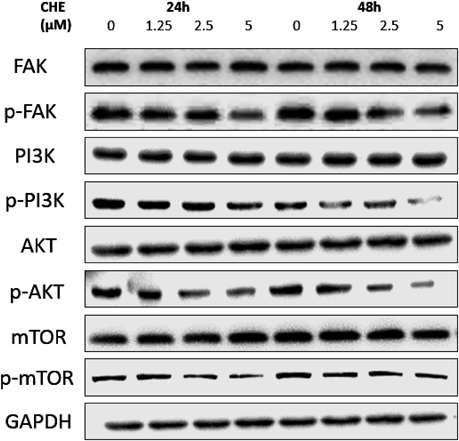
Western blot analysis show that CHE decreased the protein levels of p-FAK, p-PI3K, p-Akt, p-mTOR in a dose-dependent manner at both 24 and 48 h without affecting the levels of their inactive forms. GAPDH was used as the internal control.
To evaluate whether PI3K was involved in the inhibitory effect of CHE on cell metastasis and MMP-2 and MMP-9 expression, Hep3B cells were pretreated with a PI3K inhibitor (LY294002, 25 µM) for 1 h and then incubated with CHE (5 µM) for 24 h. The results showed that after being treated with CHE and pretreated with LY294002, the invasion of Hep3B cells was significantly inhibited accompanied by the downregulation of MMP-2 and MMP-9 (Fig. 8).
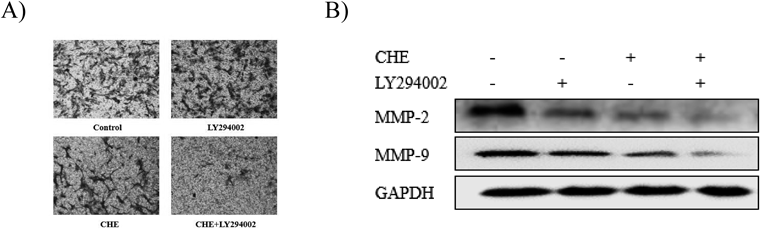
Hep3B cells were pretreated with the PI3K inhibitor LY294002 (25 µM) for 1 h and then incubated in the absence or presence of CHE (5 µM) for 24 h. A) The effect of PI3K inhibitor on cellular invasion was measured using Matrigel cell invasion assay. B) The protein levels of MMP-2 and MMP-9 were analyzed by Western blotting. The results indicated that PI3K was involved in the downregulation of MMP-2 and MMP-9 by CHE. GAPDH was used as the internal control.
MAPKs participate in one of the most important signaling pathways that mediate cellular behavior and transduce signals from the cell surface into the nucleus.29) Several MAPK pathways have been identified, including extracellular signal-regulated kinase (ERK), c-Jun N terminal kinase (JNK) and p38. Many studies indicated that the MAPK family is involved in cancer metastasis, and these three protein kinases can regulate the expression of MMPs.23,24,30,31) Western blot analysis was used to investigate the effects of CHE on MAPK signaling pathways. The results demonstrated that CHE had no effect on the protein levels of ERK1/2, JNK, and p38 after treatment with CHE at both 24 and 48 h. However, at 24 h, the protein expression of phosphorylated ERK1/2, JNK and p38 did not have a significant change, but at 48 h they were decreased in a dose-dependent manner (Fig. 9).
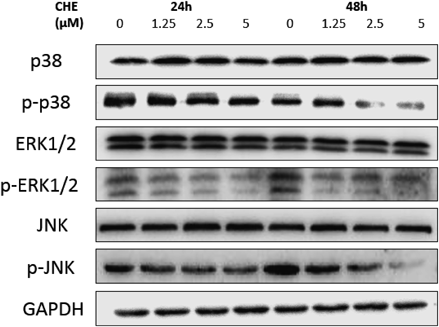
Western blot analysis show that CHE decreases the protein levels of p-ERK1/2, p-p38 and p-JNK in a dose-dependent manner at 48 h without affecting the levels of their inactivated forms. GAPDH was used as the internal control.
To evaluate whether ERK1/2, p38 and JNK were involved in the inhibitory effect of CHE on cell metastasis and MMP-2 and MMP-9 expression, Hep3B cells were pretreated with the ERK1/2 inhibitor PD98059 (10 µM), p38 inhibitor SB203580 (10 µM) and JNK inhibitor SP600125 (20 µM) for 1 h and then incubated with CHE (5 µM) for 24 h. The results showed that after being treated with CHE and pretreated with each inhibitor, the invasion of Hep3B cells was significantly inhibited accompanied by the downregulation of MMP-2 and MMP-9 (Fig. 10).

Hep3B cells were pretreated with the ERK1/2 inhibitor PD98059 (10 µM), p38 inhibitor SB203580 (10 µM) and JNK inhibitor SP600125 (20 µM) for 1 h and then incubated with CHE (5 µM) for 24 h. A) The effect of ERK1/2, p38 and JNK inhibitor on cellular invasion was measured using Matrigel cell invasion assay. B) The protein levels of MMP-2 and MMP-9 were analyzed by Western blotting. The results indicated that ERK1/2, p38 and JNK was involved in the downregulation of MMP-2 and MMP-9 by CHE. GAPDH was used as the internal control.
HCC is one of the most malignant tumors worldwide with high morbidity, mortality and poor prognosis due to recurrence and metastasis. Tumor metastasis is the main feature of malignant tumors and is responsible for more than 90% of cancer-related mortality.32) Inhibition of cancer metastasis may be an effective treatment for malignant tumors.
Cellular migration and invasion is the key to tumor metastasis. These processes are mainly mediated by the association of growth factors with cell surface receptors, activating the downstream signaling pathways and leading to cytoskeletal reorganization and increased cellular motility.33)
CHE has been shown to inhibit proliferation of various tumor cells. However, for different kinds of tumors, IC50 of CHE is quite varied. It has been reported that for SMMC cells, the IC50 at 24, 36 and 48 h was 25, 16 and 12 µM, respectively.34–36) For HL-60 cells, the IC50 at 24 h was 1.6 µM, and for HeLa cells, the IC50 at 24 h was 9 µM. In the present study, the results of the cell viability assay showed that in the concentration range from 0.625 to 5 µM, CHE had no significant inhibitory effect on the viability of Hep3B cells at either 24 or 48 h.
Cancer metastasis involves several steps, including the proliferation of the primary tumor, the degradation of the ECM, invasion through the tumor stroma, intravasation, diffusion to distal sites, extravasation and colonization at metastatic sites.37) Cell motility is the primary step in this process.38) In the present study, wounding healing assay and transwell migration and invasion assays were carried out to elucidate the effect of CHE on cellular motility, migration and invasion. The results indicated that CHE significantly inhibited the motility, migration and invasion of Hep3B cells in a dose-dependent manner at both 24 and 48 h. In addition, the range of the effective concentration was from 1.25 to 5 µM, which was not toxic for Hep3B cells. The above results indicated that CHE can inhibit the migration and invasion of Hep3B cells without exerting cytotoxicity.
The metastatic ability of tumor cells is dependent on changes in the cytoskeleton—the depolymerization and polymerization of actin. The cytoskeleton, especially the microfilaments, is an important part of the filopodia, lamellipodia, stress fibers and focal adhesions. It plays a crucial role in cell movement. Fluorescein isothiocyanate (FITC)-phalloidin is able to specifically bind with F-actin, which allows the examination of changes in the morphology and distribution of F-actin in the cells. The results of the present study showed that CHE could effectively destroy the dynamic behavior of Hep3B cells, thus inhibiting their ability to migrate and invade.
During the process of cancer metastasis, the degradation of the ECM is critical for tumor invasion. Interfering with this step may be an effective strategy for cancer therapy. MMPs are a family of zinc-dependent proteolytic enzymes. The members of this family have a number of physiological functions such as ECM modification.39) Several members of the MMP family have been identified, yet MMP-2 and MMP-9 have been proven to be most closely linked with the degradation of the ECM and are most highly expressed in metastatic carcinoma.40–42) In this study, RNA interference experiment was carried out to clarify whether MMP-2 and MMP-9 was associated with the cell metastasis in Hep3B cells. We have observed that knockdown of MMP-2 and MMP-9 could inhibit the cell migration and invasion ability in Hep3B cells. From these results we can clearly see the roles of MMP-2 and MMP-9 in Hep3B cell metastasis. And after Hep3B cells were treated with CHE for 24 and 48 h, MMP-2 and MMP-9 were downregulated both at mRNA and protein levels in a dose-dependent manner, which indicated that CHE suppressed the metastasis of Hep3B cells through the inhibition of expression of MMP-2 and MMP-9.
FAK is a kind of tyrosine kinase. It is highly expressed in a variety of cancers including HCC and plays roles in tumor formation and progression.43–45) In many types of cancer, FAK is thought to be involved in the regulation of dynamic changes of the cytoskeleton and dissociation of focal adhesions. Activation and phosphorylation of FAK is an important way for tumor cells to attain a migratory phenotype. According to the results of cytoskeletal staining, change in the expression of FAK and p-FAK expression was potentially one of the mechanisms of by which the cytoskeletal structures of Hep3B cells is altered by CHE. Once FAK is phosphorylated, it acts as a scaffold and dissolves intercellular junctions and regulates focal adhesion signaling, which is related to MMP-mediated matrix degradation involving MMP-2 and MMP-9.35,46–48) The Western blotting results in this study showed that CHE obviously decreased the phosphorylation of FAK without affecting its inactive form at the concentration of 5 µM both at 24 and 48 h. And these results are consistent with cell cytoskeletal changes.
The PI3K/AKT/mTOR signaling pathway participates in many cellular processes such as differentiation, proliferation and metastasis. In several studies, this signal transduction pathway was shown to participate in the regulation of MMP-2/-9. PI3K is the key kinase in this pathway that has both serine/threonine protein kinase activity and phospholipid kinase activity. AKT is the major downstream target of PI3K. It not only phosphorylates endothelial nitric oxide synthase (eNOS) to promote angiogenesis but also promotes the metastasis of tumor cells through a variety of mechanisms. mTOR is a highly conserved serine/threonine protein kinase. It is a hub of a variety of important intracellular signal transduction pathways, which regulate many physiological processes such as cell proliferation, division and migration. In addition, these physiological processes are closely related to tumorigenesis. In many kinds of tumor cells, mTOR is regulated by the PI3K/AKT signaling pathway.49) The Western blotting results from the present study showed that CHE reduced the extent of phosphorylation of PI3K, AKT and mTOR. In addition, the protein level of PI3K, AKT and mTOR remained unchanged after treatment with several concentrations of CHE. CHE combined with a PI3K inhibitor (LY294002) significantly reduced the invasion of Hep3B cells through the downregulation of MMP-2 and MMP-9. It has been indicated that PI3K has a role in the inhibition of the metastasis of Hep3B cells by CHE.
MAPKs are able to transmit signals from the cell surface to interior. Analysis of human HCC cell lines by oligonucleotide microarray showed that the sustained activation of the MAPK pathway is potentially related to invasion and migration of HCC cells.50) ERK1/2, JNK and p38 MAPK, the main members of the MAPK family, were proved in many studies to be related with the overexpression of MMPs in HCC.51,52) In this study, the protein expression of ERK1/2, p-ERK1/2, p38, p-p38, JNK, p-JNK were measured by Western blotting. The results showed that CHE downregulated the expression of p-ERK1/2, p-p38 and p-JNK in a dose-dependent manner at 48 h but at 24 h, such change was not obvious. However the expression of ERK1/2, p38 and JNK were not affected. CHE combined with the ERK1/2 inhibitor PD98059, p38 inhibitor SB203580 and JNK inhibitor SP600125 respectively resulted in a synergistic effect. Hep3B cell invasion was reduced significantly with the downregulation of MMP-2 and MMP-9. Based on the results of this part, MAPKs signal pathway was involved in the inhibition of Hep3B cells metastasis by CHE at 48 h.
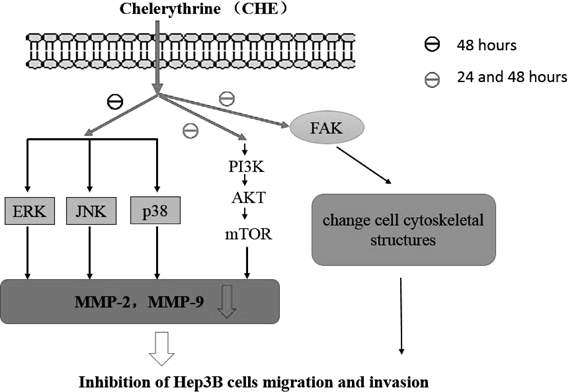
Our results showed that CHE inhibited the migration and invasion of Hep3B cells in a dose-dependent manner and also altered the cell cytoskeletal structures at both 24 and 48 h without the cytotoxic effect. Meanwhile, CHE decreased the gene and protein expression of MMP-2/9. Furthermore, CHE also suppressed the expression of p-FAK, p-PI3K, p-AKT, p-mTOR at both time points and suppressed the expression of p-ERK1/2, p-JNK and p-p38 at 48 h without affecting the levels of their inactive forms. However, whether the inhibitory effect of CHE on cell metastasis is time-dependent still needs further study. In conclusion, CHE could alter cell cytoskeletal structures of the human HCC cell line Hep3B through reducing the expression of p-FAK and inhibit the metastasis of Hep3B cells by downregulating the expression of MMP-2 and MMP-9 through the PI3K/Akt/mTOR and MAPK signaling pathways. More concretely, the PI3K/Akt/mTOR signaling pathway plays a major role in this process, it was inhibited by CHE both at 24 and 48 h. And MAPK signaling pathway was inhibited by CHE only at 48 h (Fig. 11). These results show that CHE has a potent anti-metastatic effect on Hep3B cells.
Chelerythrine (CHE, Lot: R18A6F3) and 10-hydroxycamptothecine (HPTC, Lot: Y24F8C29666) were purchased from Shanghai Yuanye Biological Technology Co., Ltd. MTT and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Corp. (St. Louis, MO, U.S.A.). Matrigel was purchased from Corning Corp. (NY, U.S.A.).
Cell CultureThe human HCC cell lines Hep3B and HepG2 were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, HyClone, Logan, UT, U.S.A.) supplemented with 10% fetal bovine serum (FBS, GIBCO, Grand Island, NY), 100 U/mL penicillin, and 100 U/mL streptomycin (Sigma, St. Louis, MO, U.S.A.) in a humidified atmosphere with 5% CO2 at 37°C.
Cell Viability AssayCell viability was evaluated by the MTT assay. Cells were seeded into a 96-well culture plate for 24 h at a density of 4×103 cells/well in 100 µL culture medium. The cells were incubated with or without serial dilutions of CHE. After 24 and 48 h, 10 µL of MTT (5 mg/mL) was added, and the cells were incubated for 4 h. Then, 150 µL/well dimethyl sulfoxide was used to dissolve the formazan crystals, and the absorbance was detected at 490 nm with a microplate reader (BioTek, Winooski, VT, U.S.A.).
RNA Interference StudiesThe siRNA was designed and chemical synthesized by Sangon Biotech (Shanghai) Co., Ltd. The company provided 4 sequences of each siRNA and we chose the most effective one for the following experiments. The target sequence against MMP-2 selected was sense GGA GAUACA AUGAA GUAAA TT and anti-sense UUUACUUCAUUGUAUCUCCT T. And that against MMP-9 selected was sense CCG GGA ACGUAUCUGGA AAT T and anti-sense UUUCCA GAUACGUUCCC GGT T. The negative control was randomly synthesized and its sequence was sense UUCUCCG AAC GUGUCACUGTT and anti-sense ACGUGAC ACGUUCGG AGA ATT. siRNA transfection was performed using Nanoscale micro poly Transfection Reagent according to the manufacturer’s instructions.
Wound Healing AssayCell motility was evaluated by wound healing assay. Cells were seeded into a 24-well culture plate at 1×105 cells/well. When the cells formed a confluent monolayer, a scratch was created with a 200-µL pipette tip. After being washed twice with phosphate buffered saline (PBS), all cells in the plate were treated with fresh medium with or without CHE at a final concentration of 0, 1.25, 2.5 and 5 µM for 24 and 48 h. Wound closure was observed and measured using an inverted microscope (OlympusX71, Tokyo, Japan). Cell migration was calculated as the percentage of the remaining cell-free area compared with the area of the initial wound. Each experiment was performed triplicate.
Transwell Migration and Invasion AssayCell migration and invasion assays were performed using transwell chambers (Corning) with a pore size of 8 µm. All the cells using for these two assays were incubated with serum-free medium for 24 h. For the migration assay, cells were seeded at a density of 5×104/well into the upper chambers in serum-free medium and treated with 0.0125% DMSO (as the vehicle) or with CHE (1.25, 2.5 and 5 µM), and in the lower chamber, 500 µL of 10% FBS-containing medium was added. After incubated for 24/48 h, a cotton swab was used to remove the cells on the upper chamber, and the migrated cells on the bottom surface were fixed with 100% methanol and stained with 0.1% crystal violet. Cell numbers were scored under an inverted microscope (OlympusX71) in three random fields. For the invasion assay, the upper chamber was coated with 50 µL Matrigel (Corning) and incubated at 37°C for 30 min. Each plate was assayed twice including vehicle, and each experiment was performed in triplicate.
Cytoskeleton StainingCells were seeded on 6-well culture plates on glass slides at a density of 1×105/well and cultured for 24 h. Then, the cells were treated with various concentrations of CHE (0 µM as the control, 1.25, 2.5 and 5 µM). After incubation of 24 and 48 h, the cells were washed twice in PBS, fixed with 4% paraformaldehyde for 15 min. The glass slides with cells were taken out, washed twice with PBS and incubated in a solution containing FITC-phalloidin (1 : 200 dilution) for 2 h at room temperature. Then, the cells were washed twice with PBS and stained with 4′-6-diamidino-2-phenylindole (DAPI) for 10 min at room temperature and protected from light. Then, the cells were washed twice with PBS before the glass slides with cells were mounted with microslides using Fluoromount-G. The cells were visualized with a fluorescence microscope.
Western Blot AnalysisCells (4×105/dish) were seeded on a 6-cm dishes, treated with or without CHE at various concentrations for 24 or 48 h and lysed in RIPA buffer. The total protein concentrations were measured by Bradford protein assay reagent (Bio-Rad, Hercules, CA, U.S.A.). Protein from each sample was separated using 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, U.S.A.). The membranes were blocked with 5% bovine serum albumin (BSA) for 1.5 h and then immunoblotted overnight with primary antibodies at 4°C. Primary anti-human MMP-2, MMP-9, FAK, p-FAK, PI3K, p-PI3K, AKT, p-AKT, mTOR, p-mTOR, ERK1/2, p-ERK1/2, JNK, p-JNK, p38, p-p38 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were used. After washing with TBST, the membranes were incubated with the appropriate secondary antibodies (peroxidase-conjugated AffiniPure goat anti-rabbit immunoglobulin G (IgG), 1 : 5000 in 3% BSA) for 2 h and then visualized using enhanced chemiluminescence reagents according to the manufacturer’s instructions (Thermo Scientific).
Real-Time Quantitative PCRTotal RNA from Hep3B cells was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer’s instructions. The quality of the total RNA was assessed using Nanodrop 2000. Reverse transcription was performed using Prime Script RT Master Mix (TaKaRa, Japan). In addition, SYBR Green qPCR Master Mix (TaKaRa) was used for real-time quantitative PCR analysis. The nucleotide sequences of the primers used in this study are as follows: GAPDH, forward 5′-CTA CGT CCC TAC TAC AAG AC-3′ and reverse 5′-CTG CTT CAC CAC CTT CTT GA-3′ as the internal control; MMP-2, forward 5′-TGA TCT TGA CCA GAA TAC CAT CGA-3′ and reverse 5′-GGC TTG CGA GGG AAG AAG TT-3′; MMP-9, forward 5′-CCT GGA GAC CTG AGA ACC AAT C-3′ and reverse 5′-CCA CCC GAG TGT AAC CAT AGC-3′.
Statistical AnalysisAll data are presented as the means±standard deviation (S.D.) for three independent experiments. One-way ANOVA was used for multiple comparisons, and Student’s t-test was used for single comparisons. Statistical analyses were performed with SPSS 21.0 (SPSS Inc., Chicago, IL, U.S.A.). p<0.05 was set as the level of significance.
This study was supported by the Qianbowen teacher studio fund (P20802).
The authors declare no conflict of interest.