2018 Volume 41 Issue 1 Pages 106-114
2018 Volume 41 Issue 1 Pages 106-114
To evaluate the capability of human-induced pluripotent stem cell-derived hepatocytes (h-iPS-HEP) in drug metabolism, the profiles of the metabolites of fentanyl, a powerful synthetic opioid, and acetylfentanyl, an N-acetyl analog of fentanyl, in the cells were determined and analyzed. Commercially available h-iPS-HEP were incubated with fentanyl or acetylfentanyl for 24 or 48 h. After enzymatic hydrolysis, the medium was deproteinized with acetonitrile, then analyzed by LC/MS. Desphenethylated metabolites and some hydroxylated metabolites, including 4′-hydroxy-fentanyl and β-hydroxy-fentanyl, were detected as metabolites of fentanyl and acetylfentanyl in the medium. The main metabolite of fentanyl with h-iPS-HEP was the desphenethylated metabolite, which was in agreement with in vivo results. These results suggest that h-iPS-HEP may be useful as a tool for investigating drug metabolism.
Human primary hepatocytes (h-PRM-HEP) are widely used for drug metabolism studies because they are highly active in phase I and phase II drug metabolism.1) h-PRM-HEP are regarded as one of the best tools for the investigation of the metabolism of drugs; however, there are problems with their use, including maintaining a stable supply of cells of a consistent quality and occasional significant drops in cell viability resulting from the fragility of the cells.2)
Induced pluripotent stem (iPS) cells, first developed in 2006, can differentiate into many different types of cells, including neurons, cardiomyocytes, and hepatocytes.3) Human iPS cell-derived hepatocytes (h-iPS-HEP) reportedly have catalytic activity from various CYP enzymes, in addition to liver-specific functions, such as albumin secretion and ammonia metabolism.4) Furthermore, h-iPS-HEP are robust and can be supplied stably, making these cells a potentially useful tool for drug metabolism studies.
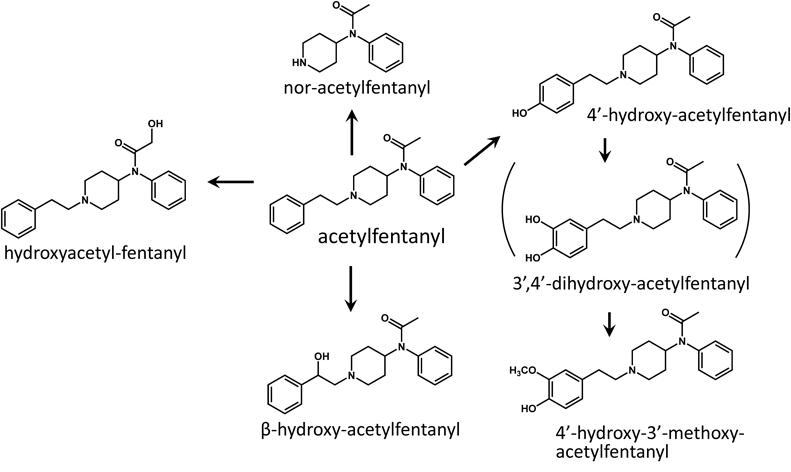
In this study, we investigated the ability of h-iPS-HEP to metabolize drugs using fentanyl and acetylfentanyl (Fig. 1) as model drugs. Fentanyl is a powerful synthetic opioid, which is used as an analgesic in patients with cancer.5) Acetylfentanyl is an N-acetyl analog of fentanyl, which is used as a substitute for recreational opioid drugs, such as heroin.6) Fentanyl is known to be metabolized by desphenethylation, 4′-hydroxylation, and hydroxylation of the N-propionyl group.7) Acetylfentanyl is metabolized in a similar manner to fentanyl.8) We evaluated how accurately the in vivo patterns of fentanyl and acetylfentanyl metabolism can be reproduced using h-iPS-HEP.

Authentic standards of fentanyl, acetylfentanyl, and their metabolites were synthesized in our laboratory. cis-3-Methylfentanyl was synthesized in our laboratory by the method reported previously.9) β-Glucuronidase/aryl sulfatase (from Helix pomatia; β-glucuronidase, 32 units/mL; aryl sulfatase, 102 units/mL) was purchased from Merck (Darmstadt, Germany). h-iPS-HEP (Cellartis™ enhanced h-iPS-HEP, differentiated from ChiPSC18 and ChiPSC22), Cellartis HEP Coat, and Cellartis HEP supplement were purchased from TaKaRa Bio Inc. (Kusatsu, Japan). h-iPS-HEP (ReproHepato™), ReproHepato culture medium, and ReproHepato assay medium were purchased from ReproCELL Inc. (Yokohama, Japan). h-PRM-HEP and KLC-SuM medium were purchased from Kurabo Industries (Osaka, Japan). InVitroGRO CP medium and InVitroGRO HT medium were purchased from BioreclamationIVT (New York, NY, U.S.A.). Y-27632 was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Matrigel™ was purchased from Corning (Corning, NY, U.S.A.). Phosphate-buffered saline (PBS), Williams Medium E and Leibovitz’s L-15 medium were purchased from Thermo Fisher Scientific (Waltham, MA, U.S.A.). All other reagents used were of analytical grade.
Synthesis of Authentic Standards of Drugs and MetabolitesAll synthesized standards were confirmed by positive electrospray ionization (ESI) mass spectrometry and 1H-NMR. ESI mass spectra were obtained from a LCQ FLEET ion trap mass spectrometer (Thermo Fisher Scientific). 1H-NMR spectra were measured on a JNM-ECA600 NMR spectrometer (JEOL, Akishima, Japan). Tetramethylsilane was used as an internal standard.
N-Phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]propanamide (Fentanyl)Fentanyl was synthesized according to the method of Siegfried.10) Briefly, 1-(2-phenylethyl)-4-piperidone and aniline were condensed in the presence of 3 Å molecular sieves. The product was reduced by sodium borohydride to give N-phenyl-1-(2-phenylethyl)-4-piperidinamine (despropionyl-fentanyl, 0.897 g, 65% yield from 1 g of 1-(2-phenylethyl)-4-piperidone), and this was then propionylated with propionyl chloride to give fentanyl. The free base of fentanyl was converted to the hydrochloride salt using hydrochloric acid (84 mg, 63% yield from 100 mg of despropionyl-fentanyl). Acetylfentanyl (N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]acetamide) hydrochloride was synthesized by the same method (90 mg, 70% yield from 100 mg of despropionyl-fentanyl).
Fentanyl (free base)—1H-NMR (CDCl3) δ: 1.02 (3H, t, J=7.3 Hz), 1.96 (2H, q, J=7.3 Hz), 1.97–2.02 (2H, m), 2.18 (2H, qd, J=3.3 Hz, 13.0 Hz), 2.80 (2H, qd, J=2.2 Hz, 11.6 Hz), 3.08–3.13 (2H, m), 3.19–3.23 (2H, m), 3.60 (2H, br d, J=10.8 Hz), 4.79 (1H, tt, J=3.8 Hz, 12.3 Hz), 7.08–7.12 (2H, m), 7.20–7.32 (5H, m), 7.40–7.49 (3H, m).
Acetylfentanyl (free base)—1H-NMR (CDCl3) δ: 1.78 (3H, s), 1.98–2.02 (2H, m), 2.20 (2H, qd, J=3.3 Hz, 13.1 Hz), 2.80 (2H, qd, J=2.1 Hz, 11.7 Hz), 3.07–3.13 (2H, m), 3.19–3.23 (2H, m), 3.60 (2H, br d, J=10.8 Hz), 4.78 (1H, tt, J=3.5 Hz, 12.2 Hz), 7.08–7.12 (2H, m), 7.20–7.33 (5H, m), 7.40–7.49 (3H, m).
N-Phenyl-N-(4-piperidinyl)propanamide (Nor-fentanyl)1-Benzyl-4-piperidone and aniline were condensed in the presence of 3 Å molecular sieves, followed by reduction with sodium borohydride and propionylation with propionyl chloride. Debenzylation of the product by catalytic hydrogenation under acidic conditions (hydrochloric acid in methanol) using 5% palladium on carbon as a catalyst gave nor-fentanyl hydrochloride (352 mg, 70% yield from 356 mg of 1-benzyl-4-piperidone). Nor-acetylfentanyl (N-phenyl-N-(4-piperidinyl)acetamide) hydrochloride was synthesized by the same method (286 mg, 69% yield from 309 mg of 1-benzyl-4-piperidone).
Nor-fentanyl hydrochloride—1H-NMR (CD3OD) δ: 0.99 (3H, t, J=7.2 Hz), 1.59 (2H, qd, J=4.2 Hz, 13.0 Hz), 1.98 (2H, q, J=7.2 Hz), 2.08 (2H, br d, J=13.8 Hz), 3.11 (2H, td, J=2.6 Hz, 13.4 Hz), 3.36–3.42 (2H, m), 4.77 (1H, tt, J=3.6 Hz, 12.6 Hz), 7.23–7.27 (2H, m), 7.46–7.54 (3H, m).
Nor-acetylfentanyl hydrochloride—1H-NMR (CD3OD) δ: 1.60 (2H, qd, J=4.0 Hz, 13.0 Hz), 1.76 (3H, s), 2.09 (2H, br d, J=13.8 Hz), 3.10 (2H, td, J=3.0 Hz, 13.5 Hz), 3.36–3.43 (2H, m), 4.77 (1H, tt, J=3.6 Hz, 12.6 Hz), 7.25–7.30 (2H, m), 7.47–7.56 (3H, m).
N-Phenyl-N-[1-[2-(4-hydroxyphenyl)ethyl]-4-piperidinyl]propanamide (4′-Hydroxy-fentanyl)4-Benzyloxyphenylacetic acid was reduced by lithium aluminum hydride to give 4-benzyloxyphenylethyl alcohol. 4-Benzyloxyphenylethyl alcohol was treated with methanesulfonyl chloride to convert the alcohol to the methansulfonic acid ester.11) Condensation of the ester with nor-fentanyl,12) followed by debenzylation gave 4′-hydroxy-fentanyl hydrochloride (34 mg, 59% yield from 40 mg of nor-fentanyl hydrochloride). 4′-Hydroxy-acetylfentanyl (N-phenyl-N-[1-[2-(4-hydroxyphenyl)ethyl]-4-piperidinyl]acetamide) hydrochloride (24 mg, 41% yield from 40 mg of nor-acetylfentanyl hydrochloride), 4′-hydroxy-3′-methoxy-fentanyl (N-phenyl-N-[1-[2-(4-hydroxy-3-methoxyphenyl)ethyl]-4-piperidinyl]propanamide) hydrochloride (26 mg, 42% yield from 40 mg of nor-fentanyl hydrochloride), and 4′-hydroxy-3′-methoxy-acetylfentanyl (N-phenyl-N-[1-[2-(4-hydroxy-3-methoxyphenyl)ethyl]-4-piperidinyl]acetamide) hydrochloride (28 mg, 44% yield from 40 mg of nor-acetylfentanyl hydrochloride) were synthesized by the same method.
4′-Hydroxy-fentanyl (free base)—1H-NMR (CDCl3) δ: 1.01 (3H, t, J=7.1 Hz), 1.96 (2H, q, J=7.1 Hz), 1.98–2.05 (2H, m), 2.11–2.21 (2H, m), 2.74–2.84 (2H, m), 3.01–3.16 (4H, m), 3.55–3.64 (2H, m), 4.74–4.84 (1H, m), 6.78 (2H, d, J=8.7 Hz), 7.03 (2H, d, J=8.7 Hz), 7.09 (2H, d, J=7.2 Hz), 7.40–7.50 (3H, m).
4′-Hydroxy-acetylfentanyl (free base)—1H-NMR (CDCl3) δ: 1.78 (3H, s), 1.97–2.06 (2H, m), 2.13–2.22 (2H, m), 2.74–2.84 (2H, m), 3.00–3.15 (4H, m), 3.55–3.63 (2H, m), 4.74–4.83 (1H, m), 6.79 (2H, d, J=7.8 Hz), 7.02 (2H, d, J=7.8 Hz), 7.10 (2H, d, J=6.6 Hz), 7.40–7.49 (3H, m).
4′-Hydroxy-3′-methoxy-fentanyl (free base)—1H-NMR (CDCl3) δ: 1.02 (3H, t, J=7.5 Hz), 1.96 (2H, q, J=7.5 Hz), 1.98–2.05 (2H, m), 2.18 (2H, qd, J=3.7 Hz, 13.0 Hz), 2.75–2.85 (2H, m), 3.03–3.17 (4H, m), 3.59 (2H, br d, J=12.0 Hz), 3.87 (3H, s), 4.79 (1H, tt, J=3.5 Hz, 12.2 Hz), 6.66 (1H, dd, J=1.5 Hz, 7.8 Hz), 6.77 (1H, d, J=1.5 Hz), 6.83 (1H, d, J=7.8 Hz), 7.09–7.13 (2H, m), 7.40–7.50 (3H, m).
4′-Hydroxy-3′-methoxy-acetylfentanyl (free base)—1H-NMR (CDCl3) δ: 1.78 (3H, s), 1.98–2.06 (2H, m), 2.19 (2H, qd, J=3.5 Hz, 13.1 Hz), 2.75–2.84 (2H, m), 3.03–3.17 (4H, m), 3.59 (2H, br d, J=10.8 Hz), 3.87 (3H, s), 4.78 (1H, tt, J=3.4 Hz, 12.2 Hz), 6.66 (1H, dd, J=1.8 Hz, 8.1 Hz), 6.77 (1H, d, J=1.8 Hz), 6.83 (1H, d, J=8.1 Hz), 7.09–7.12 (2H, m), 7.40–7.50 (3H, m).
N-Phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]-3-hydroxypropanamide (ω-Hydroxy-fentanyl)To a solution of 96 mg of 3-benzyloxypropionic acid in 1 mL of toluene, 2 drops of N,N-dimethylformamide and 129 mg of thionyl chloride were added and the mixture was heated at 90°C for 1.5 h. The reaction mixture was added dropwise to a solution of 30 mg of despropionyl-fentanyl in 1 mL of pyridine. After the addition, water was added to the reaction mixture, the mixture was acidified with 3 M hydrochloric acid, then extracted with chloroform. The extract was purified by flash chromatography (column, silica gel; solvent, n-hexane/ethyl acetate). Debenzylation of the product by catalytic hydrogenation under acidic conditions using 5% palladium on carbon as a catalyst gave 25 mg of ω-hydroxy-fentanyl hydrochloride (60% yield from despropionyl-fentanyl).
ω-Hydroxy-fentanyl (free base)—1H-NMR (CDCl3) δ: 1.98–2.03 (2H, m), 2.18 (2H, t, J=5.4 Hz), 2.23 (2H, qd, J=3.5 Hz, 13.3 Hz), 2.75–2.84 (2H, m), 3.08–3.14 (2H, m), 3.19–3.24 (2H, m), 3.61 (2H, br d, J=11.4 Hz), 3.74 (2H, t, J=5.4 Hz), 4.78 (1H, tt, J=3.4 Hz, 12.2 Hz), 7.09–7.14 (2H, m), 7.20–7.33 (5H, m), 7.42–7.50 (3H, m).
N-Phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]-2-hydroxypropanamide ((ω-1)-Hydroxy-fentanyl)To a solution of 50 mg of despropionyl-fentanyl in 2 mL of dichloromethane, 40 µL of triethylamine, and 40.3 mg of 2-acetoxypropionyl chloride were added and stirred for 2 h at room temperature. To the reaction mixture, 20 mL of 0.1 M hydrochloric acid was added, and then extracted with chloroform. The solvent was evaporated to dryness under vacuum and the residue was treated with a small amount (ca. 15 drops) of 1 M sodium hydroxide in methanol for 5 min to remove the acetyl group. The solution was neutralized with 1 M acetic acid in methanol, then water was added and the solution was basified with 28% aqueous ammonia, then extracted with chloroform. The residue was recrystallized from methanol to give 41 mg of (ω-1)-hydroxy-fentanyl (64% yield from despropionyl-fentanyl). Hydroxyacetyl-fentanyl (N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]-2-hydroxyacetamide) (free base) was synthesized by the same method (16 mg, 27% yield from 50 mg of despropionyl-fentanyl).
(ω-1)-Hydroxy-fentanyl (free base)—1H-NMR (CDCl3) δ: 1.09 (3H, d, J=6.5 Hz), 1.87–1.93 (1H, m), 2.09–2.14 (1H, m), 2.18 (1H, qd, J=3.9 Hz, 13.3 Hz), 2.36 (1H, qd, J=4.2 Hz, 13.2 Hz), 2.75–2.85 (2H, m), 3.08–3.14 (2H, m), 3.19–3.25 (2H, m), 3.57–3.66 (2H, m), 4.75 (1H, tt, J=3.7 Hz, 12.2 Hz), 7.11–7.33 (7H, m), 7.45–7.53 (3H, m).
Hydroxyacetyl-fentanyl (free base)—1H-NMR (CDCl3) δ: 2.04 (2H, br d, J=13.8 Hz), 2.28 (2H, qd, J=3.6 Hz, 12.9 Hz), 2.77–2.86 (2H, m), 3.09–3.14 (2H, m), 3.20–3.24 (2H, m), 3.60–3.64 (2H, m), 3.70 (2H, s), 4.76 (1H, tt, J=3.4 Hz, 12.3 Hz), 7.10–7.14 (2H, m), 7.20–7.33 (5H, m), 7.47–7.52 (3H, m).
N-Phenyl-N-[1-(2-hydroxy-2-phenylethyl)-4-piperidinyl]propanamide (β-Hydroxy-fentanyl)A mixture of 40 mg of nor-fentanyl hydrochloride, 28 mg of phenacyl chloride, and 15 mg of sodium bicarbonate in 1.2 mL of water–2-butanone (1 : 5) was heated at 90°C for 6 h. Water was added to the reaction mixture, and the mixture was basified with 28% aqueous ammonia, then extracted with ethyl acetate. The extract was purified by flash chromatography (column, silica gel; solvent, chloroform/ethyl acetate). The purified product, β-hydroxy-fentanyl was converted to the hydrochloride salt (18 mg, 31% yield from 40 mg of nor-fentanyl hydrochloride). β-Hydroxy-acetylfentanyl (N-phenyl-N-[1-(2-hydroxy-2-phenylethyl)-4-piperidinyl]acetamide) hydrochloride was synthesized by the same method (34 mg, 58% yield from 40 mg of nor-acetylfentanyl hydrochloride).
β-Hydroxy-fentanyl (free base)—1H-NMR (CDCl3) δ: 1.02 (3H, t, J=7.3 Hz), 1.97 (2H, q, J=7.3 Hz), 1.98–2.02 (1H, m), 2.04–2.10 (1H, m), 2.19 (1H, qd, J=3.7 Hz, 13.0 Hz), 2.27 (1H, qd, J=3.9 Hz, 13.1 Hz), 2.88–2.98 (2H, m), 2.98–3.04 (1H, m), 3.16–3.23 (1H, m), 3.79–3.86 (2H, br), 4.83 (1H, tt, J=3.8 Hz, 12.4 Hz), 5.34 (1H, dd, J=2.4 Hz, 10.2 Hz), 7.02–7.13 (2H, br), 7.28–7.33 (1H, m), 7.33–7.38 (4H, m), 7.41–7.50 (3H, m).
β-Hydroxy-acetylfentanyl (free base)—1H-NMR (CDCl3) δ: 1.79 (3H, s), 1.97–2.03 (1H, m), 2.05–2.10 (1H, m), 2.21 (1H, qd, J=3.9 Hz, 13.1 Hz), 2.29 (1H, qd, J=3.7 Hz, 13.3 Hz), 2.88–2.98 (2H, m), 2.98–3.04 (1H, m), 3.16–3.22 (1H, m), 3.79–3.86 (2H, br), 4.82 (1H, tt, J=3.5 Hz, 12.3 Hz), 5.35 (1H, dd, J=1.8 Hz, 10.2 Hz), 7.10–7.14 (2H, br), 7.28–7.33 (1H, m), 7.33–7.38 (4H, m), 7.41–7.50 (3H, m).
Incubation of Drugs with h-iPS-HEP (Cellartis™)Cellartis enhanced h-iPS-HEP (≥1.23×107 viable cells/vial, from ChiPSC18 or ChiPSC22) were thawed in 40 mL of warm InVitroGRO HT medium that contained 5 µM of Y-27632. After centrifugation at 100×g for 2 min, the supernatant was removed, then the cells were resuspended in 15 mL of warm InVitroGRO CP medium that contained 5 µM of Y-27632. A 1 mL portion of the cell suspension was dispensed into each well of a 24-well microplate coated with Cellartis HEP Coat and incubated at 37°C and 5% CO2. After 1, 3, and 5 d from the start of incubation, the medium was changed to 0.5 mL of warm fresh Williams Medium E that contained 2% Cellartis HEP supplement solution and 0.5% dimethyl sulfoxide. After changing the medium at day 5, the drug (fentanyl hydrochloride or acetylfentanyl hydrochloride dissolved in PBS) was added to each well of the plate at a final concentration of 10 µM, and incubated for 24 or 48 h. The medium was collected and stored at −30°C until analysis.
Incubation of Drugs with h-iPS-HEP (ReproHepato™)ReproHepato h-iPS-HEP (8.2×106 cells/vial) was thawed in 49 mL of warm Leibovitz’s L-15 medium. After centrifugation at 350×g for 5 min, the supernatant was removed, then the cells were resuspended in 11 mL of warm ReproHepato culture medium. A 640 µL portion of the cell suspension was dispensed into each well of a 24-well microplate coated with Matrigel, and incubated at 37°C and 5% CO2. After 1, 3, and 5 d from the start of incubation, the medium was changed to 0.5 mL of warm fresh ReproHepato culture medium. After 6 d from the start of incubation, the medium was changed to 0.5 mL of warm fresh ReproHepato assay medium, then the drug (fentanyl hydrochloride or acetylfentanyl hydrochloride dissolved in PBS) was added to each well of the plate at a final concentration of 10 µM, and incubated for 24 or 48 h. The medium was collected and stored at −30°C until analysis.
Incubation of Drugs with h-PRM-HEPh-PRM-HEP (2×106 viable cells/vial) was thawed in 20 mL of warm Leibovitz’s L-15 medium. After centrifugation at 170×g for 2 min, the supernatant was removed, then the cells were resuspended in 2 mL of warm KLC-SuM medium and the cell viability was measured by the trypan blue dye exclusion method. The cell suspension was diluted to 5×105 viable cells/mL with KLC-SuM medium and 0.5 mL of the cell suspension was dispensed into each well of a 24-well microplate. The drug (fentanyl hydrochloride or acetylfentanyl hydrochloride dissolved in PBS) was added to each well of the plate at a final concentration of 10 µM, and incubated for 3 h with shaking (90 rpm) at 37°C and 5% CO2. The medium was collected and stored at −30°C until analysis.
Identification of the MetabolitesTo a 25 µL sample of the medium, 15 µL of 0.25 M acetate buffer (pH 5.0) containing β-glucuronidase/aryl sulfatase (β-glucuronidase, 0.01 unit) was added and then incubated at 60°C for 1.5 h. To the reaction mixture, 250 µL of acetonitrile was added and the mixture was vortexed for 5 s. After centrifugation at 10000×g for 5 min, the supernatant was evaporated to dryness under a nitrogen stream. The residue was reconstituted with 50 µL of the initial mobile phase and centrifuged at 10000×g for 5 min, then the supernatant (10 µL) was analyzed by LC-ion trap MS under scan and product ion analysis modes. The conditions of analysis were as follows: apparatus, Accela LC system connected to LCQ FLEET ion trap mass spectrometer (Thermo Fisher Scientific); column, CORTECS C18 (2.1×50 mm, 2.7 µm, Waters, Milford, MA, U.S.A.) maintained at 40°C; mobile phase composition, 0.1% formic acid (A) and methanol (B); linear gradient mode, 20% B for 1 min, 20% to 80% B over 8 min, 80% B for 2 min, and 80% to 20% B over 0.1 min; flow rate, 0.2 mL/min; MS interface, positive ESI; analysis mode, scan (m/z 100–500) and product ion analysis (normalized collision energy, 35%; precursor ions, protonated molecules of drugs and putative metabolites).
Quantitation of the MetabolitesTo a 25 µL sample of the medium, 15 µL of 0.25 M acetate buffer (pH 5.0) containing β-glucuronidase/aryl sulfatase (β-glucuronidase, 0.01 unit) was added and then incubated at 60°C for 1.5 h. To the reaction mixture, 10 µL of internal standard solution (cis-3-methylfentanyl hydrochloride, 50 ng/10 µL water) was added, then 250 µL of acetonitrile was added and the mixture was vortexed for 5 s. After centrifugation at 10000×g for 5 min, 50 µL of the supernatant was mixed with 200 µL of 0.1% formic acid. This was centrifuged at 10000×g for 5 min, then the supernatant (10 µL) was analyzed by LC-triple quadrupole MS. The conditions of analysis were as follows: apparatus, NANOSPACE SI-2 LC system (Shiseido, Tokyo, Japan) connected to TSQ Quantum triple quadrupole mass spectrometer (Thermo Fisher Scientific); column, mobile phase composition, flow rate, and MS interface were the same as for identification of the metabolites; analysis mode, selected reaction monitoring (SRM). SRM parameters are listed in Table 1.
| Compound | Monitoring ion (m/z) | Collision energy (eV) | Calibration curve (µM) | |
|---|---|---|---|---|
| Precursor ([M+H]+) | Product | |||
| Fentanyl | 337.1 | 188.2 | 21 | 0.021–11 |
| Nor-fentanyl | 233.1 | 84.2 | 17 | 0.030–15 |
| ω-Hydroxy-fentanyl | 353.1 | 188.2 | 21 | 0.021–5.1 |
| (ω-1)-Hydroxy-fentanyl | 353.1 | 188.2 | 20 | 0.023–5.7 |
| 4′-Hydroxy-fentanyl | 353.1 | 121.1 | 32 | 0.021–5.1 |
| β-Hydroxy-fentanyl | 353.1 | 204.2 | 19 | 0.021–5.1 |
| 4′-Hydroxy-3′-methoxy-fentanyl | 383.1 | 151.2 | 30 | 0.019–4.8 |
| Acetylfentanyl | 323.1 | 188.2 | 20 | 0.022–11 |
| Nor-acetylfentanyl | 219.1 | 84.2 | 18 | 0.031–16 |
| Hydroxyacetyl-fentanyl | 339.1 | 188.2 | 20 | 0.024–5.9 |
| 4′-Hydroxy-acetylfentanyl | 339.1 | 121.1 | 31 | 0.021–5.3 |
| β-Hydroxy-acetylfentanyl | 339.1 | 321.3 | 16 | 0.021–5.3 |
| 4′-Hydroxy-3′-methoxy-acetylfentanyl | 369.1 | 151.2 | 28 | 0.020–4.9 |
| cis-3-Methylfentanyl (Internal standard) | 351.1 | 202.2 | 24 | — |
Authentic standards of fentanyl, acetylfentanyl, and their metabolites were added to the InVitroGRO CP medium and processed as described above to obtain the calibration curves. Excellent linearity was obtained over the concentration range 0.008–4 µg/mL (e.g. 0.021–11 µM for fentanyl hydrochloride, Table 1) for fentanyl, acetylfentanyl, nor-fentanyl, and nor-acetylfentanyl and 0.008–2 µg/mL for the other compounds, with a correlation coefficient of 0.99.
The culture media obtained from h-iPS-HEP and h-PRM-HEP were treated with conjugate-hydrolyzing enzymes, then deproteinated with acetonitrile, and analyzed by LC-ion trap MS to identify the metabolites. The total ion chromatograms (TIC) and extracted ion chromatograms (EIC) obtained from the deproteinized media of h-iPS-HEP (Cellartis, from ChiPSC22) and h-PRM-HEP incubated with fentanyl are shown in Fig. 2. Peaks corresponding to the desphenethylated metabolite (nor-fentanyl, F-2), ω-hydroxy-fentanyl (F-3), (ω-1)-hydroxy-fentanyl (F-4), 4′-hydroxy-fentanyl (F-5), and β-hydroxy-fentanyl (F-6) were detected, as the metabolites of fentanyl, in the chromatograms obtained from h-iPS-HEP (a) and h-PRM-HEP (b). The peak from 4′-hydroxy-3′-methoxy-fentanyl (F-7) was detected in the chromatogram from h-PRM-HEP (b), but not in the chromatogram from h-iPS-HEP (a). The product ion spectra of the peaks F-1–F-7 are shown in Fig. 3. All metabolites were identified by comparison of the retention times and mass spectra with authentic standards. The proposed metabolic pathways for fentanyl are shown in Fig. 4. β-Hydroxy-fentanyl and 4′-hydroxy-3′-methoxy-fentanyl were the first to be identified as metabolites of fentanyl in the present study. 4′-Hydroxy-3′-methoxy-fentanyl is thought to be formed via methylation of the intermediate metabolite, 3′,4′-dihydroxy-fentanyl. 4′-Hydroxy-3′-methoxy-fentanyl was not formed in any h-iPS-HEP, indicating that the activity of the enzyme involved in the methylation (i.e., catechol-O-methyltransferase, COMT) was negligible in h-iPS-HEP.

(a), Human iPS cell-derived hepatocytes (Cellartis h-iPS-HEP, from ChiPSC22); (b), Human primary hepatocytes (h-PRM-HEP). F-1, fentanyl; F-2, nor-fentanyl; F-3, ω-hydroxy-fentanyl; F-4, (ω-1)-hydroxy-fentanyl; F-5, 4′-hydroxy-fentanyl; F-6, β-hydroxy-fentanyl; F-7, 4′-hydroxy-3′-methoxy-fentanyl.

The protonated molecule of each compound was selected as the precursor ion. F-1, fentanyl; F-2, nor-fentanyl; F-3, ω-hydroxy-fentanyl; F-4, (ω-1)-hydroxy-fentanyl; F-5, 4′-hydroxy-fentanyl; F-6, β-hydroxy-fentanyl; F-7, 4′-hydroxy-3′-methoxy-fentanyl.

The TICs and EICs obtained from h-iPS-HEP (Cellartis, from ChiPSC22) and h-PRM-HEP incubated with acetylfentanyl are shown in Fig. 5. The peaks corresponding to the desphenethylated metabolite (nor-acetylfentanyl, AF-2), 4′-hydroxy-acetylfentanyl (AF-3), hydroxyacetyl-fentanyl (AF-4), and β-hydroxy-acetylfentanyl (AF-5) were detected in the chromatograms obtained from h-iPS-HEP (a) and h-PRM-HEP (b). As for fentanyl, the 4′-hydroxy-3′-methoxy metabolite (4′-hydroxy-3′-methoxy-acetylfentanyl, AF-6) was detected in the medium of the h-PRM-HEP, but not in the medium of the h-iPS-HEP. The product ion spectra of the peaks AF-1–AF-6 are shown in Fig. 6. The proposed metabolic pathways for acetylfentanyl are shown in Fig. 7. Melent’ev et al. have reported that acetylfentanyl was metabolized by desphenethylation, deacetylation, and hydroxylation of the phenylethyl moiety followed by further hydroxylation and methylation of one of the hydroxyl groups.8) However, this group did not confirm the exact chemical structure of the metabolites. In this study, we confirm the exact structure of the metabolites of acetylfentanyl for the first time. β-Hydroxy-acetylfentanyl and hydroxyacetyl-fentanyl are novel metabolites of acetylfentanyl. In previous reports, the N-desacyl metabolite (despropionyl-fentanyl) was found to be formed from both fentanyl and acetylfentanyl8,13); however, this desacyl metabolite was not detected in any cell culture systems in the present study.

(a), Cellartis h-iPS-HEP (from ChiPSC22); (b), h-PRM-HEP; AF-1, acetylfentanyl; AF-2, nor-acetylfentanyl; AF-3, 4′-hydroxy-acetylfentanyl; AF-4, hydroxyacetyl-fentanyl; AF-5, β-hydroxy-acetylfentanyl; AF-6, 4′-hydroxy-3′-methoxy-acetylfentanyl.

The protonated molecule of each compound was selected as the precursor ion. AF-1, acetylfentanyl; AF-2, nor-acetylfentanyl; AF-3, 4′-hydroxy-acetylfentanyl; AF-4, hydroxyacetyl-fentanyl; AF-5, β-hydroxy-acetylfentanyl; AF-6, 4′-hydroxy-3′-methoxy-acetylfentanyl.
The deproteinated culture media were analyzed by LC-triple quadrupole MS under SRM mode to quantitate the metabolites. The time–courses of the formation of the main metabolites of fentanyl in Cellartis h-iPS-HEP (ChiPSC18) are shown in Fig. 8. The amounts of all of the metabolites of fentanyl increased with the incubation time. Similar results were obtained for the other h-iPS-HEPs. The metabolite profiles of fentanyl in h-iPS-HEP (48 h incubation) and h-PRM-HEP (3 h incubation) are shown in Fig. 9. The majority of fentanyl (60–85%) remained unmetabolized after incubation with the hepatocytes. The main metabolite of fentanyl formed by h-iPS-HEP was nor-fentanyl, and the amounts ranged from 3.3–14.5% of the initial amount of fentanyl. This metabolite was also the main metabolite formed by h-PRM-HEP (13.8% of the initial amount of fentanyl). 4′-Hydroxy-fentanyl (0.2–2.4%) and β-hydroxy-fentanyl (0.9–3.5%) were the next largest amounts of metabolites formed in h-iPS-HEP and h-PRM-HEP. ω-Hydroxy-fentanyl and (ω-1)-hydroxy-fentanyl were minor metabolites. 4′-Hydroxy-3′-methoxy-fentanyl was formed only in h-PRM-HEP; however, the amount was very small (less than 0.2% of the initial amount of fentanyl). According to a previous report, the urinary excretion of nor-fentanyl and 4′-hydroxy-fentanyl, 24 h after continuous infusion of fentanyl to patients (2.1–3.0 mg/kg body weight), accounted for 8–25 and 3–6% of the initial amount of fentanyl, respectively.7) The in vivo metabolite profile of fentanyl in this report was in good accordance with the in vitro metabolite profiles obtained in our study. In both cases, the first and second largest amounts of fentanyl metabolites formed were nor-fentanyl and 4′-hydroxy-fentanyl, respectively. It is unclear why β-hydroxy-fentanyl has never been identified in biological specimens from fentanyl users when it was formed in relatively large amounts in h-iPS-HEP and h-PRM-HEP. The metabolite profiles of fentanyl varied widely between the h-iPS-HEP products. This was because of differences in the expression of the drug-metabolizing enzymes in each h-iPS-HEP product. According to the data sheets from the manufacturer, Cellartis h-iPS-HEP from ChiPSC18 has substantially higher CYP3A4 activity (more than 10 times) than h-iPS-HEP from ChiPSC22. As expected, more nor-fentanyl, which is produced by CYP3A4 from fentanyl,14) was formed in Cellartis h-iPS-HEP from ChiPSC18 than in those from ChiPSC22. Although the data are limited, above results suggested that the activity of the drug-metabolizing enzyme in h-iPS-HEP was well correlated with the production of the metabolite.

Each value is the average of two determinations.

Each value is the average of two determinations.
The time–courses of the formation of the main metabolites of acetylfentanyl in Cellartis h-iPS-HEP (ChiPSC18) are shown in Fig. 10. As for fentanyl, the amounts of the main metabolites of acetylfentanyl increased with the incubation time. The metabolite profiles of acetylfentanyl in h-iPS-HEP (48 h incubation) and h-PRM-HEP (3 h incubation) are shown in Fig. 11. The main metabolites of acetylfentanyl formed by h-iPS-HEP and h-PRM-HEP were nor-acetylfentanyl and 4′-hydroxy-acetylfentanyl, and the amounts ranged from 2.7–10.1 and 0.5–8.5% of the initial acetylfentanyl, respectively. As for fentanyl, more of the desphenethylated metabolite (nor-acetylfentanyl) was formed in Cellartis h-iPS-HEP from ChiPSC18 than in those from ChiPSC22, indicating that this metabolic reaction was also catalyzed by CYP3A4. The 4′-hydroxy-3′-methoxy metabolite was formed only in h-PRM-HEP. β-Hydroxy-acetylfentanyl was the metabolite formed in the third largest amount in h-iPS-HEP and h-PRM-HEP; however, this metabolite has never been detected in in vivo samples. Careful analysis of samples from fentanyl and acetylfentanyl users may result in the detection of β-hydroxy metabolites of these drugs in vivo in the future. The quantitative values of the metabolites of fentanyl and acetylfentanyl were not affected by enzymatic hydrolysis, indicating that the metabolites did not undergo any conjugation.

Each value is the average of two determinations.

Each value is the average of two determinations.
h-iPS-HEP had drug-metabolizing activity comparable to h-PRM-HEP, and showed a similar pattern for fentanyl metabolite formation as seen in vivo, indicating that these cells may be potentially useful for the prediction of in vivo drug metabolism. In addition, h-iPS-HEP have the advantage that the cells are robust (cell viability after thawing was more than 90%) and can be supplied stably. However, some enzymatic activities (O-methylation and N-deacylation) were not detected in h-iPS-HEP. Further study is needed to clarify the ability of h-iPS-HEP to metabolize drug molecules.
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 15K08895. We thank Victoria Muir, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
The authors declare no conflict of interest.