2018 Volume 41 Issue 10 Pages 1620-1626
2018 Volume 41 Issue 10 Pages 1620-1626
External stimuli, such as radiation, induce inflammatory cytokine and chemokine production in skin, but the mechanisms involved are not completely understood. We previously showed that the P2Y11 nucleotide receptor, p38 mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) all participate in interleukin (IL)-6 production induced by γ-irradiation. Here, we focused on the transient receptor potential vanilloid 4 (TRPV4) channel, which is expressed in skin keratinocytes and has been reported to play a role in inflammation. We found that irradiation of human epidermal keratinocytes HaCaT cells with 5 Gy of γ-rays (137Cs: 0.75 Gy/min) induced IL-6 and IL-8 production. HaCaT cells treated with TRPV4 channel agonist GSK1016790A also showed increased IL-6 and IL-8 production. In both cases, IL-6/IL-8 production was not increased at 24 h after stimulation, but was increased at 48 h. ATP was released from cells exposed to γ-irradiation or TRPV4 channel agonist, and the release was suppressed by TRPV4 channel inhibitors. The γ-irradiation-induced increase in IL-6 and IL-8 production was suppressed by apyrase (ecto-nucleotidase), NF157 (selective P2Y11 receptor antagonist) and SB203580 (p38 MAPK inhibitor). GSK1016790A-induced inhibitor of kappa B-alpha (IκBα) decomposition, which causes NF-κB activation was suppressed by NF157 and SB203580, and γ-irradiation-induced IκBα decomposition was suppressed by TRPV4 channel inhibitors. Our results suggest that γ-irradiation of keratinocytes induces ATP release via activation of the TRPV4 channel, and then ATP activates P2Y11 receptor and p38 MAPK-NF-κB signaling, resulting in IL-6/IL-8 production.
It is well-known that stimuli such as ultraviolet or gamma (γ-) irradiation can cause skin inflammation due to overproduction of cytokines and chemokines.1) Indeed, γ-irradiation, which is commonly used as a treatment for cancer, can cause a range of side effects, such as depilation, skin dryness, erythema, pigmentation, bubbles, erosion, skin ulcer, necrosis and even carcinogenesis.2–5) However, the mechanism through which γ-irradiation induces production of cytokines/chemokines in epidermal cells is not fully understood.
ATP is released from human epidermal keratinocyte-derived HaCaT cells in response to various stimuli6) and induces epidermal cell proliferation, differentiation and apoptosis7) through autocrine and paracrine signal transduction via the P2 receptor, which is expressed on the cell membrane.8,9) Extracellular ATP also induces production of interleukin (IL)-6 (pro-inflammatory cytokine) and IL-8 (chemokine) in epidermal cells.10) In addition, we have reported an important role in P2Y11 receptor in lipopolysaccharide (LPS)-induced IL-6 production in human monocyte THP-1 cells.11) Further, we have also reported the involvement of P2Y11 receptor in IL-6 production by interferon-γ, silica nanoparticles, or γ-irradiation in HaCaT cells.12–14) P2Y11 receptors are also involved in the induction of IL-6 production in response to UVA irradiation in HaCaT cells,15) and activation of P2Y11 receptors induces IL-8 production in human monocyte-derived dendritic cells.16) However, little work has been done on the mechanism of γ-irradiation-induced IL-8 production or the mediators of ATP release in HaCaT cells.
We previously reported the involvement of P2Y11 receptor, p38 mitogen-activated protein kinase (MAPK), and nuclear factor-kappa B (NF-κB) in γ-irradiation-induced IL-6 production in HaCaT cells.14) γ-Irradiation induces release of ATP, which activates P2Y11 receptor, although we found that P2Y11 receptor is not activated immediately after γ-irradiation, but only at about 18 h after irradiation. Taking account of these findings, we focused here on the transient receptor potential vanilloid 4 (TRPV4) channel, which is expressed in skin keratinocytes and has been reported to play a role in inflammation, as a candidate for mediating ATP release in response to γ-irradiation.
The TRPV4 channel is a nonselective cation channel that was identified as an osmotic pressure sensor in 2000.17,18) Subsequent studies revealed not only hypotonic stimulation, but also resulted in the discovery of other irritant receptors activated by stimulants such as arachidonic acid and its metabolites,19) endocannabinoid,20) and mechanical stimulation.21) TRPV4 is also activated by thermal stimulation22,23) (27–35°C). It is expressed in various locations, including the nervous system, kidneys, skin, blood vessels, lungs and bladder,24,25) and was recently shown to be involved in production of inflammatory mediators,26) including IL-627–29) and IL-8.30) It has also been reported to be involved in ATP release.31–33) Therefore, in this work we investigated the role of TRPV4 channel in ATP release after γ-irradiation of HaCaT cells, as well as the signalling pathway leading to IL-6/IL-8 production.
Apyrase, SB203580, and GSK2193874 were purchased from Sigma-Aldrich (U.S.A.). NF157 was purchased from Tocris Bioscience (U.K.). RN-1734 and GSK1016790A were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Cell Culture and IrradiationThe culture of immortalized human-derived epidermal keratinocytes HaCaT cells and γ-irradiation were performed as described previously.14) HaCaT cells were kindly supplied by Drs. M. Ichihashi and M. Ueda (Kobe University School of Medicine, Kobe, Japan) with the permission of Dr. N. E. Fusening (German Cancer Research Center, Heidelberg, Germany).34,35) The cells were irradiated with γ-rays from a Gammacell 40 (137Cs source) (Nordin International, Inc., Japan; 0.75 Gy/min) at room temperature.
ImmunoblottingImmunoblotting was performed as described previously.14) In brief, cells were washed with phosphate-buffered saline (PBS) (Wako, Japan) twice and incubated in lysis buffer containing 1% Triton X-100, protease inhibitor cocktail, and PHOSSTOP® (Sigma-Aldrich) at 4°C for 30 min. The cell lysate was centrifuged at 10000×g for 15 min, and the supernatant was mixed with 2x Laemmli sample buffer (Wako) and 10 mM dithiothreitol. Samples were incubated at 95°C for 10 min. Samples (1.5 µg protein) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the bands were transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were incubated overnight at 4°C in TBST (0.1% Tween-20, 10 mM Tris–HCl, 0.1 M NaCl) containing 1% bovine serum albumin (BSA) (Wako), further incubated overnight at 4°C with anti-TRPV4 antibody (Sigma-Aldrich) or mouse anti-inhibitor of kappa B-alpha (IκBα) (L35A5) monoclonal antibody (mAb) (1 : 1000) (Cell Signaling Technology, Inc., U.S.A.), washed with TBST for 30 min, incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin G (IgG) antibody (1 : 20000) (Cell Signaling Technology, Inc.) or HRP-conjugated anti-mouse IgG antibody (1 : 20000) (Cell Signaling Technology, Inc.) for 1.5 h at room temperature, and then washed again with TBST for 30 min. Membranes were also incubated for 1 h at room temperature with peroxidase-conjugated anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mAb (1 : 50000) (Wako), to confirm equal loading. Specific proteins were visualized by using ImmunoStar®LD (Wako), and bands were analyzed with Image Studio 4.0 for C-DiGit Scanner (LI-COR, U.K.).
Cytokine ProductionHaCaT cells (1.0×105 cells/mL) were seeded and incubated for 24 h. After incubation, the cells were stimulated by exposure to γ-irradiation or GSK1016790A. The concentration of IL-6 in culture medium was measured by enzyme-linked immunosorbent assay (ELISA) as described previously.14) The concentration of IL-8 was measured with human IL-8 ELISA Ready-SET-Go!®(2nd Generation) (eBioscience, U.S.A.) according to the manufacturer’s instructions.
Measurement of Extracellular ATPThe concentration of ATP in culture medium was measured as described previously.14) In brief, HaCaT cells (1.5×105 cells/mL) were seeded and incubated for 24 h. The culture medium was changed to phenol red-free medium containing 0.5% FBS for 1 h before irradiation. The culture medium was harvested at 24 h after irradiation. The medium was centrifuged at 600×g for 1 min at 4°C. The supernatant (10 µL) was added to wells of a white 96-well plate, and then 100 µL of Luciferase/Luciferin Reagent (Promega) was injected into each well. The chemiluminescence was measured with a WALLAC ARVO SX multilabel counter (PerkinElmer, Inc., U.S.A.).
StatisticsResults were expressed as mean±standard error (S.E.). The statistical significance of differences between control and other groups was calculated using Dunnett’s test. Multiple groups were compared using ANOVA followed by pairwise comparisons with Bonferroni’s post hoc analysis. Calculations were done with the Instat version 3.0 statistical software package (Graph Pad Software, U.S.A.). The criterion of significance was p<0.05.
First, expression of TRPV4 channel in HaCaT cells was confirmed (Fig. 1A). Then, HaCaT cells were irradiated with 5 Gy of γ-rays or stimulated with TRPV4 channel agonist GSK1016790A, and we measured IL-6 and IL-8 production. Though IL-6 and IL-8 production was unchanged at 24 h after stimulation, it was significantly increased at 48 h (Figs. 1B, C). This is in agreement with the results of our previous study on IL-6 production,14) in which we demonstrated the involvement of ATP-mediated P2Y11 receptor activation and p38 MAPK signaling in γ-irradiation-induced IL-6 production. Since IL-8 production induced by γ-irradiation showed similar time dependence, we speculated that the similar pathways were involved. This was confirmed by the findings that the γ-irradiation-induced increase in IL-6/IL-8 production was suppressed by apyrase (ecto-nucleotidase), NF157 (a selective P2Y11 receptor antagonist) and SB203580 (a p38 MAPK inhibitor) (Figs. 2A–C).

(A) HaCaT cells (1.0×105 cells/mL) were seeded and cultured for 48 h. Relative TRPV4 channel level was analyzed by immunoblotting. (B, C) HaCaT cells (1.0×105 cells/mL) were seeded and cultured for 24 h. Cells were γ-irradiated (5 Gy) or stimulated with GSK1016790A (1 µM), and the culture supernatant was harvested at 24 h or 48 h after stimulation (γ-irradiation or GSK1016790A). The IL-6 and IL-8 concentrations were measured. Each value represents the mean±S.E. (n=4). Significant differences between non-irradiation and irradiation (5 Gy), or between control and GSK1016790A are indicated with asterisks (** p<0.01, *** p<0.001).
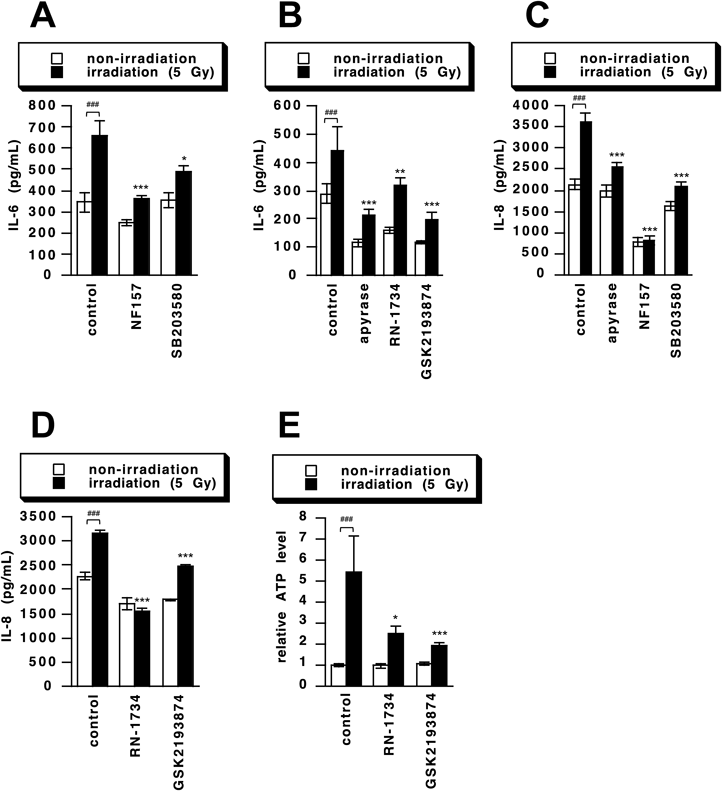
(A–D) HaCaT cells (1.0×105 cells/mL) were seeded and cultured for 24 h. Cells were γ-irradiated (5 Gy) and the culture supernatant was harvested 48 h thereafter. Inhibitors (apyrase 10 U/mL, NF157 50 µM, SB203580 10 µM, RN-1734 20 µM and GSK2193874 1 µM) were added 1 h before γ-irradiation. IL-6 (A and B) or IL-8 (C and D) concentration was measured. Each value represents the mean±S.E. (n=4). (E) HaCaT cells (1.5×105 cells/mL) were seeded and incubated for 24 h. Cells were γ-irradiated (5 Gy) for 24 h. Inhibitors (RN-1734 20 µM and GSK2193874 1 µM) were added 1 h before γ-irradiation. The ATP concentration in culture supernatants was measured as described in Materials and Methods. Each value represents the mean±S.E. (n=8). Significant differences between non-irradiation and irradiation (5 Gy) are indicated with sharps (### p<0.001) and significant differences from control γ-irradiation (5 Gy) are indicated with asterisks (* p<0.05, ** p<0.01, *** p<0.001).
We have reported that ATP release from γ-irradiated cells,14) and it was recently shown that TRPV4 channel stimulation causes ATP release in esophageal keratinocytes, gastric epithelia and odontoblasts.31–33) Therefore, we next investigated the role of TRPV4 in γ-irradiation-induced IL-6 and IL-8 production by using two TRPV4 channel inhibitors, RN-1734 and GSK2193874. Both inhibitors suppressed the γ-irradiation-induced increase in production of IL-6 and IL-8 (Figs. 2B, D). Further, the amount of ATP released from HaCaT cells was increased at 24 h after γ-irradiation, and the γ-irradiation-induced ATP release was suppressed by RN-1734 and GSK2193874 (Fig. 2E). As reported in previous paper,14) the concentration of ATP in culture medium was detected at pM order, because the released ATP was metabolized by ecto-nucleotidase and diluted in culture medium though pericellular ATP concentration would be much higher. To determine the involvement of TRPV4 channel in the ATP release, we here showed ATP release as a ratio of control. These results indicate that the TRPV4 channel is involved in γ-irradiation-induced IL-6 and IL-8 production and ATP release. Thus, we next investigated whether stimulation of the TRPV4 channel would induce production of IL-6 and IL-8. Indeed, the amount of ATP released from HaCaT cells were increased at 24 h after stimulation with a TRPV4 agonist, GSK1016790A. The GSK1016790A-induced ATP release was suppressed by RN-1734 and GSK2193874 (Fig. 3A). We also investigated the signaling pathways, and found that the GSK1016790A-induced IL-6 and IL-8 production was suppressed by apyrase, NF157, SB203580, RN-1734 and GSK2193874 (Figs. 3B–E). These results strongly suggest that the TRPV4 channel is involved in IL-6 and IL-8 production by regulating γ-irradiation-induced ATP release.

(A) HaCaT cells (1.5×105 cells/mL) were seeded and incubated for 24 h. Cells were stimulated with GSK1016790A (1 µM) for 24 h. Inhibitors (RN-1734 20 µM and GSK2193874 1 µM) were added 1 h before stimulation with GSK1016790A (1 µM). The ATP concentration in culture supernatants was measured as described in Materials and Methods. Each value represents the mean±S.E. (n=8). (B–E) HaCaT cells (1.0×105 cells/mL) were seeded and cultured for 24 h. Cells were stimulated with GSK1016790A (1 µM) and the culture supernatant was harvested 48 h thereafter. Inhibitors (apyrase 10 U/mL, NF157 50 µM, SB203580 10 µM, RN-1734 20 µM and GSK2193874 1 µM) were added 1 h before stimulation with GSK1016790A (1 µM). IL-6 (B and C) or IL-8 (D and E) concentration was measured. Each value represents the mean±S.E. (n=4). Significant differences between control and GSK1016790A stimulation are indicated with sharps (# p<0.05, ### p<0.001) and significant differences from control GSK1016790A (inhibitor untreated stimulation group) are indicated with asterisks (* p<0.05, ** p<0.01, *** p<0.001).
It has been reported that NF-κB is involved in γ-irradiation-induced IL-6 production in various cells,36–39) including HaCaT cells.14) Therefore, we investigated whether TRPV4 channel is involved in the activation of NF-κB in irradiated cells. Activation of NF-κB occurs via decomposition of IκBα, which is an inhibitor of NF-κB. Therefore, we evaluated the activation of NF-κB by measuring relative IκBα level. We found that GSK1016790A promotes the decomposition of IκBα, supporting the idea that activation of the TRPV4 channel leads to activation of NF-κB. GSK1016790A-induced IκBα decomposition was suppressed by NF157 and SB203580 (Figs. 4A, B). In addition, IκBα was decreased by γ-irradiation, and the decrease was suppressed by RN-1734 and GSK2193874 (Figs. 4C, D). These results suggest that TRPV4 channel activation induces P2Y11 receptor activation and p38 MAPK-mediated NF-κB activation. Since we have previously shown that the IL-6 production is mediated through P2Y11 receptor-p38 MAPK-NF-κB pathway in γ-irradiatted HaCaT cells,14) activation of the TRPV4 channel appears to act as a trigger of this pathway by regulating ATP release.

HaCaT cells (1.0×105 cells/mL) were seeded, cultured for 24 h, and then stimulated with GSK1016790A (1 µM) (A, B) or γ-irradiated (5 Gy) (C, D) for 24 h. Inhibitors (NF157 50 µM (A), SB203580 10 µM (B), RN-1734 20 µM (C), and GSK2193874 1 µM (D)) were added 1 h before stimulation (GSK1016790A or γ-irradiation). Relative IκBα level was analyzed by immunoblotting (% of control or non-irradiation). Each value represents the mean±S.E. (n=3 or 4). Significant differences between the control GSK1016790A (inhibitor-untreated stimulation) group and inhibitor treatment group or between the control γ-irradiation (5 Gy) group and inhibitor treatment group are indicated with asterisks (* p<0.05, ** p<0.01).
ATP release pathways include maxi anion channel, pannexin hemichannel, connexin hemichannel, exocytosis concentrated into vesicle by vesicle nucleotide transporter (VNUT), and other anion channel(s).40) Among them, involvement of anion channels or hemichannels in radiation-induced ATP release has been reported. γ-Irradiation-induced ATP release has also been reported to involve maxi anion channels in HaCaT cells.41) On the other hand, hemichannels and VNUT are involved in TRPV4 channel-induced ATP release,31,42) and there is one report describing the involvement of TRPV4 as a regulator of intracellular Ca2+ in human esophageal epithelial cells.30) Here, we provide the first evidence that TRPV4 channel-regulated ATP release is involved in γ-irradiation-induced IL-6 and IL-8 production in keratinocytes. However, further work will be needed to determine the mechanism of γ-irradiation-induced TRPV4 activation and the pathway of ATP release.
Since cytokines and chemokines promote cancer and metastasis,43,44) our present findings could be helpful in developing ways to reduce these undesired side effects of radiotherapy. In addition, the TRPV4 channel might be a novel therapeutic target for γ-irradiation-induced inflammation.
Parts of this work were supported by Grants-in-Aid for Pharmaceutical Scientific Research (to MT) from Takeda Science Foundation.
The authors declare no conflict of interest.