2018 Volume 41 Issue 1 Pages 123-131
2018 Volume 41 Issue 1 Pages 123-131
Ketamine (KT) is a chiral anesthetic agent, (R)- and (S)-enantiomers of which differ in their pharmacological properties. KT has become one of the most commonly used illicit drugs in the world, thus, rapid and feasible on-site testing is required to crack down on the illicit use. Although immunochemical approach with specific antibodies is promising for this purpose, in practice anti-KT antibodies are difficult to obtain. We here disclose generation of monoclonal antibodies against KT. Mice were immunized with either (a) commercially-available or (b) in-house-prepared KT-albumin conjugates. Splenocytes from these mouse groups (a and b) were separately fused with P3/NS1/1-Ag4-1 myeloma cells. After standard screening and cloning, we established 5 hybridoma clones: 2 were derived from group-a mice [generating Ab-KT(a)#2 and #37] and 3 were from group-b mice [generating Ab-KT(b)#9, #13, and #45]. These antibodies exhibited practical performance in competitive enzyme-linked immunosorbent assay systems. When (±)-KT·hydrochloride (HCl) was used as the competitor, dose–response curves showed midpoint values of 30 and 70 ng/assay (a-series antibodies) and 2.0–3.0 ng/assay (b-series antibodies). Remarkably, the a-series antibodies were specific for (S)-KT·HCl, while the b-series antibodies were specific for (R)-KT·HCl. Ab-KT(a)#2 (Ka, 7.5×107 M−1) and Ab-KT(b)#45 (Ka, 7.7×108 M−1) exhibited the highest enantioselectivity for each group, and cross-reactivity with the (R)- and (S)-antipodes was 1.3 and 1.7%, respectively. The hybridomas established here are also valuable as a source of genetic information for the anti-KT antibodies, which is required for progressing to next-generation technologies using genetically engineered antibodies.
Ketamine (KT), a chiral phencyclidine derivative that functions as a noncompetitive N-methyl-D-aspartate receptor antagonist, has been used as an anesthetic agent in both humans and veterinary medicine.1) However, KT has become one of the most commonly used illicit drugs in the world, and is popular particularly among young populations as a “club drug.”2,3) In Japan, KT is normally supplied as hydrochloride salt of a racemic mixture of (R)-KT and (S)-KT (Fig. 1A) for clinical use, both of which differ in their pharmacological properties. The (S)-enantiomer shows more potent analgesic and anesthetic effects than the (R)-antipode. The (S)-enantiomer is responsible for the psychotomimetic effects whereas the (R)-antipode induces a state of relaxation.1,4,5) Therefore, the (S)-enantiomer is now commercially available in some countries, and its use has increased in recent years.1,5) After administration in humans, KT is mostly biotransformed to norketamine (NKT), the N-demethylated active metabolite, which is then dehydrogenated to generate dehydronorketamine (DNKT) via hydroxylation of the cyclohexanone ring6,7) (Fig. 1B).

In Japan, KT has been controlled under the Narcotics and Psychotropics Control Law since 2007. Rapid, feasible, and reliable on-site testing is required to crack down on illicit KT use and distribution. Immunochemical approaches with specific antibodies are more suitable for this purpose than chromatographic methods because they require neither expensive and fixed instruments, nor time-consuming sample-pretreatment steps. Indeed, several immunoassay kits for testing KT are now available for purchase, using anti-KT antibodies, which would have been generated in these companies.8–11) Recently, advanced immunosensors for KT have also been reported. These studies involved the use of anti-KT antibodies for immobilization on electrodes12) or quartz crystal microbalance chips,13) which might have been donated or purchased from these companies.
However, it has been difficult in practice to obtain anti-KT antibodies when we plan for developing our own analytical methods. Although 1 patent report described the generation of polyclonal anti-KT antibodies against novel haptenic derivatives that the applicants synthesized,14) scientific reports describing the production of anti-KT antibodies are scarce. We therefore generated novel monoclonal antibodies against KT by immunizing mice with 2 kinds of haptenized immunogens, either a commercially available or an in-house-prepared KT-albumin conjugate: the use of immunogens of different types should enlarge the probability of obtaining useful antibodies. Interestingly, the resulting antibodies showed remarkable stereoselectivity for either the (R)- or (S)-KT enantiomer, and performed acceptably in enzyme-linked immunosorbent assays (ELISAs) with practical sensitivity for determining circulating racemic KT contents.
(±)-KT·hydrochloride (HCl) was supplied by Central Customs Laboratory (Kashiwa, Japan). (R)-(−)-KT·HCl and (S)-(+)-KT·HCl (both with an enantiomeric excess of >99%) were supplied from our laboratory.15,16) Solutions of (±)-NKT·HCl and (±)-DNKT·HCl were obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). One immunogenic conjugate with KT linked to bovine serum albumin (BSA), referred to hereafter as KT-BSA(a), was purchased from GenWay Biotech (San Diego, CA, U.S.A.).
BuffersThe following buffers were used in this study: PB, 50 mM sodium phosphate buffer (pH 7.3); PBS, PB containing 9.0 g/L NaCl; G-PBS, PBS containing 1.0 g/L gelatin; T-PBS, PBS containing 0.050% (v/v) Tween 20; M-PBS, PBS containing 20 g/L skim milk.
In-House Preparation of the KT-BSA(b) Immunogenic ConjugateAnother immunogenic conjugate, abbreviated as KT-BSA(b), was prepared as follows.
(a) Synthesis of Haptenic Derivative(±)-KT·HCl (80 mg) was dissolved in 5% Na2CO3 and extracted with CHCl3 to obtain the (±)-KT free base (Fig. 2A). Sodium hydride (55 mg) and 3-bromopropionic acid ethyl ester (150 μL) were added to the (±)-KT in N,N-dimethylformamide (1.5 mL), and the mixture was stirred at 60°C for 40 min. The mixture was diluted with water and then extracted with AcOEt. After removing the solvent, the residue was chromatographed on a silica gel (CHCl3/MeOH=15 : 1) to isolate ester (1) as a colorless oil (40.3 mg). [α]D −0.1 (c=0.40, MeOH). 1H-NMR (600 MHz, CD3OD) δ: 7.71 (1H, d, J=7.8 Hz, 3′-H), 7.46 (1H, t, J=7.8 Hz, 4′-H), 7.43 (1H, d, J=7.8 Hz, 6′-H), 7.36 (1H, t, J=7.8 Hz, 5′-H), 4.04 (2H, dd, J=14.4, 7.2 Hz, 10-H), 3.14 (1H, dd, J=14.4, 3.0 Hz, 3α-H), 2.44 (1H, ddt, J=12.8, 7.8, 5.3 Hz, 6-H), 2.26–2.39 (2H, m, 8-H), 2.07–2.11 (1H, m, 5-H), 1.96–2.03 (1H, m, 7-H), 1.97 (3H, s, 2-CH3), 1.75–1.78 (2H, m, 4α,β-H), 1.53–1.60 (2H, m, 3β-H, 7-H), 1.35–1.43 (1H, m, 5-H), 1.18 (3H, t, J=7.2 Hz, 11-H). 13C-NMR (151 MHz, CD3OD) δ: 213.6, 175.2, 136.8, 135.2, 132.5, 132.1, 130.8, 128.3, 72.7, 61.4, 48.4, 40.0, 38.7, 32.4, 28.8, 26.2, 23.0, 14.6. High resolution (HR)-MS (electrospray ionization (ESI)-Orbitrap) m/z: [M+H]+ Calcd for C18H25ClNO3 338.1517. Found 338.1514.
To a solution of ester 1 (40.3 mg) in MeOH (1.0 mL), 5% KOH (0.20 mL) was added and the mixture was stirred at 60°C for 30 min. The mixture was diluted with water and extracted with CHCl3. The aqueous phase was acidified with HCl, and then run on a column packed with Supelpak-2. After washing with water, the adsorbed substances were eluted with MeOH. Removal of the solvent yielded the acid KT-COOH (2) as a colorless solid (14.3 mg). 1H-NMR (500 MHz, C5D5N) δ: 7.60 (1H, t, J=8.0 Hz, 6′-H), 7.42 (1H, t, J=8.0 Hz, 3′-H), 7.37 (1H, t, J=8.0 Hz, 5′-H), 7.26 (1H, t, J=8.0 Hz, 4′-H), 3.06 (1H, d, J=10.5 Hz, 3-H), 2.55–2.58 (1H, m, 6-H), 2.47–2.51 (1H, m, 7-H), 2.06 (3H, s, 2-CH3), 1.88–1.99 (2H, m, 5-H, 7-H), 1.52–1.63 (3H, m, 3-H, 4-H), 1.33–1.41 (1H, m, 5-H). 13C-NMR (125.5 MHz, C5D5N) δ: 212.5, 138.1, 136.2, 132.0, 131.1, 129.6, 127.5, 72.0, 48.2, 40.2, 37.9, 29.1, 26.7, 22.4. HR-MS (ESI-Orbitrap) m/z: [M+H]+ Calcd for C16H21ClNO3 310.1201. Found 310.1203.
(b) Conjugation of KT-COOH and AlbuminKT-COOH was converted to its N-succinimidyl ester (3) according to a reported method.17) Crude ester 3 (6.6 mg, 16 µmol) was reacted with BSA (Sigma-Aldrich) (15 mg, 0.23 µmol) in a mixture of 1,4-dioxane (1.5 mL), PB (1.5 mL), and pyridine (1.0 mL) with stirring at room temperature for 3 h, then overnight at 4°C. The reaction mixture was treated as described previously (protein precipitation with acetone and subsequent dialysis),18) and the desired conjugate KT–BSA(b) was obtained as a 1.0-mg/mL solution in saline. The KT:albumin-coupling molar ratio was determined to be 28, based on titration of the residual amino groups with sodium 2,4,6-trinitrobenzene sulfonate.19)
Preparation of Enzyme-Labeled KTβ-Galactosidase (GAL)-labeled KT (KT-GAL) was prepared as described previously.20) Briefly, a solution of GAL (EC 3.2.1.23; Roche Diagnostics, Basel, Switzerland) (2.0 mg) in PB (200 µL) was added to the active ester 3 (46 µg) in 1,4-dioxane (200 µL), and the mixture was stirred at 4°C for 4 h. The solution was subjected to a PD-10 column (GE Healthcare Japan, Tokyo, Japan), which was equilibrated with PB–EtOH (4 : 1) and eluted with the same buffer. Fractions that showed GAL activity were collected and dialyzed for 2 d against cold PB. The resulting solution was adjusted to 45.7 µg/mL (in terms of GAL) in G-PBS and stored at 4°C until use.
Immunization, Cell Fusion, and Monoclonal Antibody ProductionAll experiments on animals were carried out in Kobe Pharmaceutical University in accordance with guidelines and regulations established in the university, and all experimental protocols were approved by the Institutional Animal Care and Use Committee.

Synthesis pathway (A) and the HMBC and NOESY correlations for the compound 1 (B). The most stable conformation of compound 1 calculated, viewed from 2 different angles, are also shown (C). A restricted Hartree–Fock SCF calculation was performed at the 3–21G* level using the Spartan program (Spartan’14 for Windows; Wavefunction, Irvine, CA, U.S.A.).
Female BALB/c and A/J mice (8 weeks of age) were immunized with KT-BSA(a) or KT-BSA(b) (25 µg/mouse) biweekly for a total of 4 or 5 times, as summarized in Fig. 3. After screening of the titer for anti-KT antibodies in serum, 1 or 2 mice that showed stronger immune responses were administered intraperitoneal and intrasplenic injections21) of the conjugate (25 µg total). After 3 d, splenocytes were collected from the mice and cell fusion22) with P3/NS1/1-Ag4-1 (NS1) myeloma cells23) was performed according to our standard procedure.18,24,25) Briefly, splenocytes (1–3×108 cells) were fused with 1/5 number of NS1 cells using a 40% polyethylene glycol 4000 solution containing dimethyl sulfoxide and poly-L-arginine. Fused cells were cultured for approximately 2 weeks at 37°C in HAT (hypoxanthine-aminopterin-thymidine) medium supplemented with 10% briclone (DS Pharma Biomedical, Osaka, Japan) under 5% CO2/95% air. Culture supernatants were then screened by ELISA, as described below. Hybridomas producing anti-KT antibodies were expanded in HT (hypoxanthine-thymidine) medium and then cloned by limiting dilution. Finally, the cloned hybridomas were cultured for 7–10 d, and the monoclonal antibodies in the supernatant were characterized. The heavy and light chain isotypes were determined using the ImmunoPure Monoclonal Antibody Isotyping Kit II (Thermo Fisher Scientific, Waltham, MA, U.S.A.).
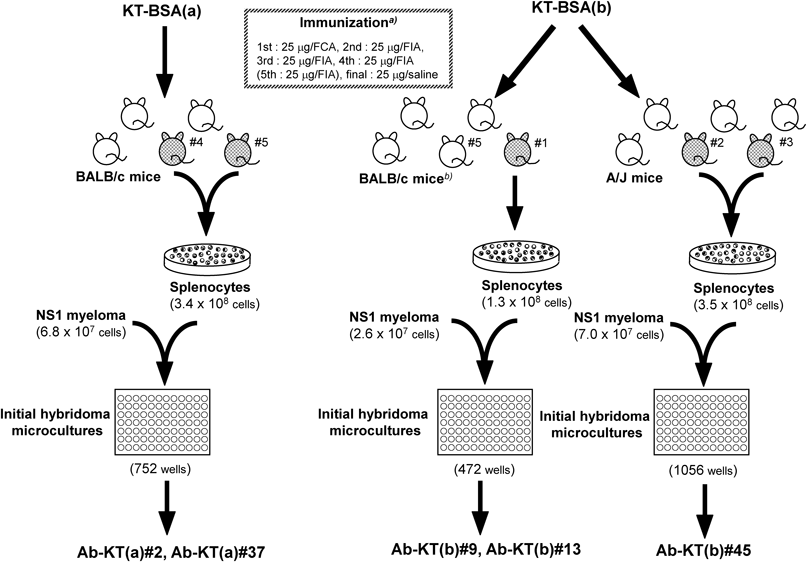
a) Each mouse was immunized with subcutaneous injection of 25 µg KT-BSA at multiple sites on the back with an emulsion of Freund’s complete adjuvant (FCA) or incomplete adjuvant (FIA) and saline (1 : 1, 0.2 mL). The selected mice were received the final immunization with 25 µg KT-BSA dissolved in saline (see text). b) The #5 mouse that showed the strongest humoral response was dead, thus the splenocytes were collected only from the #1 mouse that showed the second strongest response.
The ELISA procedures used in this study are illustrated in Fig. 4. In both assays described below, the concentrations of antibodies were adjusted to give bound enzyme activities at B0 (the reactions without standard KT·HCl or its analogs) of approximately 1.0–1.5 absorbance units after a 30-min enzyme reaction.

Costar 96-well microplates (#3590; Corning, Corning, NY, U.S.A.) were coated overnight at room temperature with 1.0 µg/mL KT-BSA(a) in 0.10 M carbonate buffer (pH 8.6) (100 µL/well) and blocked with M-PBS at 37°C for 60 min. After washing the wells 3 times with T-PBS, various concentrations of KT·HCl (or analogs) dissolved in G-PBS (50.0 µL/well) and a monoclonal anti-KT antibody (the hybridoma supernatants were used) diluted with G-PBS or an antiserum diluted with PBS containing 5.0% BSA (100 µL/well) were added, mixed, and incubated at 37°C for 60 min. Subsequently, wells were washed and probed with peroxidase (POD)-labeled goat anti-mouse immunoglobulin G (IgG) (Fc-specific) antibody (160 ng/mL; Jackson ImmunoResearch, West Grove, PA, U.S.A.) diluted in G-PBS (100 µL/well). After incubation at 37°C for 30 min, the wells were washed, and the captured POD activity was determined colorimetrically at 490 nm using o-phenylenediamine as a hydrogen donor.26)
(b) Characterization of Anti-KT “b-Series” Antibodies Derived from Mice Immunized with KT-BSA(b)Costar microplates (#3590) were coated overnight at 4°C with a 5.0-µg/mL solution of affinity-purified goat anti-mouse IgG antibody (Jackson ImmunoResearch) in PBS (100 µL/well). After washing 3 times with PBS, the wells were blocked with M-PBS at 37°C for 60 min. The wells were washed 3 times with T-PBS, and then a monoclonal antibody (the hybridoma supernatants were used) or antiserum against KT diluted with G-PBS (100 µL/well) was added to the wells. After incubation at 37°C for 60 min, the solutions were removed and the wells were washed similarly. Then, 0.50 µg/mL KT-GAL (100 µL/well) and various concentrations of KT·HCl (or analogs) (50.0 µL/well), both diluted in G-PBS, were added, mixed, and incubated at 37°C for 60 min. The wells were washed, and the captured GAL activity was determined colorimetrically at 405 nm using o-nitrophenyl β-D-galactopyranoside as the substrate.20)
Preparation of Fab Fragments and Determining Their Antigen-Binding ParametersFab fragments of Ab-KT(a)#2 and Ab-KT(b)#45 were prepared with the Pierce Fab Preparation Kit (Thermo Fisher Scientific). Affinity and dissociation rate constants (ka and kd) and equilibrium affinity and dissociation constants (Ka and Kd) against KT residues in the “homologous KT-BSA” (the conjugate from which the antibody was derived) were determined for these Fab fragments at 25°C using BLItz (ForteBio, Fremont, CA, U.S.A.), a bio-layer interferometry (BLI) sensor. Streptavidin-coated biosensor tips, which were saturated with biotin-labeled KT-BSA, prepared by reaction with EZ-Link NHS-LC-Biotin (Thermo Fisher Scientific), were dipped into 4.0-µL antibody solutions in G-PBS [10, 20, 50, or 100 nmol/L for Ab-KT(a)#2; 50, 100, 200, or 500 nmol/L for Ab-KT(b)#45]. Association of the antibodies with the KT residues was monitored for 300 s, and then dissociation was measured for 300 s in G-PBS.
Because of a lack of immunogenicity, KT molecules had to be linked with suitable carrier macromolecules to generate anti-KT antibodies. A previous patent report described the synthesis of 4 haptenic derivatives each having a different linker structure for coupling with the carrier.14) Three of them (type A; Fig. 5) had the linker on the nitrogen, and another (type B; Fig. 5) had the linker on the carbon at the position 6 (C6: the α-carbon of the cyclohexanone ring). One of the type-A compounds was coupled to BSA and used to immunize sheep. The resulting antisera reacted almost equally to KT and NKT (Fig. 1B): this binding property is reasonably explained based on the nitrogen-masked hapten structure of the conjugate.

“X” refers to functional groups used for conjugation with carrier proteins, and “R” refers to spacers that linked the KT moiety and functional group X.
For our hapten synthesis, (±)-KT was alkylated with a β-bromoester to introduce a linker structure (Fig. 2A). Protons and carbons of the compound 1 were assigned by 1H–1H correlation spectroscopy (COSY) and 1H–13C heteronuclear single quantum coherence (HSQC) and multiple bond coherence (HMBC) (Fig. 2B) experiments. The relative configuration of compound 1 was established as depicted in Fig. 2A on the basis of nuclear Overhauser effect correlated spectroscopy (NOESY) experiments (Fig. 2B). For a better understanding of the conformation of 1, ab initio (HF/3–21G*) calculations were performed. The most stable conformation of 1 (Fig. 2C) was in good agreement with its spectral data. Interestingly, the ester substituent on C6, which was deduced to a trans configuration with respect to the o-chlorophenyl group, is bending downwards to face the o-chlorophenyl group. Saponification of the ethyl ester 1 provided KT-COOH 2, which was then converted to the N-succinimidyl ester 3: this was obtained as a solid and could be stored stably for years at 4°C. The ester 3 was reacted with BSA and GAL separately, to prepare immunogen [KT-BSA(b)] and enzyme-labeled KT (KT-GAL), respectively.
Generation of Monoclonal Anti-KT AntibodiesOutline of the monoclonal antibody production is illustrated in Fig. 3. BALB/c mice, the first strain chosen as the spleen donor during the hybridoma preparation, were immunized with 2 immunogens: the commercially available hapten-carrier conjugate [KT-BSA(a)] and the in-house-prepared immunogen [KT-BSA(b)] described above. A/J mice were also selected for immunization with the latter immunogen, based on our previous success in preparing hybridomas secreting practical antibodies against several small molecules.20,27) The immunization schedule for this study is summarized in Fig. 3. Seven days after the fourth or fifth immunization, blood was collected individually from each mouse, and the titers of the anti-KT antibodies in the sera were examined by the ELISA system, as illustrated in Fig. 4 (see in Materials and Methods as well).
For sera from the mice immunized with KT-BSA(a) (a-series sera), it was necessary to use the same conjugate, i.e., KT-BSA(a), to capture the target antibodies because no information for the chemical structure of haptenic derivative was available. The antigen–antibody binding reaction was performed in assay buffer containing 5.0% BSA to reduce hapten-independent binding due to accompanying anti-BSA antibodies in the sera. The antibody dilution curves are shown in Fig. 6A. Unusually high binding was observed, most of which might have been due to the anti-BSA antibodies that were not completely prevented. However, subsequent inhibition testing by addition of the (±)-KT·HCl standard indicated the presence of anti-KT antibodies. The antigen–antibody binding ability of sera from mice immunized with KT-BSA(b) (b-series sera) was monitored with the homologous haptenic derivative (KT-COOH in Fig. 2A) labeled with β-galactosidase (KT-GAL): in this case, anti-BSA antibodies, if any, should not cause positive signals. The dilution curves showed that all mice generated anti-KT antibodies (Fig. 6B), and inhibition test results suggested that the antibodies might provide practical dose-dependent responses against (±)-KT·HCl (data not shown).

Based on these data, mice were selected as spleen donors (Fig. 3) and their splenocytes were fused with NS1 myeloma cells.22,23) Three fusion experiments (Fig. 3) afforded 2 antibody-secreting hybridoma clones (#2 and #37) derived from mice immunized with KT-BSA(a) and 3 clones (#9, #13, and #45) derived from mice immunized with KT-BSA(b). The “a-series antibodies” from the former 2 hybridoma clones, designated Ab-KT(a)#2 and Ab-KT(a)#37, were composed of γ1 heavy chains and κ light chains. The “b-series antibodies” from the latter 3 clones, designated Ab-KT(b)#9, Ab-KT(b)#13, and Ab-KT(b)#45, were composed of γ1 heavy chains and λ light chains. We note that these 5 antibodies had different amino acid sequences both for the heavy and light chain variable domains (data not shown).
Characterization of the Monoclonal Anti-KT Antibodies by ELISA(a) SensitivityAlthough the (S)-enantiomer is available in some countries,1) racemic mixtures of KT·HCl are supposed to be still circulating for illicit use. Therefore, ELISAs were first performed using (±)-KT·HCl as the analyte with our monoclonal antibodies. The assay systems using the a-series or b-series antibodies are shown in Fig. 4. Dose–response curves are shown in Fig. 7. The midpoint, which is the dose of (±)-KT·HCl that inhibits antibody binding to the immobilized- or enzyme-labeled KT by 50% (the same meaning as IC50: a useful index of assay sensitivity), was 30 and 70 ng/assay for the a-series antibodies [Ab-KT(a)#2 and Ab-KT(a)#37, respectively] (Fig. 7A). The b-series antibodies [Ab-KT(b)#9, Ab-KT(b)#13, and Ab-KT(b)#45] generated more sensitive dose–response curves with much lower midpoint values (3.0, 2.0, and 2.1 ng/assay, respectively) (Fig. 7B). The limit of detection (LOD) was determined for the most effective antibodies in each series [i.e., Ab-KT(a)#2 and Ab-KT(b)#45], selected based on the sensitivity and enantioselectivity (see below). The LODs for Ab-KT(a)#2 and Ab-KT(b)#45 were 1.5 and 0.21 ng/assay, based on the amount of (±)-KT·HCl required to give a bound absorbance 2 standard deviations below the average (n=10) of the B0 absorbance. The unit “X g/assay” used in this study means that a total of X g (mass) of analyte or cross-reactive analogs was added to the assay chamber (microwells) for the competitive antigen–antibody reactions.

The a-series monoclonal antibodies were used for measuring (±)-KT·HCl and (S)-KT·HCl (A), and the b-series monoclonal antibodies were used for measuring (±)-KT·HCl and (R)-KT·HCl (B). The vertical bars indicate the standard deviation (n=4).
Enantioselectivity of these antibodies was then examined using (R)-KT·HCl and (S)-KT·HCl standards, and evaluated as the cross-reactivity in ELISAs, where the reactivity of (±)-KT·HCl was taken as 100% (Fig. 8). The a-series antibodies bound preferentially to the (S)-enantiomer, as shown by cross-reactivity that exceeded 100%. Remarkably, Ab-KT(a)#2 showed ca. 200% cross-reactivity: when reactivity to the (S)-enantiomer was set at 100%, cross-reactivity with the (R)-antipode was 1.3%. In contrast, the b-series antibodies were specific for the (R)-enantiomer as shown by >160% reactivity. The most (R)-specific antibody, Ab-KT(b)#45, showed 1.7% cross-reactivity with the (S)-antipode when the reactivity of the (R)-enantiomer was normalized to 100%.

Cross-reactivity was determined by the 50% displacement method40) according to the following equation: Cross reactivity (%)=(X/Y)×100; where X is the midpoint (ng/assay) of (±)-KT·HCl, and Y is the midpoint (ng/assay) of a KT analog, cross-reactivity of which is to be determined.
The a-series antibodies significantly cross-reacted with NKT·HCl (ca. 30%), but showed almost negligible values for DNKT·HCl (<4%). The b-series antibodies, Ab-KT(b)#9, #13, and #45, showed rather fluctuating but significant cross-reactivity with NKT·HCl (35, 25, and 7.0%, respectively), while <4% cross-reactivity was observed with DNKT·HCl. Cross-reactivity with some compounds that are structurally unrelated to KT was examined for Ab-KT(a)#2 and Ab-KT(b)#45. Both antibodies showed negligible cross-reactivity towards p-acetamidophenol, acetylsalicylic acid, creatine, creatinine, caffeine, and uric acid (<1 and <0.1%, respectively).
(c) Binding ParametersThe association and dissociation rate constants (ka and kd, respectively) and the equilibrium affinity and dissociation constants (Ka and Kd, respectively) of Ab-KT(a)#2 and Ab-KT(b)#45 (examined as the Fab fragment) against the KT-residues were as follows: Ab-KT(a)#2, ka=3.0×105 M−1 s−1, kd=4.0×10−3 s−1, Ka=7.5×107 M−1, Kd=1.3×10−8 M; Ab-KT(b)#45, ka=1.1×106 M−1 s−1, kd=1.5×10−3 s−1, Ka=7.7×108 M−1, Kd=1.3×10−9 M. Both antibodies showed the practical nanomolar-order affinity. Ab-KT(b)#45 exhibited a 10-fold greater Ka value than Ab-KT(a)#2, consistent with the fact that this antibody showed more sensitive dose–response curves in the ELISA (Fig. 7). We observed extremely high ka values (>105 M−1 s−1), which are within the maximum range of ka values deduced for antigen–antibody reactions based on the diffusion coefficients of the reactant molecules.28) This property should allow quick binding against immobilized antigens, which is a great advantage for on-site analytical systems involving immunochromatographies or immunosensors.
Production of practical hybridoma-based monoclonal antibodies targeting small molecules (i.e., haptens) is not straightforward and requires considerable expertise. To generate specific antibodies, suitable haptenized immunogens should be used with characteristic functional groups on the target hapten externally exposed, being adequately distant from the carrier macromolecules.20) In addition, using haptens with smaller molecular mass usually increases the difficulty in generating high-affinity antibodies,29,30) although some exceptional instances have been reported: e.g., monoclonal antibodies against 3-methylindole (Mr 131.2) with Ka values of >108 M−1 were produced previously.31) This difficulty mainly arises from the fact that such small compounds (Mr<300, as a rough indication), linked with carrier macromolecules, often generate antibodies that recognize epitopes composed of both hapten residues and linker structures bridging the hapten and carrier (and sometimes, even part of the carrier structure). Consequently, such antibodies cannot tightly capture the unmodified hapten molecule alone. It is therefore not surprising that monoclonal antibodies against the small KT molecule (Mr 237.7) are not readily available.
Initially, when exploring methods to synthesize KT derivatives for linking with carriers, we attempted the reaction of (±)-KT with O-(carboxymethyl)hydroxylamine under standard conditions, but failed to obtain the desired oxime. Then, we performed a reaction with ethyl 3-bromopropionate, which mainly substituted on the α-carbon of the carbonyl group (compound 1; Fig. 2A), not on the nitrogen in the methylamino group. Thus, the haptenic derivative KT-COOH 2, which was obtained after subsequent saponification, was the one categorized as a type-B hapten (Fig. 5). Subsequent saponification of compound 1 provided the haptenic derivative 2 (KT-COOH).
Mice were immunized with 2 immunogens: the commercially available hapten-carrier conjugate [KT-BSA(a)], and the in-house-prepared conjugate linking KT-COOH and BSA [KT-BSA(b)]. Both conjugates successfully induced desirable humoral immune responses with a standard immunization schedule using Freund’s adjuvant, yielding hybridoma clones secreting anti-KT antibodies. Three functional groups containing heteroatoms (carbonyl, methylamino, and o-chlorophenyl groups) and 2 rigid ring structures (phenyl and cyclohexanone rings) are present in KT. Despite its rather small molecular size, these features might have allowed for stable complex formation with the surface receptors of relevant B-cells.
The a-series antibodies, Ab-KT(a)#2 and Ab-KT(a)#37 derived from KT-BSA(a), were specific to the (S)-enantiomer of KT·HCl, while the b-series antibodies, Ab-KT(b)#9, Ab-KT(b)#13, and Ab-KT(b)#45 derived from KT-BSA(b), preferably bound to the (R)-enantiomer. As is well recognized, specificity of anti-hapten antibodies is closely related with the structure of the hapten-carrier conjugate used for immunization. Unfortunately, the chemical structure of the commercially-available KT-BSA(a) is not publicly available. However, such distinct differences in specificity of the resulting antibodies suggested that the KT derivative used to prepare KT-BSA(a) was structurally rather different from KT-COOH used to prepare KT-BSA(b). Because KT-COOH is racemic, both the (R)- and (S)-KT residues should have been linked to BSA. In the initial event of the antibody production in vivo, individual B-cells should recognize a single hapten residue, i.e., either the (R)- or (S)-KT residue on the carrier molecule. For KT-BSA(b), the (R)-KT redidue might have exhibited stronger immunogenicity, resulting in the preferential proliferation of plasma cells secreting (R)-KT-specific antibodies.
The a-series and b-series antibodies discriminated NKT·HCl somewhat comparably (ca. 30% for the a-series and 7.0–35% for the b-series, setting values obtained with (±)-KT·HCl at 100%). These findings indicated that the immunogen KT-BSA(a) employed a haptenic derivative that was different from the type A compounds (i.e., N-substituted derivatives; Fig. 5), considering the specificity of antibodies previously generated from a type A haptenic derivative (mentioned above).14) The KT molecule has a limited number of positions where substituents can be feasibly introduced. The haptenic derivative linked in the KT-BSA(a) conjugate, which induced (S)-KT-specific antibodies, might have been a derivative of (S)-KT that have a linker structure on α-carbon of the cyclohexanone like our KT-COOH. If this is true, then, the results for the a-series antibodies shown above indicate that the (S)-KT residue is not suitable for inducing high-affinity antibodies: this possibility is compatible with the model proposed above to explain why KT-BSA(b) induced (R)-KT-specific antibodies. Such distinct differences in immunogenic properties between a pair of enantiomers have been recognized for several chiral drugs as reviewed previously.32)
In the competitive ELISA, the best antibody in the a-series, Ab-KT(a)#2, facilitated antigen measurements at ca. 10–1000 ng/assay for (±)-KT·HCl and ca. 5.0–500 ng/assay for (S)-KT·HCl (Fig. 7A). The best antibody in the b-series, Ab-KT(b)#45, enabled more sensitive measurements with the range of ca. 0.20–50 ng/assay for (±)-KT·HCl, and ca. 0.010–50 ng/assay for (R)-KT·HCl (although different ELISA protocols were used for the a-series and b-series antibodies) (Fig. 7B). Ab-KT(b)#45 also exhibited dose–response curves for (S)-KT·HCl, due to cross-reaction, covering the range of ca. 5.0–1000 ng/assay (not shown in the Figure), which was similar to that obtained with Ab-KT(a)#2. Presently, KT for illegal drug use is supposed to be distributed as (±)-KT·HCl or (S)-KT·HCl. Considering the measurable ranges shown above, Ab-KT(b)#45 is the most useful antibody among the 5 antibodies developed here for on-site analysis aimed cracking down on illicit KT use. The combined use of Ab-KT(a)#2 and Ab-KT(b)#45 might enable separate monitoring of (R)- and (S)-KT disposition after administration of the racemic KT mixture in clinical and preclinical studies. Very recently, (R)-KT has been shown to exert longer-lasting antidepressant effects than (S)-KT in animal models of depression.33) The (R)-enantiomer might be promising as a new antidepressant drug, and the (R)-specific Ab-KT(b)#45 might help the development researches.
Here, we provide the first description of producing monoclonal anti-KT antibodies with practical binding properties. The present antibodies will be useful for constructing various immunochemical systems suitable for on-site analyses involving immunochromatographies and immunosensors. The hybridoma clones that we established are also valuable as a source of genetic information for these antibodies, which is necessary for extending next-generation technologies using genetically engineered antibodies.34–36) The genes encoding the variable domains of the antibodies referred above have already been cloned, and relevant single-chain Fv fragments (scFvs) that with KT-binding ability have been prepared in other laboratories (unpublished data). In vitro evolution on these scFvs might create improved antibodies with higher affinity enabling more sensitive assays.37–40)
We thank Drs. Atsuko Takeuchi and Chisato Tode (Kobe Pharmaceutical University) for determining MS and NMR spectra. (±)-KT·HCl was supplied from Central Customs Laboratory (Kashiwa, Japan). This work was supported in part by Ushio Inc. (Chiyoda-ku, Tokyo, Japan).
The authors declare no conflict of interest.