2018 年 41 巻 1 号 p. 65-72
2018 年 41 巻 1 号 p. 65-72
In a previous work, we reported the regulatory role of the triterpenoids on 5-hydroxytryptamine (5-HT)3A receptors activity in Xenopus laevis oocytes (Eur. J. Pharmacol., 615, 2009, Lee et al.). In the present report, we studied the modulation of triterpenoids on the activity of the human nicotinic acetylcholine receptor type α3β4. Two-electrode voltage clamp experiments were used to test acetylcholine mediated inward current (IACh). Treatment with triterpenoids (dehydroeburicoic acid, 6α-hydroxypolyporenic acid C and pachymic acid) inhibited IACh in a concentration dependent and reversible manner. The IC50 values for pachymic acid, dehydroeburicoic acid, and 6α-hydroxypolyporenic acid C were 14.9, 37.7, and 20.9 µM, respectively. The inhibitory regulation of IACh by each triterpenoid showed in a non-competitive manner on the activity of α3β4 nicotinic acetylcholine receptors. These results show that triterpenoids (pachymic acid, dehydroeburicoic acid, 6α-hydroxypolyporenic acid C) can be used as agents to modulate the activity of nicotinic acetylcholine receptor type α3β4. Furthermore, molecular docking studies of 6α-hydroxypolyporenic acid C on α3β4 nicotinic acetylcholine receptors in silico showed that this molecule interacted predominantly with residues at cavities in the α3 subunit and β4 subunit. This docking assays indicated four potential binding sites for this ligand in the extracellular region at sensor domain of α3β4 nicotinic acetylcholine receptors. In point mutagenesis of those whose alanine substitution, 6α-hydroxypolyporenic acid C potency decreased on W25A of α3 subunit or N109A of β4 subunit in both mutants. The double mutation of W25A of α3 subunit and N109A of β4 subunit was significantly attenuated inhibitory effects by 6α-hydroxypolyporenic acid C. All taken together, this study revealed that molecular basis of α3β4 nicotinic acetylcholine receptors by triterpenoids and provides a novel potent interaction ligand
Triterpenoids are classified as nature compounds and synthesized materials from triterpenes modified by squalene cyclization or acyclic carbon substitution in Fig. 1. Triterpenoids isolated from various plants are generally used for clinical purposes in Far East Asia.1) In particular, triterpenoids showed inhibitory effects on tumor growth in the dermal tissue of mice with second step tumoral calcinosis and 12 tetradecanoyl-phobol acetate derived infection.2) Furthermore, triterpenoids like as pachymic acid and dehydrotumulosic acid potently modulated PLA2 from snake toxin.3) Pachymic acid with a methyl-group at the 24th carbon also showed antiemetic effects in amphibians and was purified from the fungus Fomitopsis.4,5)
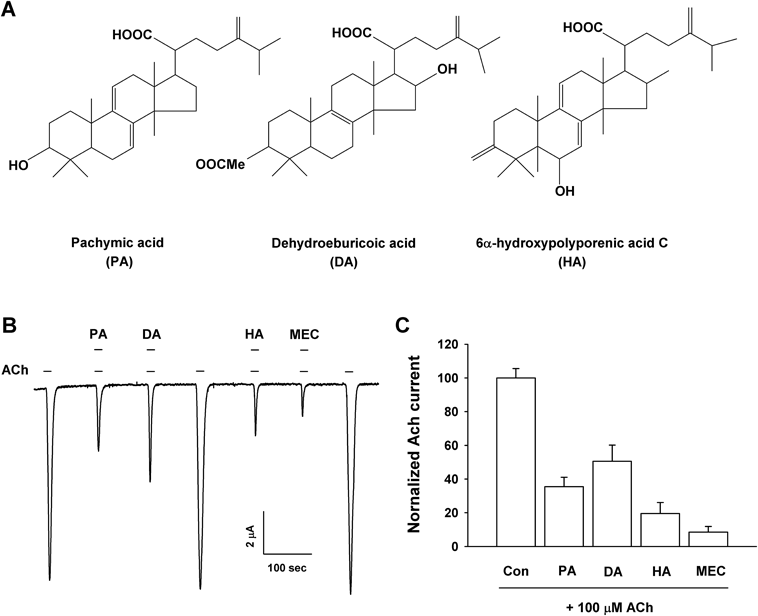
(A) The chemical structure of Pachymic acid, Dehydroeburicoic acid, and 6α-hydroxypolyporenic acid C. (B) Acetylcholine (100 µM) was treated first, and then which 100 µM acetylcholine was co-applied with 100 µM triterpenoids (PA, DA and HA). Treatment with mecamylamine, potent nACh receptor antagonists, blocked ACh induced current on the α3β4 nACh receptors at −80 mV holding potential in Xenopus oocytes. The represent data was indicated the means±S.E.M. (n=12−15 oocytes). (C) Co-treatment of triterpenoids and mecamylamine with acetylcholine exhibits inhibitory effects in summary histograms.
The acetylcholine receptor widely distributed throughout the human body and it has been studied in neuronal and muscular systems. In particular, nicotinic acetylcholine receptors are activated by the agonist acetylcholine, allowing cation movement into cytoplasm and then lead to depolarization. The nicotinic acetylcholine receptors consist of alpha and/or beta subunits. The α7, α9, and α10 sub-families were consisted homomeric receptors, but other alpha subunits should be combined with beta subunits to complete heterogenic with the critical conformation necessary to form channels according to the muscle type and neuronal region.6) The muscular nicotinic acetylcholine receptor channels are α1β1δγ subunits for the early development form or α1β1δε subfamilies for the older form,7) whereas the nervous nicotinic receptor are alpha (α2−α10) and beta (β2−β4) subunits.6) In a previous report, we showed that triterpenoids (pachymic acid (PA), dehydroeburicoic acid (DA) and 6α-hydroxypolyporenic acid C (HA)) inhibited 5-hydroxytryptamine (5-HT)3A receptor channel activity in expressed Xenopus laevis oocytes.8) However, the study of triterpenoids induced nicotinic acetylcholine (nACh) receptor channel activity regulation was not reported.
Accordingly, we showed PA, DA and HA inhibited the inward peak currents (IACh) by acetylcholine in the expressed human α3β4 nACh receptor subfamily complimentary RNA in Xenopus oocytes with a two-electrode voltage clamp system (TEVC). TEVC has various advantages such as heterologous expression of ion channels for many biochemical studies.9,10) This study also showed that the effect of triterpenoids was mediated through non-competition with the ACh binding site and compared these results with the modulation induced by triterpenoids. Our study revealed that PA, DA and HA inhibited IACh in a voltage-independent, dose-dependent and reversible manner.
The triterpenoids and chemical compounds were dissolved by dimethyl sulfoxide (DMSO) and then stock solution was diluted with a buffer medium before using. The plasmid DNAs of human neuronal nACh receptor subtype α3 and β4 were obtained from OriGene. The DMSO was less than 0.01% in final treatment solution. The mecamylamine hydrochloride (≤100%) and acetylcholine chloride (≥99%) were purchased from Sigma and Aldrich. Triterpenoids (≥98%) were purchased from Wuhan ChemFaces Biochemical and was made into 250 mM stock in DMSO.
Preparation of X. laevis Oocytes and Mutagenesis of nACh ReceptorsThe handling of Xenopus laevis oocytes and microinjection were described in previous study.11) Briefly, frogs caring procedures were followed by the Chonnam National University animal caring institution guidelines (CNU IACUC-YB-2016-07, July 2016). The removed oocyte from X. laevis were collagenized with shaking for 2 h in Ringer solution. The matured oocytes were selected and incubated in ND96 containing: 96 mM NaCl, 1 mM MgCl2, 2 mM KCl, 1.8 mM CaCl2, and 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) at pH 7.5 with antibiotics. The introduction of complementary RNAs into the vegetal or animal pole of each single oocyte was carried out using a micro-injector (VWR Scientific, CA, U.S.A.). Two electrode voltage clamp experiments were carried out after 48 h for each of the RNA-injected oocytes. The α3 and β4 subunit mutants of nACh receptors were made by MAX-QuikChange site-directed mutagenesis protocol (Stratagene, CA, U.S.A.), along through turbo Pfu DNA polymerase and desired mutation primers.
Molecular Docking StudiesMolecular docking studies was carried out on Intel core i5, 2.20 GHZ PC with 8 GB RAM running the Windows 7 64 bit operating system, using Autodock Tools (version 1.5.6) by The Scripps Research Institute (La Jolla, CA, U.S.A.). The protein structure of α3β4 nACh receptors was obtained from the Protein Data Bank (ID code 5T90), and the three dimensional (3D) structure of the ligand (HA) was obtained from Pubchem.12) The protein–ligand complex was programmed using AutoDock Tools and considered with minimized binding energy, inhibition constant, and intermolecular energy. The complex was analyzed using Ligplot (ver. 4.5.3) by EMBL-EBI, and Pymol (ver. 1.8.4.2) by Schrödinger. Ligplot showed interactions between the protein and the ligand. Pymol was used to measure the distance between the complex and mutagenesis of amino acids of α3β4 nACh receptors.
Data RecordingOocyte was put in a perfusion chamber (Warner Instruments) and flowed with ND96 medium at 1 mL/min. Each single oocyte was then penetrated with two microelectrodes filled with electrolyte solution. The microelectrodes resistance was from 0.5 to 0.8 MΩ. The electrophysiological experiment was performed at room temperature with Oocyte Clamp Amplifier (OC-725C; Warner Instruments) and data acquisition were performed using Digidata 1320 and pClamp 9 (Molecular Devices, CA, U.S.A.). For this study, the holding potential was clamped at −80 mV in each oocyte. The ramp experiment of the current voltage relationship was shed from −90 to +60 mV for the α3β4 nACh channel receptors. The stock solution of 250 mM of triterpenoids and used chemical compounds were prepared with DMSO and then they were diluted to each low concentration for actual use with ND96 bath buffer.
Data AnalysisTo acquire dose dependent curves for the effects of triterpenoid on IACh, the induced peak currents at various concentrations of each triterpenoid were plotted using the Hill equation. Origin Pro 7.0 was used to apply the Hill equation, which is y/ymax=[A]n/([A]n+[IC50]n), where y is the peak amplitude at a given dose of triterpenoid, ymax is the induced peak current, IC50 is the dose of triterpenoid that produces a half maximal effect, [A] is the triterpenoid concentration, and n is the interaction coefficient. All other values were presented as the means±standard error of the mean (S.E.M.). The significance among the mean of the control and application values were determined using one-way ANOVA with Tukey tests of Origin pro 7.0 statistic software. Values with p<0.01 were considered to be statistically significant.
We evaluated the effects of triterpenoids on the acetylcholine induced inward current (IACh) using α3β4 nicotinic acetylcholine receptors expressed in Xenopus oocytes with a two-electrode voltage-clamp recording system. The application of acetylcholine (100 µM) to the recording buffer elicited a huge inward current in the expressed cells injected with the α3β4 nicotinic acetylcholine channel receptor subfamily, indicating that nicotinic acetylcholine channel receptor subtypes were systemically expressed in this recording experiment (Fig. 1). The addition of oocytes with single PA, DA or HA had any no regulatory effects on α3β4 nACh channel current at a −80 mV holding potential (data not shown). In contrast, combined application of the expressed cells with PA, DA or HA (each 100 µM) and 100 µM acetylcholine produced a significantly reduced peak IACh compared to inward peak currents in the presence of only acetylcholine (Fig. 1; n=12–15 from five different frogs). The regulatory suppression of peak IACh by PA, DA and HA was reversible. We verified the inhibitory effect with a representative non-competitive nACh channel receptor antagonist, mecamylamine (10 µM), on α3β4 nACh receptor channel-expressing oocytes. The inhibition of peak IACh was 64.5±5.5, 49.5±9.7, 80.4±6.5, and 91.5±3.4% by PA, DA, HA (each 100 µM) and 10 µM mecamylamine, respectively.
Concentration–response studies showed that co-application with acetylcholine and various concentrations of PA, DA and HA concentration-dependently inhibited IACh in oocytes expressing nicotinic type α3β4 acetylcholine channel receptors (Fig. 2). The IC50 values were 24.9, 37.7, 20.9, and 3.1 µM for PA, DA, HA and mecamylamine, respectively (n=10–15 from six different frogs). The Hill coefficient was 1.1±0.1, 1.2±0.3, 1.1±0.2, and 1.1±0.2 for PA, DA, HA and mecamylamine, respectively. These results indicate that PA, DA and HA regulate α3β4 nicotinic acetylcholine receptors in a concentration-dependent manner (Fig. 2).
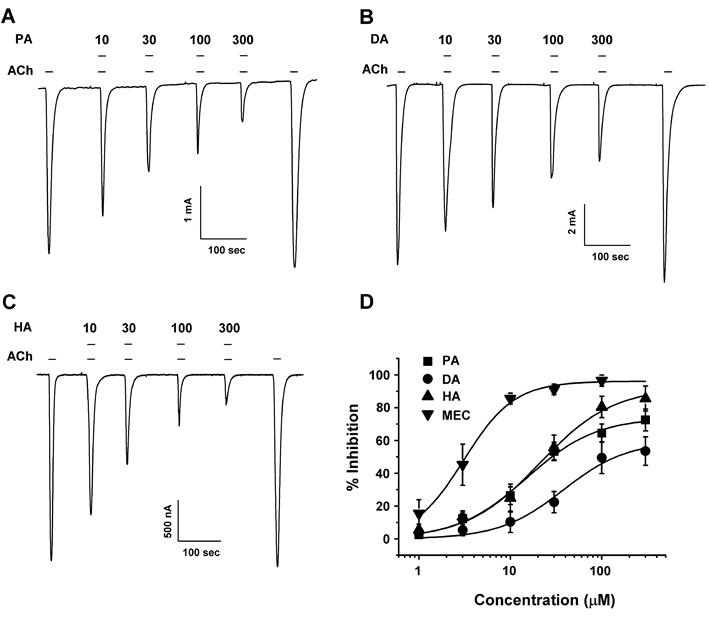
(A−C) Acetylcholine-mediated inward current in oocytes expressing human α3β4 nicotinic acetylcholine receptors was elicited at a holding potential of −80 mV for the indicated time in the presence of 100 µM acetylcholine, after which the indicated concentrations of triterpenoids (PA, DA and HA) were co-applied with acetylcholine. Traces are representative of eight separate oocytes from four different frogs. (D) Concentration response curves for the effect of triterpeonids on oocytes expressing the α3β4 nicotinic acetylcholine receptor. The percent inhibition of IACh by PA (■), DA (●), HA (▲) and mecamylamine (▼) were normalized based on the peak inward current induced by acetylcholine and that of the peak inward current elicited by acetylcholine plus triterpenoids or mecamylamine. Each represent point showed the mean±S.E.M. (n=10–15/group). Additional half inhibitory concentration, Hill coefficient, and Imax values are presented in Table 1.
| PA | DA | HA | MEC | |
|---|---|---|---|---|
| Imax | 74.0±3.2 | 58.5±4.1 | 92.3±5.6 | 96.1±2.4 |
| IC50 | 24.9±2.3 | 37.7±4.2 | 20.9±3.2 | 3.1±1.1 |
| nH | 1.1±0.1 | 1.2±0.3 | 1.1±0.2 | 1.1±0.2 |
Values represent the means ±S.E.M. (n=10−15/group). Currents were elicited at a holding potential of −80 mV. IC50, Hill’s coefficient, and Imax were determined as described in Materials and Methods.
To further investigate the mechanism by which PA, DA or HA inhibited IACh in cells that expressed nicotinic type α3β4 acetylcholine channel, we experimented the current–voltage relationship for ACh single treatment to evaluate the current elicited with treatment through acetylcholine+triterpenoids (PA, DA or HA). The current–voltage relationship for the eluted current induced by ACh with a voltage ramp from −90 to +60 mV showed superficial Ach induced-inward rectified currents at over 0 mV in cells expressing α3β4 nACh channel, as shown in Fig. 3A. The reverse potential was approximately 0 mV with both acetylcholine only treatment and with a combination of acetylcholine+triterpenoids (PA, DA or HA). The results indicate that acetylcholine elicited the peak current by cation influx through the channels, which was not interrupted by the application of triterpenoids. These investigations further revealed that the inhibition by PA, DA and HA on IACh in the cells expressing α3β4 nACh receptors was independent at the tested holding potentials (data not shown). At the membrane holding potentials tested, PA inhibited IACh by 68.5±6.5, 62.5±5.6, 65.6±8.2, and 57.2±9.2%, at −120, −90, −60, and −30 mV (n=8–13, from four different frogs), DA suppressed IACh by 55.5±3.5, 51.6±8.2, 55.2±4.5, and 52.7±8.6% (n=8–10, from four different frogs), and HA suppressed IACh by 88.2±5.1, 82.8±5.0, 80.2±9.2, and 83.2±12.5% respectively (n=8–13, from four different frogs).
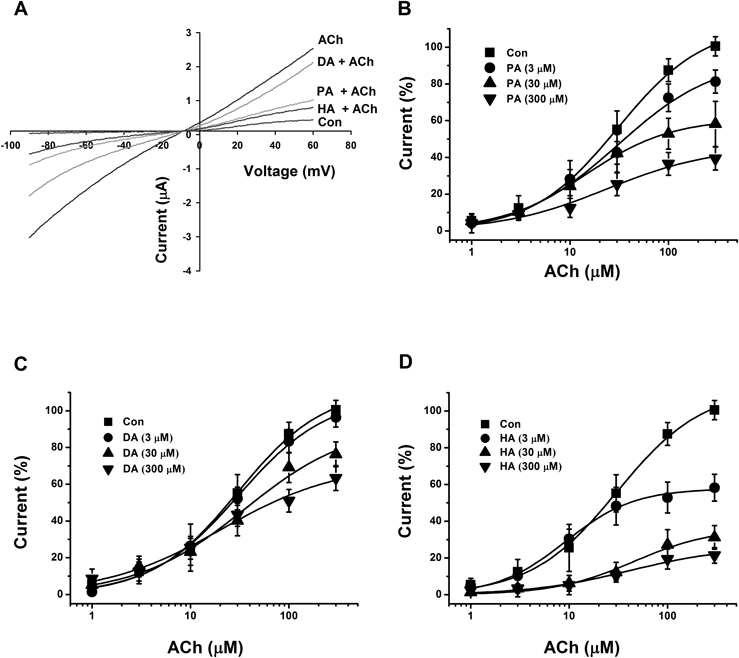
(A) The representative current–voltage dependency curves were gained using 1 s for voltage ramps from −90 to +60 mV at −80 mV holding potential. The voltage application was treated after application of 100 µM acetylcholine in the presence or absence of 100 µM PA, DA or HA. Each point represents the mean±S.E.M. (n=8–12/group). (C−D) Acetylcholine mediated–inward current induced by the indicated concentrations of acetylcholine in the absence (■) or presence of 3, 30, 300 µM PA (B), DA (C), and HA (D). Each oocyte was held at −80 mV and then treated acetylcholine with or without PA, DA or HA for 3 min to be exposed sufficiently. Each represent point was showed the mean±S.E.M. (n=9–12/group). The half efficient concentration, Vmax, and Hill coefficient values were described in Materials and Methods.
We evaluated the pharmacological mechanism through which PA, DA and HA suppress IACh in cells expressing α3β4 nACh receptors. The effects of PA, DA and HA (3, 30 or 300 µM) on the IACh evoked by various acetylcholine concentrations are shown in Fig. 3. Co-application of PA, DA or HA (30 µM) with different concentrations of acetylcholine did not significantly shift the dose–response curve of acetylcholine to the positive side (EC50 from 30.6±4.4 to 27.6±10.1, 30.7±5.2 and 27.4±5.3 µM, and Hill-coefficient from 0.9±0.1 to 0.8±0.2, 0.9±0.1 and 1.2±0.1 for PA, DA and HA, respectively). Thus, PA, DA and HA significantly modulated the currents elicited by 3, 30 or 300 µM of acetylcholine in a manner unrelated to the acetylcholine concentration (n=9–12 from four different frogs, Fig. 3).
To further examine the possible interaction mode between triterpenoids and the α3β4 nicotinic acetylcholine receptors, we employed the covalent docking homology modeling of wild-type and mutants (Fig. 4). It is noteworthy that the best-fit docking results showed that HA forms strong hydrogen bonds with wild-type but not with mutants (Fig. 5). The W25, R94, and V109 residues in α3 subunits and F106, Y107, and N109 residues in β4 subunits were designated as the active site residues, and the active radium was set as 5 Å from the active site residues. Molecular docking revealed that HA could fit into this pocket, interacting with previously unidentified residues: notably positively charged amino acids from α3β4 nACh receptors and hydroxyl group of HA. In Fig. 5C, HA interacted with six residues of this receptors, which each W25 (distance=3.6 Å), R94 (2.9 Å), and V109 (3.7 Å) residue of α3 subunit interacts with HA, which each F106 (5.2 Å), Y107 (3.8 Å) and N109 (3.9 Å) residue of β4 subunit interacts with HA, respectively. To confirm that the activity of each residue, we tested the ability of this compound to regulate the current of α3β4 nicotinic acetylcholine receptor mutants in which each residues was replaced by an alanine residue. The inhibitory effects of HA on each of mutant channels is shown in Fig. 6 and Table 2. The W25A of α3 subunit or N109A of β4 subunit mutant showed significant attenuation of inhibitory effects by HA, double mutation (W25A of α3 subunit and N109A of β4 subunit mutant) to alanine of those abolished the inhibitory activity of HA. These results indicate that HA-induced regulation of α3β4 nicotinic acetylcholine receptor channel activity is closely related to the W25 residue of α3 subunit and N109 residue of β4 subunit.
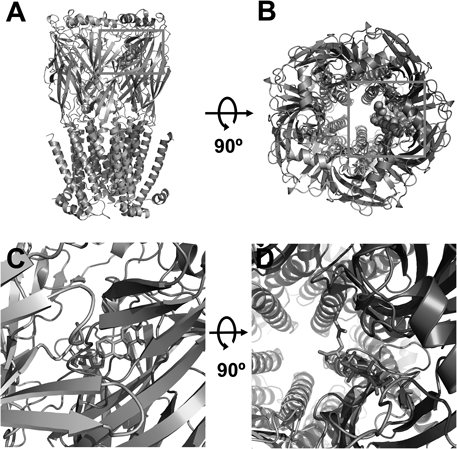
(A and C) Side views of the docked HA in complex with α3β4 nicotinic acetylcholine receptors and (B and D) top views of docking model.
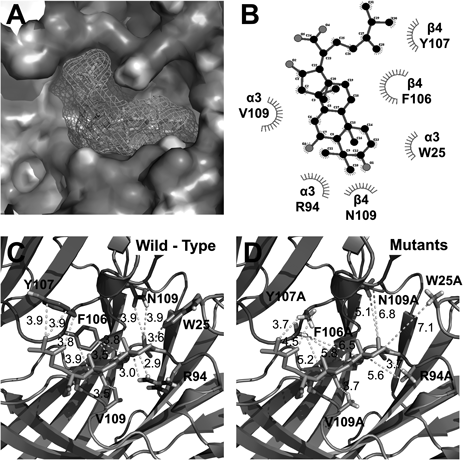
(A) HA located in binding pocket in extracellular area between segments 1 and 2 of α3β4 nicotinic acetylcholine receptors. (B) 2D schematic presentation of the predicted binding mode of HA in the ligand binding pocket. The ligands and important residues are shown. (C and D) Binding interface and HA of the wild type (C) and the four mutant channels, which mutations disturb the interaction of HA to varying degrees.
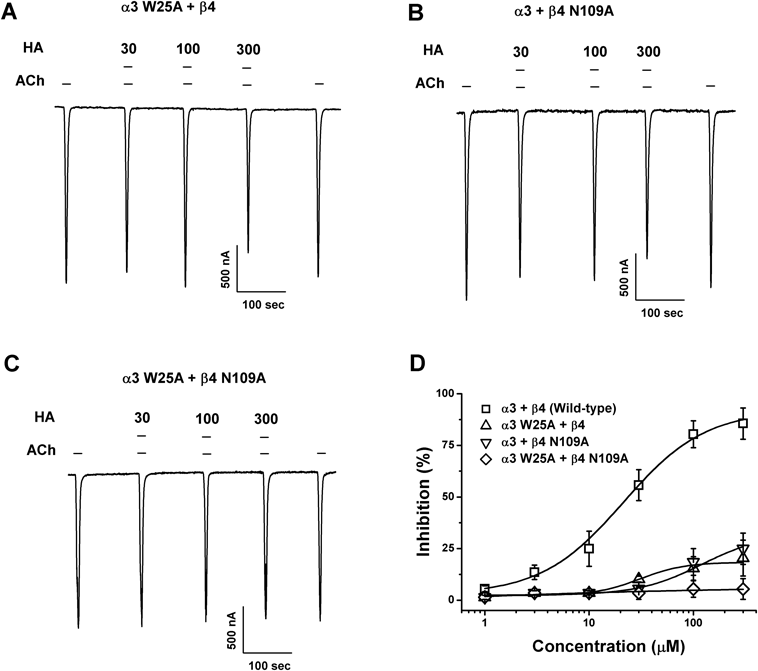
(A−C) Acetylcholine-mediated inward current in oocytes expressing human mutant α3β4 nicotinic acetylcholine receptors was elicited at a holding potential of −80 mV for the indicated time in the presence of 100 µM acetylcholine, after which the indicated concentrations of 6α-hydroxypolyporenic acid C (HA) were co-applied with acetylcholine. (D) Concentration response curves for the effect of HA on oocytes expressing mutants. The percent inhibition of IACh on each mutant were normalized based on the peak inward current induced by acetylcholine and that of the peak inward current elicited by acetylcholine plus 6α-hydroxypolyporenic acid C (HA). Each represent point showed the mean±S.E.M. (n=6–11/group). Additional half inhibitory concentration, Hill coefficient, and Imax values are described in Results.
| Subunit mutants | Imax | IC50 | nH |
|---|---|---|---|
| α3+β4 | 92.3±5.6 | 20.9±3.9 | 1.1±0.2 |
| α3 W25A+β4 | 18.4±6.3 | 30.7±13.9 | 1.6±0.5 |
| α3 R94A+β4 | 68.3±5.2 | 21.5±14.2 | 1.5±0.2 |
| α3 V109A+β4 | 55.2±3.2 | 23.5±10.9 | 1.2±0.4 |
| α3+β4 F106A | 45.2±5.6 | 26.3±9.9 | 1.3±0.5 |
| α3+β4 Y107A | 70.5±7.5 | 33.9±8.9 | 1.3±0.5 |
| α3+β4 N109A | 32.2±17.5 | 80.9±45.2 | 1.4±1.1 |
| α3 W25A+β4 N109A | 7.4±6.5 | 15.5±8.5 | 0.2±2.3 |
Values represent the means ±S.E.M. (n=6−11/group). Currents were elicited at a holding potential of −80 mV. IC50, Hill’s coefficient, and Imax were determined as described in Materials and Methods.
In this report, we demonstrated that (a) co-treatment of triterpenoids (pachymic acid, dehydroeburicoic acid, 6α-hydroxypolyporenic acid C) and acetylcholine inhibited IACh in oocytes expressing α3β4 nACh receptors in a reversible manner, (b) the inhibition of IACh by the triterpenoids was concentration-dependent, (c) the inhibition of IACh by PA, DA and HA showed a noncompetitive relationship and a voltage independent condition. We showed the modulation of PA, DA and HA on IACh in cells expressing α3β4 nACh receptors. One possible mechanism is that triterpenoids may play a role as open channel modulators for α3β4 nACh receptors. Actually, open channel modulators like anesthetics and hexamethonium are potent voltage dependent agents as they change the transmembrane mobility at their electrical fields and interact with voltage sensors or sensitive regions.13–15) According to our results, the inhibition of acetylcholine current by triterpenoids in the oocytes was not voltage dependent, suggesting that these triterpenoids may not be open channel modulators. The other hypothetical reasoning is that triterpenoids may act as competitive modulators by interrupting the attachment of agonists to their binding sites on α3β4 nACh receptors. In this study, competition experiments for the triterpenoids showed that the presence of PA, DA and HA did not affect the competitive requirements of acetylcholine in the oocytes expressing α3β4 nicotinic acetylcholine receptors (Fig. 4). Therefore, the results suggest that triterpenoids may play a role as noncompetitive modulators of α3β4 nACh receptors, which have important roles in various location-dependent regions.
In previous report, we reported the regulatory role of the triterpenoids on 5-HT3A receptors activity in Xenopus laevis oocytes1) and, we suggest the modulation of triterpenoids on the activity of the human nicotinic acetylcholine receptor type α3β4 in the present report. Graihe R et al. reported that the predominant intracellular location of α3β4 nACh receptors and the predominant expression of the 5-HT3A receptors in dendritic surface loci.16) They suggested that α3β4 nACh receptors remained intracellularly, like as waiting, for a signal that could trigger their transport to the cell surface, as is the case for α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)-selective ionotropic glutamate receptors in hippocampal neuron. Therefore, intracellular pool corresponded to receptors that were folded in a native conformation and then excitatory signal transduction occurs, this receptor is activated by its localization in the cell membrane. We report that PA, DA or HA modulate both currents of α3β4 nACh receptors and 5-HT3A receptors. Taken together these results, triterpenoids regulated the 5-HT3A receptors activity on resting state and then regulated the α3β4 nACh currents after excitatory signal transduction on hippocampal neuron. As well as, α3β4 nACh receptors are widely localized in the myenteric neurons in the intestine and mediate the excitation of cholinergic transmission.17) The α3β4 nACh receptors are densely expressed on adrenal chromaffin cells and play a tremendous role in catecholamine release in the peripheral nervous system.18) The core of the habenulo–interpeduncular routes has a higher density of α3β4 nACh receptors than many other regions in the central nervous system, and is connected with the afferent neuronal pathway of the interpeduncular nucleus. In summary, we demonstrated herein that PA, DA or HA modulate acetylcholine currents in oocytes expressing neuronal α3β4 nACh channel receptors. These results may suggest their potential as new noncompetitive antagonists against α3β4 nACh receptors.
This research was financially supported by the Ministry of Trade, Industry and Energy (MOTIE) and the Korea Institute for Advancement of Technology (KIAT) through the Research and Development for Regional Industry (R0004860).
The authors declare no conflict of interest.