2018 Volume 41 Issue 11 Pages 1678-1684
2018 Volume 41 Issue 11 Pages 1678-1684
L-Theanine, a green tea amino acid derivative, has cardiovascular qualities. The focus of the current evaluation was to examine the suppression of L-theanine on cultured vascular smooth muscle cell (VSMC) proliferation and migration that is prompted by angiotensin II (Ang II). The VSMCs were treated with non-cytotoxic concentrations of L-theanine and then stimulated with Ang II. The CCK-8 and Transwell chamber assays were monitored on the proliferation and migration rate, respectively. We discovered that L-theanine (50 and 100 µM) significantly halted Ang II-induced VSMC proliferation and migration. This was joined by a decline in the amount of cyclin D1. An additional discovery was that L-theanine lowered the proportion of S-phase cells, whereas the number of G1/G0-phase cells in Ang II-stimulated VSMCs was elevated, based on flow cytometry. Western blotting analyses indicated that L-theanine had no impact on extracellular-signal-regulated kinase 1/2 (ERK1/2) activation prompted by Ang II. Nevertheless, L-theanine significantly lowered Ang II-prompted phosphorylation of Janus kinase 2 (JAK2), c-Src tyrosine kinase, and signal transducer and activators of transcription 3 (STAT3). The outcomes revealed that L-theanine subdued the Ang II-prompted proliferation and migration of VSMC, partly via the obstruction of the JAK/STAT3 pathway instead of via just the ERK pathway.
Angiotensin II (Ang II), a main result of the renin-angiotensin-aldosterone system (RAAS), acts on vascular smooth muscle cells (VSMCs) and induces microartery contraction. VSMCs were chronic exposed to Ang II resulting in cell proliferation, hypertrophy and aging.1) Abnormal proliferation of VSMCs makes vascular remodeling a pivotal process in the pathogenesis of hypertension. In addition, it is the structural foundation of the decline of hypertension.2) Looking for drugs that can both inhibit blood pressure and inhibit proliferation of VSMCs is an important strategy to prevent hypertensive diseases.
Ang II connects to Ang II type 1 (AT1) receptors and signals via small guanosine 5′-triphosphate (GTP)-binding proteins, including the Rho family, Ras family and Rac family, which control VSMC gene expression, proliferation, migration, and cytoskeletal reorganization.3) Ang II prompts the mitogen-activated protein kinase (MAPK) pathway, such as the p42/p44 extracellular signal-related kinases (ERK1/2), c-Jun N-terminal protein kinase (JNK), and p38.4–6) Ang II also prompts some upstream tyrosine kinases, including Src and Janus kinase (JAK2)/Tyk. Signal transducers and activators of transcription (STATs) are impacted downstream.7–9)
Green and oolong teas are favored in China, and drinking 120 mL/d or more for 1 year significantly lowers the risk of hypertension in this population.10) L-Theanine (2-amino-4-[ethylcarbamoyl]) butyric acid, an innateanalogue of glutamate, is a rare amino acid that is located in green and black teas11) (Fig. 1). Previous reports showed that it is benefit to improve relaxing, cognitive performance, hepatoprotective, neuroprotective effects against cadmium and anti-tumor.12–16) Some studies have suggested that L-theanine can avert cardiovascular disease. L-Theanine administration significantly lowered the amount of 5-hydroxyindole in the brains of Wistar Kyoto (WKY) and spontaneously hypertensive rats17) (SHRs). Additionally, L-theanine counteracted the impacts of caffeine on blood pressure and lowered elevated blood pressure.18) L-Theanine improved vascular function by encouraging nitric oxide (NO) generation in endothelial cells and it reduced the risk of cardiovascular disease.19) However, the evidence whether L-theanine affects Ang II-accelerated VSMCs proliferation and migration is lacking. This evaluation examined the favorable impacts of L-theanine on VSMCs.
L-Theanine (CAS: 3081-61-6, 98% purity) and Angiotensin II (A9525) were obtained from Sigma (St. Louis, MO, U.S.A.), dissolved in sterile deionize water, and stored at 4°C. Anti-cyclin D1, anti-phospho-STAT3 (Tyr705), anti-STAT3, anti-phospho-JAK2 (Tyr1007/1008), anti-phospho-Src (Tyr416) and anti-Src were purchased from Cell Signaling Technology Inc. (Danvers, MA, U.S.A.). The immunoglobulin G (IgG) secondary antibodies conjugated to IRDye 800 were obtained from Rockland (Gilbertsville, PA, U.S.A.). Anti-ERK1/2, anti-phospho-ERK1/2 (T202/Y204) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody were purchased from Bioworld Technology, Inc. (Nanjing, China). Transwell chambers were manufactured by Millipore (Billerica, MA, U.S.A.).
Cell CultureThe VSMCs were gathered with an explant culture that originated from the thoracic aortas of Sprague–Dawley rats.20,21) The VSMCs were incubated in a humidified 5% CO2 atmosphere at 37°C and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone Laboratories Inc., U.S.A.) supplemented with 10% fetal bovine serum (FBS), 100 U/mL streptomycin and 100 U/mL penicillin. The morphology of primary cultured VSMCS was observed under optical microscope. The cells were mostly spindle shaped, and the cells in some areas were overlapped to form the VSMCs peak-valley morphology. And specific SM α-actin immunocytochemical staining in cytoplasmic conformed to VSMCs characteristics. The subcultures were set up via dissociation with 0.25% trypsin and 0.02% ethylenediaminetetraacetic acid (EDTA) after obtaining about 80% of confluence. VSMCs at passages 3–5 were utilized.
Cell Proliferation AssayCell proliferation assays were conducted with a CCK-8 Cell Counting Kit (Vazyme Biotech, Nanjing, China) based on the company’s directions. Briefly, 96-well plates were seeded with rat VSMCs (4×104/well). VSMCs were serum-deprived for 24 h prior to the 30 min treatment with L-theanine (5, 50, or 100 µM) and then incubated for 24 h with 100 nM Ang II. At the documented times, 10 µL of the CCK-8 working solution were placed into each well and incubated for 2 h at 37°C. The absorbance was quantified with an ELx 800 Universal Microplate Reader (Bio-Tek, Inc., U.S.A.) at a wavelength of 450 nm.
Cell Cycle AssayCell cycle analysis was determined by an Cell cycle Detection Kit (Vazyme Biotech, Nanjing, China) followed by flow cytometric analysis. The rat VSMCs (2×106/well) were seeded in 6-well plates. After being cultured for 24 h, VSMCs were treated with serum-free media for about 24 h to achieve synchronization at G0/G1 phase. After pretreatment with L-theanine (5, 50, or 100 µM) for 30 min, VSMCs were stimulated with Ang II. Cells were gathered with 0.25% trypsin, rinsed with phosphate buffered saline (PBS), and fixed in cold 70% ethanol at 4°C overnight. The cells were collected by centrifugation and incubated in RNaseA (1 mg/mL) at 37°C for 30 min, then stained with propidium iodide (PI, 1 µg/µL) at 4°C for 30 min. The percentages of cells (G0/G1, S, and G2+M) were analyzed on Becton Dickinson FACSCalibur.
VSMC Migration AssayThe migration of VSMCs was performed by Transwell chamber assay. The rat VSMCs (4×104/well) were seeded into Transwell culture chambers (8.0 µm pore-size; Corning, NY, U.S.A.) and then placed into a 24-well cell plates; 10% FBS was put in the lower chamber with no cells for 24 h. The cells were treatment with L-theanine (5, 50, or 100 µM) for 30 min. Next, Ang II (100 nM) was put into the lower chamber and left there for 24 h; after incubation, the upper chambers of the plate were taken out. Cells that were not migrating were cleared with a cotton swab from the upper surface of the Transwell filters. On the lower surface of the membrane, cells that were migrating fixed with 4% paraformaldehyde followed by a 2 min distilled water rinse. After staining with hematoxylin and eosin, visual-field images were arbitrarily chosen from five squares in each well using a light microscope at 100× magnification. The migrating cells were quantified and averaged; this was expressed as the relative migration rate.
Western BlottingRat VSMCs were treated with L-theanine (5, 50, or 100 µM) for 30 min before Ang II exposure. The cells were washed with cold PBS and then scraped off, lysed with lysis buffer (50 mM Tris pH 8.0; 150 mM NaCl; 0.02% sodium azide; 0.2% sodium dodecyl sulfate (SDS); 0.1 mM phenylmethylsulfonyl fluoride (PMSF); 10 mM NaF; 0.5 mM EDTA; 0.1 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA); 0.5% sodium deoxycholate; 10 µL/mL aprotinin; 1 mM β-glycerophosphate), and centrifuged at 12000 rpm at 4°C for 15 min. Identical amounts of proteins (20 µg) were separated with 12% SDS-polyacrylamide gel electrophoresis (PAGE) and moved to nitrocellulose membranes (Whatman, GE Healthcare, U.S.A.). The membranes were obstructed with 5% milk in Tris-buffered saline with 0.1% Tween-20 (TBST) at room temperature for 1 h, rinsed three times with TBST, and then incubated with primary antibodies against cyclin D1, STAT3, p-STAT3, JAK2, p-JAK2, p-Src, and Src (1 : 1000, Cell Signaling Technology), as well as ERK1/2, p-ERK1/2, and GAPDH (1 : 1000, Bioworld Biotechnology) overnight at 4°C. The membranes were rinsed three times with TBST and then incubated with anti-mouse or anti-rabbit IgG antibodies (1 : 5000, IRDye 800CW) in a dark room for 1 h at room temperature. After rinsing, the bands were detected with the Li-COR Odyssey infrared imaging system (LI-COR Biosciences, U.S.A.). Odyssey V1.2 software (LI-COR Biosciences) was used to quantify the band intensities.
Statistical AnalysisStatistical analyses were conducted with GraphPad 5.0. Data are expressed as the mean±standard error of the mean (S.E.M.) and differences were examined by one-way ANOVA followed with Tukey’s post-hoc test. Significance was defined as p<0.05.
The impacts of L-theanine on VSMC viability or Ang II-prompted VSMC proliferation were noted. The cytotoxicity of 100 µM L-theanine on VSMCs was not noted (Fig. 2A). The cells were pretreated with 5, 50, or 100 µM L-theanine for 30 min prior to Ang II exposure (100 nM). Ang II (100 nM) led to a significant elevation of cells (162.65±5.52% of controls). L-Theanine limited the proliferation of the VSMCs stimulated by Ang II in a dose-dependent manner. The inhibition rate was 29.6% when VSMCs were treated with 100 µM L-theanine.
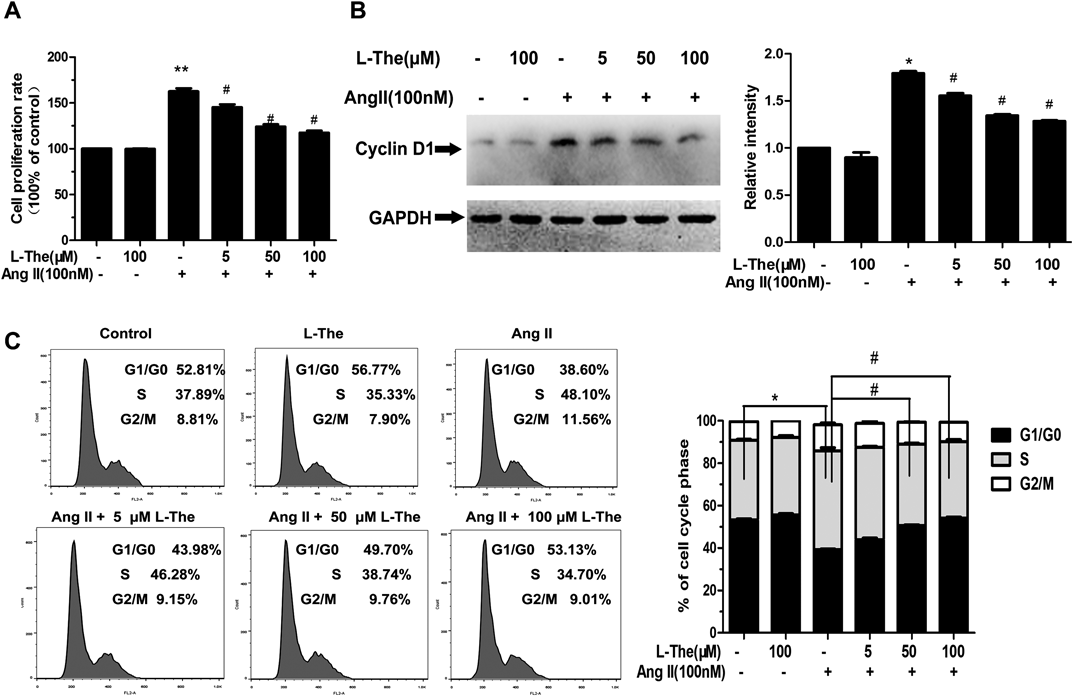
A) VSMCs were treated with serum-free media for approximately 24 h, and then were stimulated with 100 nM Ang II with or without L-theanine (5, 50, or 100 µM) for 24 h. The control group was not treated with Ang II or L-theanine. VSMC proliferation was assayed using CCK-8. B) VSMCs were treated with serum-free media for about 24 h. Cells were then given a 30-min pretreatment with or without L-theanine (5, 50, or 100 µM), followed by stimulation with 100 nM Ang II for 12 h. Western blotting with cyclin D1 antibody was used to analyze the cell lysates. Outcomes are expressed as the mean±S.E.M. of three independent experiments. * p<0.05, ** p<0.01 vs. control group; # p<0.05 vs. only Ang II-stimulated rat VSMCs group. C) VSMCs were treated with serum-free media for approximately 24 h, and then were pretreated with and without L-theanine (5, 50, or 100 µM) for 30 min. They were then stimulated with 100 nM Ang II for 24 h. The progression of the cell cycle was monitored using flow cytometry and the DNA stain PI. Each value represents as mean±S.E.M. (n=3). * p<0.05 vs. control group and # p<0.05 vs. only Ang II-stimulated rat VSMCs group (% cells in S phase) are indicated by lines.
Our outcomes revealed that Ang II-prompted cyclin D1 protein levels 1.8-fold in contrast to the control group. L-Theanine (at doses of 50 and 100 µM) significantly lowered cyclin D1 levels to 25.5 and 30.2%, respectively (p<0.05, in contrast to the Ang II group) (Fig. 2B). The outcomes verified that the lowering of cyclin D1 levels was required for an inhibitory effect of L-theanine on Ang II-triggered proliferation of VSMCs.
An additional new discovery was the inhibitory impact of L-theanine on Ang II-prompted proliferation of VSMCs based on the cell cycle assay. The percentage of cells in the G0/G1-phase following treatment with 100 µM L-theanine compared to untreated cells was 52.81±1.50 and 56.77±0.46%, respectively (p<0.05). Pretreatment with L-theanine at 5, 50, and 100 µM for 30 min before Ang II exposure significantly lowered the percentage of cells in S-phase to 46.28±1.90, 38.74±2.60, and 34.70±3.60% of controls, respectively (p<0.05). This impact was eliminated with 100 µM L-theanine by 27.90% in contrast to the Ang II group. These data showed that L-theanine inhibited Ang II-induced proliferation by reducing the cell cycle G1/S transition (Fig. 2C).
L-Theanine Suppresses Ang II-Induced VSMC MigrationTranswell assay also displayed that L-theanine suppressed the migration of VSMCs by Ang II stimulation. Cellular migration increased 2.27±0.18-folds in the Ang II group compared to the control group. At the concentration of 100 µM L-theanine and Ang II group reduced the migration of VSMC by 36.10% compared with the Ang II-treated group (p<0.05, Fig. 3).
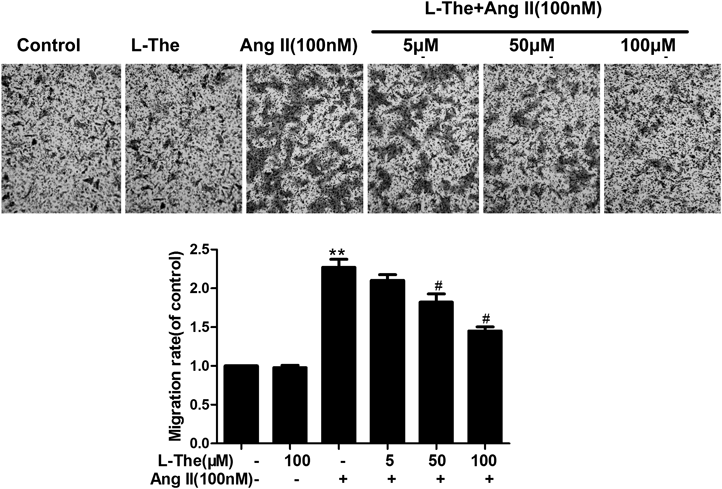
VSMCs were treated with serum-free media for approximately 24 h, and then were stimulated with 100 nM Ang II in the absence or presence of L-theanine (5, 50, or 100 µM) for 24 h. Outcomes are expressed as the mean±S.E.M. of three individual experiments. ** p<0.01 vs. control group; # p<0.05 vs. only Ang II-stimulated rat VSMCs group.
To continue to examine the possible pathways underlying the inhibition of L-theanine on Ang II-prompted VSMC proliferation and migration, we first examined the effect of L-theanine on the ERK signaling pathway. Phosphorylation ERK at the Thr202/Tyr204 site was probed by Western blot analysis. Nevertheless, L-theanine did not affect Ang II-mediated activation of ERK1/2, which indicated that it was not required for an inhibitory impact on VSMC proliferation prompted by Ang II (Fig. 4).
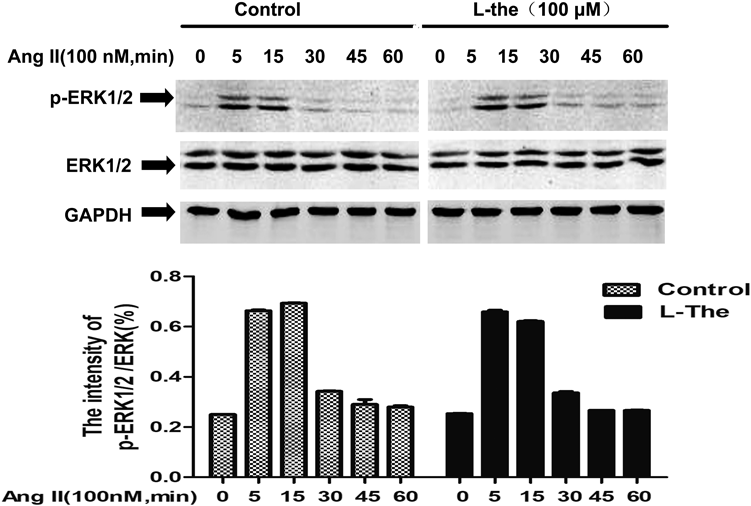
VSMCs were treated with serum-free media for about 24 h, and then pretreated with and without 100 µM L-theanine for 30 min. They were then stimulated for 0, 5, 15, 30, 45, and 60 min with 100 nM Ang II. Cell lysates were analyzed with Western blotting and antibodies as documented. Outcomes are expressed as the mean±S.E.M. of three individual experiments.
JAK/STAT signaling was activated by Ang II makes role in VSMC growth and migration. To estimate whether L-theanine inhibited the activation of JAK/STAT, we detected the activation of JAK2, STAT3 and Src by immunoblotting with the phosphorylation specific antibody. Halted VSMCs were pretreated with different concentrations of L-theanine (5, 50, and 100 µM). They were then stimulated with 100 nM Ang II for 2 h. The outcomes resulted in a similar conclusion in which L-theanine substantially lowered JAK2, STAT3, and Src activations that were prompted by Ang II in a concentration-dependent fashion (p<0.05, Fig. 5). The percentage inhibition of phosphorylated STAT3 observed with 5, 50, 100 µM L-theanine was 5.0, 25.0, and 30.1%, respectively.
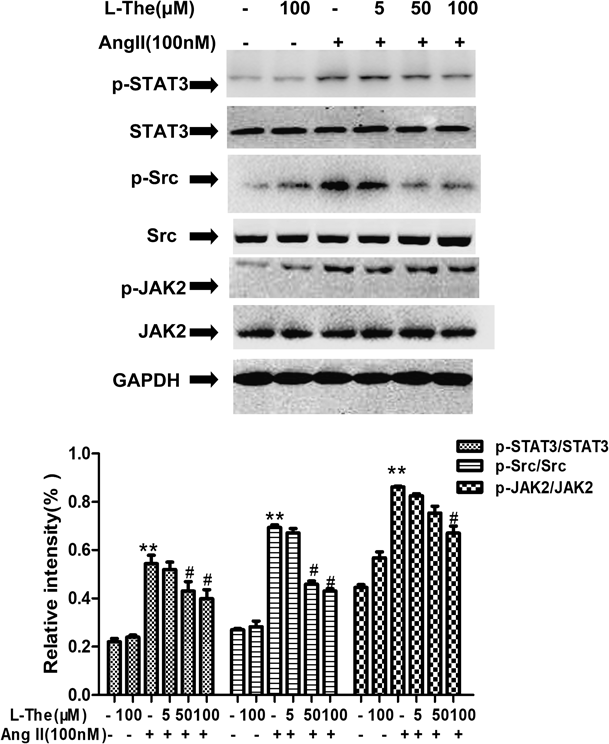
VSMCs were treated with serum-free media for about 24 h, and then pretreated with and without L-theanine (5, 50, or 100 µM) for 30 min. They were then stimulated with 100 nM Ang II for 2 h. Phosphorylated STAT 3, phosphorylated JAK2, and phosphorylated Src were immunoblotted using their respective antibodies. Outcomes are expressed as the mean±S.E.M. of three individual experiments.* p<0.05, ** p<0.01 vs. control group; # p<0.05 vs. only Ang II-stimulated rat VSMCs group.
Vascular injury is the pathological foundation of inflammation. It underlies atherosclerosis, hypertension, and ischemic heart disease that is connected to VSMC growth, calcification, migration, differentiation, and generation of extracellular matrix proteins, which are prompted mainly by Ang II.22) We chose vascular smooth muscle cells as the target model cells in this study. We evaluated the impact of L-theanine on Ang II-induced proliferation and migration in rat VSMCs and examined its underlying mechanisms.
L-Theanine is soluble in water, and it is quickly absorbed and reaches its maximum concentration in blood between 30 min and 2 h.23) In the present study, we selected three doses of L-theanine (5, 50 and 100 µM) to treat VSMCs 30 min, and these doses are not toxic to cells (Fig. 2A). The proliferation and migration of VSMCs prompted by Ang II are required events that underlie the resulting problems of vascular injury.24) CCK-8 method and Transwell chamber assay were employed to detect VSMCs proliferation and migration respectively. Our outcomes revealed that VSMC proliferation and migration can be significantly prompted at a dose of 100 nM Ang II. L-Theanine can prevent these Ang II impacts in a concentration-dependent fashion (Figs. 2B, 3).
In typical mammalian cells, cell cycle proteins control proliferation during the G1 phase of the cell cycle.25) Cyclin D1 is expressed and synthesized in the cell proliferation period, its expression level is an important mediator that reduces cell cycle G1-S phase transition.26) Therefore, we also performed immunoblotting of cyclin D1 to verify effects on proliferation. In this regard, Ang II has been shown to induce cyclin D1 in VSMCs. L-Theanine lowered the levels of cyclin D1 that were prompted by Ang II. This indicated that L-theanine controls the cell cycle from the G1 to S phase to prevent VSMC proliferation (Fig. 2C).
Although some investigations reported MAPKs pathway is mediated cell cycle proteins expression or activation.27) In blood vessels, Ang II bonded to AT1 receptors that activates ERK1/2 within 5 min.5) Contrary to the findings of L-theanine decreased expression of cyclin D1, we did not find activation of ERK1/2 was not suppressed by L-theanine and it was not time-dependent manner (Fig. 4). In human and rat VSMCs, JAK2 has a pivotal part in Ang II-prompted neointima formation after vascular damage. Also, c-Src is critically involved in Ang II-prompted vasoconstriction damage, which can cause hypertension.28,29) STAT3 is the focus of many tyrosine kinase signal pathways, such as epidermal growth factor receptor (EGFR), interleukin-6 (IL-6)/JAK, and Src. STAT3 proteins dimerize, translocate into the nucleus, and activate p21WAF1/CIP1 and cyclin D1 gene by the Src oncoprotein in mouse fioblasts.30) Qin et al. demonstrated that STAT3 directly bind to cyclin D1 promoter by Chromatin Immunoprecipitation (ChIP) assay and inhibition of STAT3 decreased cyclin D1 expression to decrease the HT-29 cell viability.31) And ATP induced cyclin D1 expression via STAT3 activation to engage in VSMCs proliferation.32) Matrix metalloproteinase-2 (MMP-2) promoter activity assessed with anti-STAT3 antibodies by chromatin immunoprecipitation, which knockdown blocked vascular adventitial fibroblast migration and phenotypic alterations.33) STAT3-knockdown of VSMCs by small interfering RNA (siRNA) inhibited Gastrin-releasing peptide (GRP)-induced migration of VSMCs.34) STAT3 activation induces abnormal high expression of the key genes such us cyclin D1 and MMP-2 related to the cell proliferation and migration. We have verified that using Western blot produces similar results that Ang II stimulates JAK2, Src and STAT3 phosphorylation in VSMCs. And L-theanine significantly inactivated the Src/STAT3 and JAK2 on Ang II-induced (Fig. 5).
Prior studies revealed that Ang II prompts nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox), leading to elevated reactive oxygen species (ROS) production, which regulates VSMC proliferation.35) ROS activate multiple signaling molecules, including JAK/STAT3.32,36) L-Theanine has antioxidant activity that counteracts chronic restraint stress and improves cognitive decline by lowering oxidative stress. We already documented that lowered antioxidant enzyme activity, including that of glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD), in the brains of cadmium-intoxicated mice were counteracted by L-theanine.15) We speculate that L-theanine reduced Ang II-induced JAK/STAT3 pathway might be due to L-theanine has antioxidant function. Further research is required to more fully explain the mechanisms of L-theanine.
In conclusion, the identification and characterization of effective compounds that can inhibit VSMCs proliferation and migration have become one hot spot of investigation to prevent hypertension. The outcomes from this evaluation indicated that L-theanine can prevent Ang II-stimulated VSMC proliferation and migration. The impacts of L-theanine were partly connected to the inhibition of the JAK/STAT3 pathway. Additional evaluations on the detailed molecular mechanisms of L-theanine may indicate clinical applications of L-theanine for treatment of cardiovascular conditions.
This research was supported by the National Nature Science Foundation of China (Grant No. 81471557), the Natural Science Research Project of Anhui Provincial Education Department (Grant No. KJ2017A908) and the Science Research Project of Chuzhou City Vocation College (Grant No. 2016zk02).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.