
- |<
- <
- 1
- >
- >|
-
Michihisa Tohda, Hiroshi Watanabe2018Volume 41Issue 11 Pages 1627-1631
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
Advance online publication: August 28, 2018JOURNAL FREE ACCESS FULL-TEXT HTMLThis review article mentions about the following points, and proposes its importance and positive thinking. 1) Wakan-yaku (Japanese oriental medicines) is covered by the national health insurance system in Japan as therapeutic drugs to be actively used in medical practice to treat illness. 2) Applications of Wakan-yaku is accomplished based on the reliable own theories which are established with long histories. 3) Promotion of studies based on these theories will be highly expected to find novel view points which breaks conventional concepts and to novel standards for developing new medicinal drugs. Although studies based on the reliable Wakan-yaku theories are not advancing satisfactorily till now, the possibilities to obtain the advanced resources for drugs and novel viewpoints for experiments by studies about Wakan-yaku theories are discussed in this review.
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (682K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (682K) Full view HTML
-
Takahisa Nakane, Hirohisa Doi, Masayoshi Hirohara, Hajime Hamashima, Y ...2018Volume 41Issue 11 Pages 1632-1637
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTMLTacrolimus ointment is used worldwide to treat atopic dermatitis. Although tacrolimus ointment is not suitable for clinical admixtures, it is often mixed with various ointments or creams, such as corticosteroids, antibacterial agents, and moisturizing agents. There is only one report of quality testing of admixtures of tacrolimus ointment with adaparene gel (Differin® Gel). In this study, we used HPLC to evaluate the pharmaceutical stability of tacrolimus mixed with eight different dermatologic ointments or creams. No decrease in the tacrolimus content was observed in any of the mixtures after 4 weeks of storage at room temperature, indicating that tacrolimus admixtures are stable.
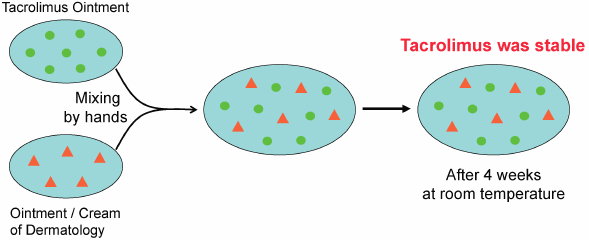 Graphical Abstract Fullsize ImageView full abstractDownload PDF (596K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (596K) Full view HTML -
Yashu Xu, Cheng Yang, Shujun Zhang, Jiajun Li, Qing Xiao, Wenxiang Hua ...2018Volume 41Issue 11 Pages 1638-1644
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
Advance online publication: August 21, 2018JOURNAL FREE ACCESS FULL-TEXT HTML
Supplementary materialNon-alcoholic fatty liver disease (NAFLD) is increasingly prevalent and represents a growing challenge in terms of prevention and treatment. The purpose of this study is to investigate the protective effects of ginsenoside Rg1 (Rg1), an active ingredient of a natural medicine, and further clarify its protective mechanisms, in a mouse model of NAFLD induced by a high-fat diet. Rg1 significantly reduced liver weight, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), liver free fatty acids (FFAs) and malondialdehyde (MDA) levels, and increased superoxide dismutase (SOD) activity. Rg1 also upregulated the expression of peroxisome proliferator-activated receptor-alpha (PPARα), which stimulated fatty acid beta oxidation and promoted the metabolism of FFAs and TG. It also suppressed the expression of CCAAT/enhancer binding protein (C/EBP) homologous protein (CHOP), cysteine-containing aspartate-specific proteases 12 (Caspase 12), and glucose-regulated protein78 (GRP78), which reduced endoplasmic reticulum (ER) stress. Furthermore, Rg1 alleviated liver inflammation by inhibiting the activation of nucleotide binding oligomerization domain (NOD)-like receptor family pyrin domain-containing 3 (NLRP3) and thus reduced the production of inflammatory cytokines, such as interleukin 1-beta (IL-1β) and interleukin 18 (IL-18). These results suggested that Rg1 may protect against NAFLD, through regulation of lipid peroxidation, ER stress and inflammasome activation.
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (2075K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (2075K) Full view HTML -
Dongjian Xia, Zhuang Zhang, Yuanli Zhao2018Volume 41Issue 11 Pages 1645-1651
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTML
Supplementary materialActeoside (ACT) has been shown to exert antioxidant and neuroprotective effects in neurodegenerative diseases. However, the effect of ACT on cerebral ischemia–reperfusion (I/R) injury is not yet clear. In this study, we found that ACT administration reduced infarct volume and brain edema, and improved neurological deficits, as indicated by the decreased modified neurological severity score. Administration of ACT strikingly reduced oxidative stress, accompanied by decreased levels of reactive oxygen species and malondialdehyde and increased levels of superoxide dismutase and catalase in a rat model of middle cerebral artery occlusion/reperfusion (MCAO/R). Furthermore, ACT administration reduced the number of terminal deoxynucleotidyl transferase uridine 5′-triphosphate (UTP) nick-end labeling-positive cells in the cerebral cortex of ischemic side of MCAO/R rats, accompanied by downregulation of B cell lymphoma 2 (Bcl-2) associated X protein and cleaved caspase-3 proteins and upregulation of Bcl-2 protein. Additionally, ACT treatment inhibited the protein kinase R/eukaryotic initiation factor-2α stress pathway in the brains of MCAO/R rats. Our results demonstrated that ACT attenuates oxidative stress and neuronal apoptosis in MCAO/R rats, suggesting that ACT may serve as a novel therapeutic candidate for the treatment of I/R brain injury.
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (1130K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (1130K) Full view HTML -
Hongtao Lu, Jiarong Ding, Wenrui Liu, Zhongjiang Peng, Wei Chen, Xueju ...2018Volume 41Issue 11 Pages 1652-1658
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTMLHydrogen has a significant protective effect on calcium oxalate-induced renal injury, but its effect on metabolic profiles is unknown. This study showed the effects of hydrogen on serum and urine metabolites in a renal injury model. Ultra-HPLC quadrupole time-of-flight-MS-based metabolomics was used to characterise metabolic variations. Twenty-five serum metabolites and 14 urine metabolites showed differences in the the nitrogen and oxygen inhalation (NO), nitrogen and oxygen inhalation combined with calcium oxalate induction (CaOx), and hydrogen inhalation combined with calcium oxalate induction (HO+CaOx) groups. Nineteen serum metabolites and 7 urine metabolites showed significant restoration to normal levels after hydrogen gas (H2) treatment. These metabolites are primarily related to amino acid metabolism, fatty acid metabolism, and phospholipid metabolism. This study showed that a comprehensive metabolomics approach is an effective strategy to elucidate the mechanisms underlying the effects of hydrogen treatment on calcium oxalate-induced renal injury.
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (2427K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (2427K) Full view HTML -
Orawin Prangsaengtong, Phatcharida Jantaree, Kriengsak Lirdprapamongko ...2018Volume 41Issue 11 Pages 1659-1666
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTMLLymphangiogenesis, the formation of lymphatic vessels from preexisting ones, promotes cancer growth and metastasis. Finding natural compounds with anti-lymphangiogenic activity will be useful for preventive treatment of lymphatic metastasis. Shikonin, an ingredient of a traditional Japanese and Chinese medicinal herb Lithospermum erythrorhizon, has been widely used in several pharmaceutical and cosmetic preparations, as well as in food colorants. Shikonin has been reported to inhibit lymphangiogenesis in vitro, but the mechanism of inhibition has not been determined. The aim of this study is to investigate the mechanism of anti-lymphangiogenesis of shikonin in primary human lymphatic endothelial cells (HMVEC-dLy). Shikonin, at non-toxic concentrations, significantly inhibited cord formation ability of lymphatic endothelial cells in a dose- and time-dependent manner. Western blotting analysis showed that shikonin decreased nuclear factor-kappaB (NF-κB) activation, as indicated by phosphorylation and nuclear translocation of NF-κB p65, and also reduced both mRNA and protein levels of hypoxia-inducible factor-1 (HIF-1)α. Use of an NF-κB inhibitor (BAY 11-7085) and HIF-1α small interfering RNA (siRNA) transfection revealed that NF-κB activation was upstream of HIF-1α expression, which controls cord formation by HMVEC-dLy. In addition, the reduction of vascular endothelial growth factor C (VEGF-C) and vascular endothelial growth factor receptor-3 (VEGFR-3) mRNA levels were also found in HMVEC-dLy that treated with shikonin. In conclusion, shikonin inhibits lymphangiogenesis in vitro by interfering the NF-κB/HIF-1α pathway and involves in suppression of VEGF-C and VEGFR-3 mRNA expression.
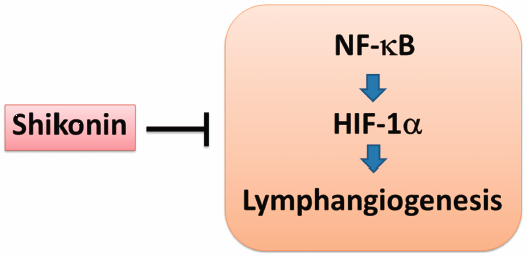 Graphical Abstract Fullsize ImageView full abstractDownload PDF (2339K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (2339K) Full view HTML -
Mayako Uchida, Tsutomu Nakamura, Takahiro Shima, Goichi Yoshimoto, Koj ...2018Volume 41Issue 11 Pages 1667-1671
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTMLChemotherapy-induced nausea and vomiting (CINV) are generally evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE). The Multinational Association for Supportive Care in Cancer (MASCC) developed the MASCC Antiemesis Tool (MAT) to facilitate recognition for CINV between patients and oncology specialists. In the present study, MAT and CTCAE were comparatively assessed in Japanese patients with hematological malignancies. A total of 61 patients were eligible for this study. The CTCAE data were collected from an electronic medical record system. The patients were asked to complete the Japanese version of MAT in the hospital, on the first and fourth days after the start of chemotherapy. The percentages of patients in whom nausea was completely controlled, with severity scores of zero, ranged from 70.5 to 82.0% for CTCAE and from 59.0 to 75.4% for MAT, during the first five days after the chemotherapy. The percentages of patients who had no vomiting ranged from 93.4 to 96.7% for CTCAE and from 90.2 to 98.4% for MAT. During the observation periods, the day-to-day response profiles of patients who received antiemetic treatment were comparable between CTCAE and MAT cohorts, and these two assessment tools showed good, positive correlations for nausea severity scores. The present study shows that the MAT is a useful tool for assessing the severity of CINV in patients with hematological malignancy, is comparable to CTCAE, and facilitates the identification of poor cancer care conditions by medical staff.
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (555K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (555K) Full view HTML -
Masataka Moriuchi, Yoshio Nakano, Yu Tsurekawa, Mariam Piruzyan, Shing ...2018Volume 41Issue 11 Pages 1672-1677
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTMLTaurine has important physiological roles as well as a wide range of pharmacological effects. Studies have suggested that taurine ameliorates diabetes, hypertension, oxidative stress, and inflammatory diseases. However, its mechanisms of action are still unclear. It has been reported that N-acyl taurine activates transient receptor potential vanilloid-1 (TRPV1), which is related to the pathogenesis of many inflammatory diseases. In this study, we hypothesized that taurine has a regulatory effect on TRPV1 activation via N-acyl taurine. To evaluate this hypothesis, we assessed the calcium influx activated by a TRPV1 agonist in human keratinocyte (HaCaT) cells and paraquat-induced oxidative stress in Caenorhabditis elegans. Our results indicate that taurine inhibits TRPV-dependent activity to overcome oxidative stress in cultured cell lines and in C. elegans.
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (1189K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (1189K) Full view HTML -
Peiling Ben, Monong Hu, Huizhen Wu, Zhengping Zhang, Yanhong Gao, Lan ...2018Volume 41Issue 11 Pages 1678-1684
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTML
Supplementary materialL-Theanine, a green tea amino acid derivative, has cardiovascular qualities. The focus of the current evaluation was to examine the suppression of L-theanine on cultured vascular smooth muscle cell (VSMC) proliferation and migration that is prompted by angiotensin II (Ang II). The VSMCs were treated with non-cytotoxic concentrations of L-theanine and then stimulated with Ang II. The CCK-8 and Transwell chamber assays were monitored on the proliferation and migration rate, respectively. We discovered that L-theanine (50 and 100 µM) significantly halted Ang II-induced VSMC proliferation and migration. This was joined by a decline in the amount of cyclin D1. An additional discovery was that L-theanine lowered the proportion of S-phase cells, whereas the number of G1/G0-phase cells in Ang II-stimulated VSMCs was elevated, based on flow cytometry. Western blotting analyses indicated that L-theanine had no impact on extracellular-signal-regulated kinase 1/2 (ERK1/2) activation prompted by Ang II. Nevertheless, L-theanine significantly lowered Ang II-prompted phosphorylation of Janus kinase 2 (JAK2), c-Src tyrosine kinase, and signal transducer and activators of transcription 3 (STAT3). The outcomes revealed that L-theanine subdued the Ang II-prompted proliferation and migration of VSMC, partly via the obstruction of the JAK/STAT3 pathway instead of via just the ERK pathway.
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (1842K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (1842K) Full view HTML -
Cheng Wei Lu, Hsi Lung Hsieh, Tzu Yu Lin, Ting Yang Hsieh, Shu Kuei Hu ...2018Volume 41Issue 11 Pages 1685-1693
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
Advance online publication: September 08, 2018JOURNAL FREE ACCESS FULL-TEXT HTMLEchinacoside is a major compound of Cistanche Herb and has glutamate release-inhibiting activity in the brain. Given the involvement of excitotoxicity caused by massive glutamate in the pathophysiology of epilepsy, we explored the antiepileptic effect of echinacoside on kainic acid-induced seizures in rats. The rats were intraperitoneally administrated echinacoside for 30 min prior to intraperitoneal injection with kainic acid. The results showed that kainic acid induced seizure-like behavioral patterns, increased glutamate concentrations, caused neuronal loss and microglial activation, and stimulated proinflammatory cytokine gene expression in the hippocampus. These kainic acid-induced alternations were found to be attenuated by echinacoside pretreatment. Furthermore, decreased Akt and glycogen synthase kinase 3β (GSK3β) phosphorylation as well as Bcl-2 expression in the hippocampus was reversed by the echinacoside pretreatment. These results demonstrate that echinacoside exert its antiepileptic and neuroprotective actions in a kainic acid rat model through suppressing inflammatory response and activating the Akt/GSK3β signaling. Therefore, the present study suggests that echinacoside is the potentially useful in the prevention of epilepsy.
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (5155K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (5155K) Full view HTML -
Yusuke Noguchi, Yugo Kawashima, Megumi Maruyama, Hiroko Kawara, Yoko T ...2018Volume 41Issue 11 Pages 1694-1700
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTMLPaclitaxel and nanoparticle albumin-bound paclitaxel are known to cause adverse events of eye disorders, such as cystoid macular edema. However, at present, the risk factors remain unclear. Therefore, risk factors for eye disorders caused by paclitaxel and nanoparticle albumin-bound paclitaxel were studied. This retrospective study targeted patients who were newly administered paclitaxel or nanoparticle albumin-bound paclitaxel at Kyoto Okamoto Memorial Hospital between April 1, 2012, and March 31, 2017. Eye disorder occurrence was defined as an event in which the pharmacist confirmed the symptoms in a patient interview and the ophthalmologist diagnosed the disorder. To analyze the risk factors, logistic regression analysis using 41 factors was performed. Of 128 subjects, 13 (10.2%) had eye disorders with symptom degrees of Grades 1 and 2. The symptoms were conjunctivitis or subconjunctival hemorrhage (3.1%), visual acuity reduction (2.3%), blurred vision and eye pain (1.6% each), eye mucus, blepharitis, stye, watering eyes, photopsia, and muscae volitantes (0.8% each). In eight patients, the conditions patients improved with spontaneously or with medication use; no improvements were observed the cases of visual acuity reduction, blurred vision, or muscae volitantes. Multivariate logistic regression analysis revealed that a cumulative dose of ≥819 mg/m2 (odds ratio: 5.34, 95% confidence interval: 1.32–21.60, p=0.019) and baseline alkaline phosphatase ≥256 U/L (odds ratio: 3.74, 95% confidence interval: 1.02–13.70, p=0.046) were significant risk factors associated with eye disorders. In conclusion, it was determined that paclitaxel- and nanoparticle albumin-bound paclitaxel-related eye disorders might be influenced by cumulative dose and baseline alkaline phosphatase.
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (288K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (288K) Full view HTML -
Jeong-Hun Lee, Dong Gyu Leem, Kyung-Sook Chung, Kyung-Tack Kim, Sang Y ...2018Volume 41Issue 11 Pages 1701-1707
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTMLPanaxydol, a polyacetylenic compound derived from Panax ginseng has been reported to suppress the growth of cancer cells. However, the molecular mechanisms underlying cell cycle arrest by this compound in non-small cell lung cancer (NSCLC) are unknown. Our study found that panaxydol treatment induced cell cycle arrest at G1 phase in NSCLC cells. The cell cycle arrest was accompanied by down-regulation of the protein expression of cyclin-dependent kinase (CDK) 2, CDK4, CDK6, cyclin D1 and cyclin E, and decrease in the phosphorylation of retinoblastoma (Rb) protein. Furthermore, up-regulation of cyclin-dependent kinase inhibitor (CDKI) p21CIP1/WAF1 and p27KIP1 was observed in panaxydol-treated NSCLC cells. In addition, panaxydol also induced accumulation of intracellular Ca2+ ([Ca2+]i). (Acetyloxy)methyl 2-({2-[(acetyloxy)methoxy]-2-oxoethyl}[2-(2-{2-[bis({2-[(acetyloxy)methoxy]-2-oxoethyl})amino]phenoxy}ethoxy)phenyl]amino)acetate (BAPTA-AM), the Ca2+ chelator, attenuated not only panaxydol-induced accumulation of [Ca2+]i, but also G1 cell cycle arrest and decrease of CDK6 and cyclin D1 protein expression level. These results demonstrated that the anti-proliferative effects of panaxydol were caused by cell cycle arrest, which is closely linked to the up-regulation of [Ca2+]i and represents a promising approach for the treatment of lung cancer.
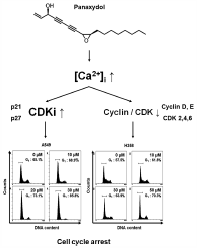 Graphical Abstract Fullsize ImageView full abstractDownload PDF (1539K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (1539K) Full view HTML -
Satomi Yamaguchi Ikeuchi, Atsushi Kambayashi, Hiroyuki Kojima, Naoto O ...2018Volume 41Issue 11 Pages 1708-1715
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTMLThe purpose of this research was to establish an in vitro dissolution testing method to predict the oral pharmacokinetic (PK) profiles and food effects of gabapentin enacarbil formulated as wax matrix extended-release (ER) tablets in humans. We adopted various biorelevant dissolution methods using the United States Pharmacopeia (USP) apparatus 2, 3 and 4 under simulated fasted and fed states. Simulated PK profiles using the convolution approach were compared to published in vivo human PK data. USP apparatus 2 and 4 underestimated the in vivo performance due to slow in vitro dissolution behaviors. In contrast, biorelevant dissolution using USP apparatus 3 coupled with the convolution approach successfully predicted the oral PK profile of gabapentin enacarbil after oral administration of a Regnite® tablet under fasted state. This approach might be useful for predicting the oral PK profiles of other drugs formulated as wax matrix-type ER tablets under fasted state.
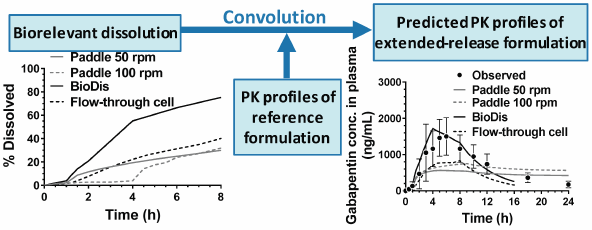 Graphical Abstract Fullsize ImageView full abstractDownload PDF (707K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (707K) Full view HTML -
 Masahiro Tsuda, Yuki Otani, Atsushi Yonezawa, Sho Masui, Yasuaki Ikemi ...2018Volume 41Issue 11 Pages 1716-1721
Masahiro Tsuda, Yuki Otani, Atsushi Yonezawa, Sho Masui, Yasuaki Ikemi ...2018Volume 41Issue 11 Pages 1716-1721
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
Advance online publication: August 30, 2018JOURNAL FREE ACCESS FULL-TEXT HTMLBiosimilar products of therapeutic antibodies have been launched all over the world. They can relieve some of the economic burden of medicines. Although clinical trials have demonstrated the equivalency of biosimilar products with their reference product, biosimilar products are not commonly used in clinical practice. One reason is that the structural difference between the reference product and a biosimilar one remains unclear. We analyzed glycoforms and amino acids of an infliximab biosimilar product approved in Japan compared to that of the reference product (Remicade®). By combination of papain digestion and LC/ time-of-flight (TOF)-MS, we established a valuable method to analyze these therapeutic antibodies. Nine glycoforms were detected in infliximab, and a difference in amino acids was observed. In the glycoforms of MMF, MGnF/GnMF, GnGn, GnGnF, AGnF/GnAF, and AAF, the relative intensities were significantly different between the reference and biosimilar product. Furthermore, we elucidated that the content rate of the C-terminal lysine was different among glycoforms. In conclusion, our analytical method can analyze not only amino acids but also carbohydrate chains of therapeutic antibodies, and will provide a useful strategy to evaluate bio-medicines including biosimilar antibodies.
 Graphical Abstract Fullsize ImageView full abstractEditor's pick
Graphical Abstract Fullsize ImageView full abstractEditor's pickHow can you know the structural difference between biosimilar and reference product? The structure of biosimilar products is not the same as their original product because of post-translational modifications. In the article by Tsuda et al., a valuable method with a papain digestion and LC/TOF-MS analysis was established. Their new method can analyze not only carbohydrate chains but also amino acids of bio-medicines including biosimilar antibodies. This technology will provide a useful strategy to evaluate bio-medicines including biosimilar antibodies.
Download PDF (879K) Full view HTML -
Takeshi Chiba, Satoru Nihei, Hideaki Komatsu, Mami Obara, Yasushi Ishi ...2018Volume 41Issue 11 Pages 1722-1726
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTMLThe objective of this study was to investigate whether improving glycemic control reduces the prevalence and progression of proteinuria among bevacizumab (BEV)-treated cancer patients with type 2 diabetes mellitus (DM). We retrospectively reviewed the medical records of 55 patients with type 2 DM who were treated with BEV between July 1 2011 and May 31 2018 at Iwate Medical University Hospital. The patients were classified based on changes in glycated hemoglobin (HbA1c) level during the 3 months following BEV administration into the “HbA1c elevated” group (+0.5% or above, n=24) and the “HbA1c non-elevated” group (indicating no change or decrease; n=31). At 3 months following BEV administration, the means of HbA1c and its change rate in the ‘HbA1c elevated’ group was significantly higher than that in the ‘HbA1c non-elevated’ group, and the prevalence of proteinuria in the ‘HbA1c elevated’ group was significantly higher than that in the ‘HbA1c non-elevated’ group. Additionally, our subjects were classified into the proteinuria group and non-proteinuria group. The mean HbA1c level in the proteinuria group was significantly higher than that in the non-proteinuria group at 3 months following BEV administration. Furthermore, the mean rates of change of HbA1c level in patients experiencing grades 1 and 2 proteinuria were +9.97±2.26 and +14.0±3.82%, respectively. These values were significantly higher than those of patients with no proteinuria (−2.15±1.96%). Our results suggest that deterioration of glycemic control contributes to the prevalence of proteinuria among BEV-treated cancer patients with type 2 DM.
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (316K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (316K) Full view HTML
-
Satoru Esumi, Yoshihisa Kitamura, Hitomi Yokota-Kumasaki, Soichiro Ush ...2018Volume 41Issue 11 Pages 1727-1731
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTMLDuloxetine is a serotonin/noradrenaline reuptake inhibitor that is used as an antidepressant. However, it is known to cause constipation as a side effect. Magnesium compounds, such as magnesium oxide and magnesium hydroxide aqueous solution, are often combined with duloxetine to ameliorate the constipation caused by duloxetine. However, there is concern that these magnesium compounds might alter the effects of duloxetine via physicochemical interactions. In this study, we attempted to clarify the interactions that take place between duloxetine and magnesium oxide using in vivo and in vitro experiments. We evaluated the influence of magnesium oxide on in vitro duloxetine concentrations using HPLC. In addition, we examined the in vivo antidepressant-like effects and serum concentrations of duloxetine in rats. In the in vitro experiment, the duloxetine concentration was significantly decreased by co-treatment with magnesium oxide. In the in vivo experiment, the antidepressant-like effects of duloxetine were not affected by the combined oral administration of magnesium oxide and a duloxetine formulation although the serum duloxetine level was significantly decreased. However, the antidepressant-like effects of a duloxetine reagent were significantly attenuated by the co-administration of magnesium oxide. These results suggest that duloxetine and magnesium oxide directly interact and that such interactions affect the absorption and antidepressant-like effects of duloxetine.
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (469K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (469K) Full view HTML -
Chie Munakata, Yuki Fuchigami, Shu Hiroishi, Ayana Haraguchi, Masayori ...2018Volume 41Issue 11 Pages 1732-1735
Published: November 01, 2018
Released on J-STAGE: November 01, 2018
JOURNAL FREE ACCESS FULL-TEXT HTMLAdministration of high doses of acetaminophen (APAP) is known to cause drug-induced liver injury (DILI) in humans. Therefore, the detection or prediction of these side-effects at an early stage using appropriate biomarkers is the need of the hour. Micro RNA (miR)-122 is expected to be a novel biomarker for liver injury. However, more evidence is required in various alternate situations such as its use in combination as APAP is often used along with anticancer drugs. In the present study, we aimed to evaluate the functions of miR-122 as a biomarker for liver injury in comparison with alanine aminotransferase (ALT) in a mice model with the APAP-induced liver injury (AILI). Consequently, there was a dose-dependent increase in miR-122 after administration of APAP intraperitoneally. Similar observations were made for ALT activity. Additionally, the expression of miR-122 increased in a more rapid manner compared to ALT activity. However, there was a variation in the miR-122 expression. Further, we investigated the drug–drug interaction between APAP and 5-fluorouracil using miR-122 and ALT in mice. As a result, the degree of AILI was not changed by the use of 5-fluorouracil in combination with APAP in mice.
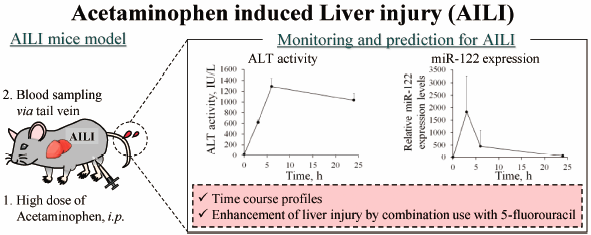 Graphical Abstract Fullsize ImageView full abstractDownload PDF (477K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (477K) Full view HTML
- |<
- <
- 1
- >
- >|