2018 Volume 41 Issue 12 Pages 1809-1817
2018 Volume 41 Issue 12 Pages 1809-1817
2,3-Dimethoxy-5-methyl-p-benzoquinone is a common chemical structure of coenzyme Q (CoQ) that conjugates different lengths of an isoprenoid side chain at the 6-position of the p-benzoquinone ring. In a series of studies to explore the cytotoxic mechanism of CoQ homologues with a short isoprenoid side chain, we found that a CoQ analogue without an isoprenoid side chain, CoQ0, showed marked toxicity against HeLa cells in comparison with cytotoxic homologues. Therefore, we examined the cytotoxic mechanism of CoQ0. Different from the cytotoxic CoQ homologues that induced apoptosis, 100 µM CoQ0 induced necrosis of HeLa cells. The CoQ0-induced cell death was accompanied by a decrease in endogenous non-protein and protein-associated sulfhydryl (SH)-groups, but this improved with the concomitant addition of compounds with SH-groups but not antioxidants without SH-groups. In addition, UV-spectrum analysis suggested that CoQ0 could rapidly form S-conjugated adducts with compounds with SH-groups by Michael addition. On the other hand, enzyme activities of both glyceraldehyde-3-phosphate dehydrogenase, which has a Cys residue in the active site, and α-ketoglutarate dehydrogenase complex, which requires cofactors with SH-groups, CoA and protein-bound α-lipoic acid, and CoA and ATP contents in the cells were significantly decreased by the addition of CoQ0 but not CoQ1. Furthermore, the decrease of an endogenous antioxidant, glutathione (GSH), by CoQ0 treatment was much greater than the predicted increase of endogenous GSH disulfide. These results suggest that CoQ0 rapidly forms S-conjugate adducts with these endogenous non-protein and protein-associated SH-groups of HeLa cells, which disrupts carbohydrate metabolism followed by intracellular ATP depletion and necrotic cell death.
2,3-Dimethoxy-5-methyl-p-benzoquinone is a common chemical structure of coenzyme Q (CoQ), and many CoQ homologues that conjugate different lengths of an isoprenoid side chain at the 6-position of the p-benzoquinone ring (Fig. S1) exist in nature.1) In most mammals including humans, CoQ10, with an isoprenoid side chain that consists of ten polymerized 2-methyl-1,3-butadienes (isoprene), is the only CoQ homologue to participate in ATP production as an electron carrier in the mitochondrial respiratory chain, but there are organisms with other CoQ homologues besides CoQ9 as the main homologue, such as rodents. In general, CoQ homologues with a longer isoprenoid side chain than CoQ5 participate in ATP production in higher organisms and are essential for maintaining their life activities, but CoQ homologues with a shorter isoprenoid side chain than CoQ5 are toxic to mammalian cells.2,3)
Many quinone compounds in nature are toxic because most exogenous quinone compounds undergo redox-cycling in the process of metabolism in cells.4) When quinone compounds are distributed in cells, they undergo one-electron reduction by intracellular one-electron-reducing enzymes such as reduced nicotinamide adenine dinucleotide phosphate (NADPH)-CYP reductase, by which semiquinone radicals are generated. Because the semiquinone radicals are very labile under aerobic conditions, they immediately oxidize to the parent quinone compounds in the presence of molecular oxygen concomitantly with generation of the superoxide anion (·O2−). The superoxide anion undergoes a dismutation reaction in the presence of intracellular superoxide dismutase, and produces hydrogen peroxide (H2O2) and molecular oxygen. Hydrogen peroxide yields another highly reactive oxygen radical, the hydroxyl radical (·OH), in the presence of ferrous ions (Fenton reaction). Furthermore, the superoxide anion can change to a hydroperoxyl radical (·OOH) in water5) and/or can react with free radical nitric oxide (NO) to generate peroxynitrite (ONOO−).6) Because reactive oxygen species (ROS), such as ·O2−, H2O2, ·OH, and ·OOH, and reactive nitrogen species (RNS), such as ONOO−, show high reactivity, they can rapidly oxidize neighboring biomolecules, such as sugars, unsaturated fatty acids, amino acids, proteins, and nucleic acids, disrupt cell homeostasis, and eventually induce cell death. Furthermore, it is also known that peroxynitrite can rapidly react with the aromatic ring of free or protein-associated tyrosine residues.7) In addition, some quinone compounds can directly alkylate biomolecules, such as DNA, in the process of metabolism and induce cell death.8) Thus, although quinone compounds are generally harmful to cells, they may be beneficial in some cases. For example, many medicines with a quinone ring in the chemical structure, such as mitomycin C and doxorubicin, are frequently used for cancer therapy by utilizing these properties.
In a previous paper, we reported that CoQ homologues with a shorter isoprenoid side chain than CoQ5 are cytotoxic compounds that can induce apoptosis by which HeLa cells are induced to undergo cell death (3). Simultaneously, we found that CoQ0, which is a CoQ analogue and does not have an isoprenoid side chain at the 6-position of the 2,3-dimethoxy-5-methyl-p-benzoquinone structure, is more toxic than CoQ homologues with an isoprenoid side chain at the 6-position. However, its cytotoxic mechanism is not clear. Therefore, we conducted this study to clarify the cytotoxic mechanism of CoQ0 against HeLa cells.
CoQ0, CoQ1, CoQ2, CoQ4, (±)-lipoic acid (LA), (±)-dihydrolipoic acid (DHLA), and CoA were purchased from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.). CoQ3 was kindly donated by Nissin Flour Milling Co. (Tokyo, Japan). HeLa cells were provided by RIKEN BRC (Tsukuba, Ibaraki, Japan) through the National Bio-Resource Project of Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. {Benzyloxycarbonyl-L-valyl-L-alanyl-[(2S)-2-amino-3-(methoxycarbonyl)propionyl} fluoromethane(z-Val-Ala-Asp(OMe)-fmk, zVADfmk) was purchased from Peptide Institute, Inc. (Osaka, Japan). 2-(1H-Indol-3-yl)-3-pentylamino-maleimide (IM-54) was purchased from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.). All other chemicals were of the highest grade commercially available.
Cell CultureHuman cervical carcinoma cell line HeLa cells were maintained in 10-cm-diameter dishes filled with a standard culture medium that consisted of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 0.1 mg/mL streptomycin. The cells were incubated at 37°C under a water-saturated atmosphere of 5% CO2 and 95% air in a CO2 incubator. One day before each experiment, the cells on the dishes were trypsinized, harvested, and suspended in the standard culture medium at 2.5×105 cells/mL. The cell suspension was seeded on a 35-mm-diameter dish at 5.0×105 cells, and the cells were pre-cultured in the CO2 incubator until the experiments.
Dispersions of CoQ0 and CoQ Homologues in Aqueous Medium and Cell TreatmentIn order to make CoQ stock solutions, CoQ0 or CoQ homologues were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 100 mM. Then, stock solution or control DMSO was added to the standard culture medium in a sterilized test tube, and the medium was vigorously mixed by vortexing. At the beginning of the experiment, standard culture medium on the pre-cultured cells was replaced by 1 mL of the medium.
Cell ToxicityIn order to assess the cytotoxicity of CoQ0 and CoQ homologues, disruptions of cell membranes were measured by the lactate dehydrogenase (LDH) assay.
A one-hundred micro-molar aliquot of medium in the culture dish was harvested and centrifuged at 250×g for 5 min at 4°C to remove suspended intact cells. In order to disrupt all intact cells on the culture dish, 0.1% triton X-100 was added to the culture medium remaining in the dish. After incubation at 37°C for 10 min, the culture medium were collected and centrifuged at 250×g for 5 min at 4°C. LDH activity of both cell-free supernatants was measured by the method of Moldéus et al.9)
Preparations of Cell-Lysate and Cell-ExtractCells were trypsinized and harvested from a 35-mm-diameter culture dish. After washing with 1 mL of ice-cold phosphate-buffered saline (PBS) twice, the cells were harvested again with centrifugation at 250×g for 5 min at 4°C and suspended in 50 µL of ice-cold NP-40 lysis buffer consisting of 0.1% Nonidet® P-40, 1 mM ethylenediaminetetraacetic acid (EDTA), 250 mM NaCl, 10% glycerol, 50 mM Tris–HCl buffer (pH 8.0), and 1% Protease Inhibitor Cocktail for Use with Mammalian Cells and Tissue Extract (Nacalai Tesque, Inc., Kyoto, Japan) to make a cell-lysate. After incubation on ice for 10 min, the cell-lysate was centrifuged at 20000×g for 10 min at 4°C, and the supernatant was collected as a cell-extract.
Non-protein and Protein-Associated Sulfhydryl Group ContentsCells were trypsinized and harvested from the culture dish. After washing with 1 mL of ice-cold PBS twice, the cells were harvested again with centrifugation at 250×g for 5 min at 4°C and suspended in 0.1 mL of PBS. Non-protein and protein-associated sulfhydryl (SH) group contents of the cells were quantified by the method of Bhatnagar.10)
Reduced and Oxidized Glutathione ContentsReduced glutathione (GSH) and oxidized glutathione (=glutathione disulfide, GSSG) contents in the cell-extract were quantified using BIOXYTECH® GSH/GSSG-412™ (OxisReserch™, Portland, OR, U.S.A.). The assay was carried out in accordance with the instruction manual.
CoA ContentThe CoA content in the cell-extract was quantified using EnzyChrom™ Coenzyme A Assay Kit (BioAssay Systems, Hayward, CA, U.S.A.). The assay was carried out in accordance with the instruction manual.
Absorption SpectrumOne micro-molar CoQ0 stock solution was dissolved in 1 mL of PBS with or without 0.025% NaBH4, 200 µM of compounds with free SH-group(s), and 200 µM of compounds with a disulfide-bond or 200 µM of antioxidants. After incubation at 37°C for 10 min, the absorption spectrum between 220 and 360 nm of the mixture was continuously measured using a UV/VIS Spectrophotometer V-560 (JASCO Co., Tokyo, Japan) with a crystal cuvette.
Enzyme ActivitiesGlyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity in the cell-extract was measured by the method of Pancholi and Fischetti,11) and the absorbance change at 340 nm and 37°C was continuously measured for 1–2 min using a UV/VIS Spectrophotometer V-560 with a crystal cuvette.
On the other hand, for the assay of α-ketoglutarate dehydrogenase complex (KGDHc) activity, the cell-extract was added to an assay mixture consisting of 50 mM Tris–HCl buffer (pH 7.4), 0.5 mM oxidized form of nicotinamide adenine dinucleotide (NAD+), 0.2 mM thiamin pyrophosphate, 40 µM CoA, 2.5 µM rotenone, and 4 mM α-ketoglutarate, and the absorbance change at 340 nm at 37°C was immediately and continuously measured for 1–2 min using a UV/VIS Spectrophotometer V-560 with a crystal cuvette. The NAD+ quantity reduced through both assays was calculated using the molar absorption coefficient of reduced nicotinamide adenine dinucleotide (NADH) at 340 nm (6.22×103 M−1·cm−1), and 1 U of both enzyme activities was defined as the amount of enzyme that reduced 1 µmol of NAD+ per minute under each assay condition.
Intracellular ATP ContentCells were trypsinized and harvested from the culture dish. After washing with 1 mL of ice-cold PBS twice, the cells were harvested again with centrifugation at 250×g for 5 min at 4°C and suspended in 0.12 mL of PBS. The ATP content of the cells was immediately quantified using the IntraCellular ATP assay kit (TOYO B-Net Co., Ltd., Tokyo, Japan) based on the luciferin-luciferase reaction. The assay was carried out in accordance with the instruction manual, and the integrated relative light intensity for 10 s was measured using a Gena Light GL-200 (Microtec Co., Ltd., Chiba, Japan).
Protein QuantityProteins were quantified using Protein Assay CBB Solution (Nacalai Tesque, Inc.) based on the method of Bradford,12) and bovine γ-globulin was used to generate a standard curve for this assay.
Statistical AnalysisData are shown as the arithmetic means±standard deviation (S.D.). The significance of differences between individual data was determined by Dunnett’s test. The p-values less than 0.05 were considered significant.
In our previous study, CoQ homologues with a shorter isoprenoid side chain than CoQ5 induced the apoptosis of HeLa cells.3) Therefore, we compared toxic intensities of CoQ0 with cytotoxic CoQ homologues.
When HeLa cells were incubated with CoQ0 or CoQ homologues, CoQ1 to CoQ4, each compound released intracellular LDH into the extracellular environment concentration- and time-dependently (Figs. 1A, B, respectively). The order of cytotoxic intensity was CoQ0≫CoQ3>CoQ1≫CoQ2>CoQ4. These results indicate that the CoQ analogue without the isoprenoid side chain at the 6-position of the p-benzoquinone ring, CoQ0, is more toxic to HeLa cells than CoQ homologues with a short isoprenoid side chain at the same position.

(A) HeLa cells were incubated with standard culture medium containing 0.1% DMSO, CoQ0, or each CoQ homologue with a short isoprenoid side chain at the indicated concentrations and 37°C for 12 h. (B) HeLa cells were incubated with standard culture medium containing 0.1% DMSO, 100 µM CoQ0, or 100 µM of each CoQ homologue with a short isoprenoid side chain at 37°C for the indicated time periods. After the incubations, cell toxicity was assessed by the LDH assay. Symbols in both figures are as follows: open diamond, DMSO; open circle, CoQ0; open triangle, CoQ1, open square, CoQ2; closed circle, CoQ3, closed triangle, CoQ4. The data are shown as the means±S.D. of four experiments and statistical analysis of data was performed using Dunnett's test. Other symbols: * (for CoQ0), # (for CoQ1), $ (for CoQ2), § (for CoQ3), and ¶ (for CoQ4) indicate significant differences at p<0.05 in comparison with DMSO-treated cells.
Because CoQ0 is a quinone compound, it may undergo one-electron reduction in cells, produce ROS, and then induce cell death. Therefore, we investigated the effects of antioxidants on CoQ0-induced cytotoxicity.
When HeLa cells were incubated with 100 µM CoQ0 at 37°C for 5 h, 18±2% of intracellular LDH was released into the culture medium (Figs. 2 and S2). However, the LDH release was almost completely suppressed by the concomitant addition of antioxidants with the free SH-group, 10 mM N-acetyl-L-cysteine (NAC) and 200 µM GSH, whereas it was not suppressed at all by 200 µM of another water-soluble antioxidant without the SH-group, ascorbic acid (Asc), and lipophilic antioxidant without the SH-group, α-tocopherol (α-Toc) or its analogue, trolox. In addition, we were unable to detect an increase in thiobarbituric acid reactive substances, which are ROS-induced oxidative stress products, even when HeLa cells were incubated with 100 µM CoQ0 for 5 h (data not shown). These results indicate that oxidative stress is not related to CoQ0-induced cell death and is not an important event in cell death. Moreover, an NAD(P)H dehydrogenase (quinone) 1 (NQO1) inhibitor, dicumarol, and a general caspase inhibitor, zVADfmk, did not suppress the CoQ0-induced cell death (Figs. 2 and S2). We already reported that 10 µM of both compounds inhibits HeLa cell death induced by CoQ homologues with a short isoprenoid side chain.3) Therefore, this result indicates that the cytotoxic mechanism of CoQ0 is distinct from that of CoQ homologues with a short isoprenoid side chain that induce apoptotic cell death. However, thirty micro-molar of a necrosis inhibitor, IM-54, also did not affect the CoQ0-induced cell death.
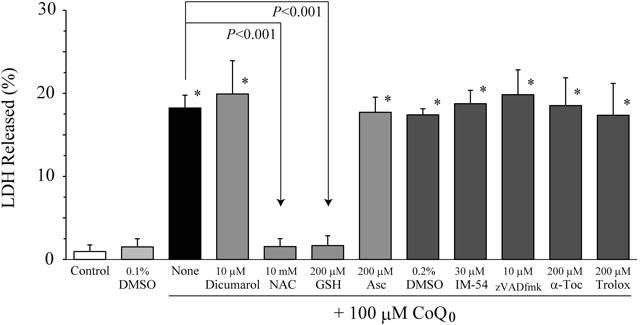
HeLa cells were pre-incubated with standard culture medium or medium containing 10 µM dicumarol, 10 mM NAC, 200 µM of water-soluble antioxidants such as GSH and Asc, 0.2% DMSO, 30 µM IM-54, 10 µM zVADfmk or 200 µM of a lipid-soluble antioxidant, α-Toc, and its water-soluble analogue, trolox, at 37°C. After 1 h, each culture medium was changed to fresh medium containing the same compound, and then 100 µM CoQ0 was immediately added to the culture medium. Separately, HeLa cells were incubated with standard culture medium or medium containing 0.1% DMSO. After incubation at 37°C for 5 h, cell toxicity was assessed by the LDH assay. The data are shown as the means±S.D. of four experiments and statistical analysis of data was performed using Dunnett’s test. p-Values in the figure indicate significant differences between data indicated by each arrow. The asterisk indicates significant differences at p<0.001 in comparison with the control.
In Figs. 2 and S2, NAC and GSH almost completely suppress CoQ0-induced toxicity of HeLa cells. Because both compounds have a free SH-group in their chemical structures in common, we investigated the effects of other SH-group-including compounds on CoQ0-induced cytotoxicity.
When HeLa cells were incubated with 100 µM CoQ0 in the presence of 100 µM of compounds with free SH-groups, i.e., NAC, GSH, Cys, DHLA, CoA, 2-mercaptoethanol (2-ME), dithiothreitol (DTT), and 2,3-dimercapto-1-propanesulfonic acid (DMPS), at 37°C for 5 h, these compounds significantly suppressed CoQ0-induced cytotoxicity (Figs. 3A and S3). However, the same concentrations of GSSG and cystine, which form a disulfide bond between the same two molecules of GSH and cysteine, respectively, did not suppress CoQ0-induced cytotoxicity. In addition, LA, which forms a disulfide bond between two SH-groups in one molecule of DHLA, also did not suppress CoQ0-induced cytotoxicity. Those results suggest that SH-groups of the compounds are important functional groups for the inhibition of CoQ0-induced toxicity against HeLa cells.

(A) The culture medium for HeLa cells was changed to fresh standard culture medium or medium containing 0.1% DMSO, 100 µM of a compound with SH-groups, such as GSH, Cys, DHLA, NAC, CoA, 2-ME, DTT, and DMPS, or 100 µM of a compound without SH-groups, such as GSSG, cystine, and LA, and then 100 µM CoQ0 was immediately added to the medium. Separately, the culture medium for HeLa cells was changed to fresh standard culture medium or medium containing 0.1% DMSO. After incubation at 37°C for 5 h, cell toxicity was assessed by the LDH assay. The data are shown as the means±S.D. of four experiments and statistical analysis of data was performed using Dunnett’s test. p-Values in the figure indicate significant differences between data indicated by each arrow, and the symbols * and # indicate significant differences at levels of p<0.001 and p<0.05 in comparison with the control, respectively. (B) One micro-liter of CoQ0 stock solution (100 mM) was dissolved in 1 mL of PBS in the presence or absence of 0.025% NaBH4 that could reduce CoQ0 to CoQ0H2 (a). On the other hand, 1 µL of the CoQ0 stock solution was dissolved in 1 mL of PBS in the presence or absence of 200 µM of GSH or GSSG (b), Cys or Cystine (c), DHLA or LA (d), NAC (e), CoA (with or without CoQ0) (f), DTT, 2-ME or DMPS (g), or antioxidants without SH-groups, such as Asc, trolox, or α-Toc (h). After incubation at 37°C for 10 min, the absorption spectrum between 220 and 360 nm was continuously measured using a spectrophotometer. (Color figure can be accessed in the online version.)
It has been reported that compounds with a p-benzoquinone structure can spontaneously form S-conjugated adducts with free SH-group-including compounds, such as GSH, by Michael addition, resulting in the reduction of the quinone-ring of each parent compound to a quinol-ring.13) Thus, we measured the change in the absorption spectrum of CoQ0 in the presence of compounds with SH-groups in order to assess whether CoQ0 forms similar adducts with the compounds.
Although the absorption spectrum of CoQ0 shows maximum absorption at 268 nm derived from the quinone-ring, the absorption immediately disappeared by the addition of a reductant, 0.025% NaBH4 (Fig. 3B-a). This result indicates that CoQ0 was reduced to the hydroquinone form, CoQ0H2. Similar disappearances were found by the concomitant addition of compounds with free SH-groups, GSH, Cys, DHLA, NAC, 2-ME, DTT, and DMPS, but not compounds without free SH-groups, GSSG, cystine, and LA (Figs. 3B-b to B-e and B-g) or other antioxidants, Asc, trolox, and α-Toc (Fig. 3B-h). Notably, among them, the spectra of CoQ0 in the presence of DHLA (Fig. 3B-d) or DTT (Fig. 3B-g) were almost the same as that of CoQ0H2. On the other hand, although CoA itself shows maximum absorption at 258 nm, absorption at 268 nm in the presence of CoQ0 was hardly affected (Fig. 3B-f). These results indicate that a quinone-ring of CoQ0 is reduced to a quinol-ring by the concomitant addition of compounds with SH-groups, suggesting that CoQ0 forms S-conjugated adducts with free SH-group(s)-including compounds by Michael addition.
CoQ0 Decreases Non-protein and Protein-Associated SH-Group Contents of HeLa CellsCoQ0-induced HeLa cell toxicity was suppressed by concomitant additions of SH-group-including compounds. Therefore, in order to clarify the CoQ0-induced cytotoxic mechanism, we investigate the effects of CoQ0 treatment on endogenous non-protein and protein-associated SH-group contents of HeLa cells.
When HeLa cells were treated with 100-µM CoQ0 for 1 and 5 h, non-protein and protein-associated SH-groups were significantly decreased (Figs. 4A, C). However, CoQ1 that conjugates an isoprene at the 6-position of the p-benzoquinone ring constituting CoQ0, did not affect the SH-group contents. In addition, the decreases in the non-protein and protein-associated SH-groups by CoQ0-treatment for 1 h were significantly suppressed by the concomitant addition of SH-group-including compounds, such as NAC, GSH, and Cys, but not antioxidants such as Asc or α-Toc (Figs. 4B, D, respectively). These results suggest that the decrease in the endogenous SH-group is due to a direct interaction between the SH-group of endogenous biomolecules and the free 6-position of the p-benzoquinone ring constituting CoQ0, but not direct oxidation of the SH-group by ROS generated. Indeed, for GSH, a representative non-protein biomolecule with the SH-group, the intracellular content was significantly decreased by CoQ0-treatment (Fig. 5A). Although GSSG, which is an oxidized form of GSH, concomitantly increased only with CoQ0 treatment for 1 h, the increase was slight compared with the expected amount from ROS-induced GSH oxidation (Fig. 5B, open bars). On the other hand, the GSSG content after 5-h treatment was almost completely depleted (Fig. 5B, closed bars). These results support the suggestion that oxidative stress is hardly involved in the decrease of intracellular SH-groups by CoQ0-treatment, or that it is only slightly involved in an early (≤1 h) decrease.

(A and C) HeLa cells were incubated with standard culture medium or medium containing 0.1% DMSO, 100 µM CoQ0, or 100 µM CoQ1 at 37°C. After 1 h (open bar) or 5 h (closed bar), non-protein (A) and protein-associated (C) SH-group contents of the cells were measured by the method described in Materials and Methods. (B and D) HeLa cells were pre-incubated with standard culture medium or medium containing 0.1% DMSO or 200 µM of antioxidants such as NAC, GSH, Cys, Asc, and α-Toc at 37°C. After 1 h, each culture medium was changed to fresh medium containing the same compound, and then 100 µM CoQ0 was immediately added to the culture medium. Separately, HeLa cells were incubated with standard culture medium or medium containing 0.1% DMSO. After incubation at 37°C for 5 h, non-protein (B) and protein-associated (D) SH-group contents of cells were measured. The data are shown as the means±S.D. of four experiments and statistical analysis of data was performed using Dunnett’s test. p-Values in the figures indicate significant differences between data indicated by each arrow, and the symbols * and # indicate significant differences at levels of p<0.001 and p<0.005 in comparison with the control, respectively.

HeLa cells were incubated with standard culture medium or medium containing 0.1% DMSO, 100 µM CoQ0, or 100 µM CoQ1 at 37°C. After 1 h (open bar) or 5 h (closed bar), GSH (A), GSSG (B), CoA (C), ATP (F) contents and GAPDH (D), and KGDHc (E) activities of cells were measured by the methods described in Materials and Methods. The data are shown as the means±S.D. of four experiments and statistical analysis of data was performed using Dunnett’s test. p-Values in the figures indicate significant differences between data indicated by each arrow.
Next, we investigated the effects of CoQ0 on CoA, which acts as an important cofactor of carbohydrate and fatty acid metabolisms, and on GAPDH and KGDHc, which are component enzymes of glycolysis and the Krebs cycle, respectively.
When HeLa cells were treated with 100 µM CoQ0 for 1 h, intracellular CoA was completely depleted (Fig. 5C, open bars). In addition, GAPDH and KGDHc activities were significantly inhibited (open bars of Figs. 5D, E, respectively,). The same results were also observed with 5-h treatment (Figs. 5C to E, closed bars). However, the same concentration of CoQ1 did not affect the CoA content or the enzyme activities of HeLa cells. Because CoA has the SH-group in its chemical structure, and GAPDH and KGDHc require SH-groups to maintain activities, these results also suggest that CoQ0 directly interacts with SH-groups of endogenous CoA and both enzymes of HeLa cells.
CoQ0 Decreases ATP Content of HeLa CellsWe finally investigated the effect of CoQ0-treatment on the ATP content of HeLa cells.
When HeLa cells were treated with 100 µM CoQ0 for 1 h, the intracellular ATP content was significantly decreased (Fig. 5F, open bars). Moreover, the decrease was more marked with the 5-h treatment with 100 µM CoQ0 (Fig. 5F, closed bars). However, the same concentration of CoQ1 did not affect the ATP content of HeLa cells. Because ATP is an essential substance for organisms to live and the content determines the cell fate,14–16) this suggests that the decrease in the ATP content of HeLa cells is an important event in CoQ0-induced cell death.
In a series of studies to explore the cytotoxic mechanism of CoQ homologues, we found that a CoQ analogue, without an isoprenoid side chain at the 6-position of the p-benzoquinone ring, CoQ0, showed marked toxicity against HeLa cells in comparison with cytotoxic CoQ homologues.3) Therefore, we were interested in the cytotoxic mechanism of CoQ0.
We first suspected that ROS, frequently produced by redox cycling of quinone compounds in cells, might be involved in the CoQ0-induced toxicity against HeLa cells, because CoQ0 is a quinone compound. When the HeLa cells were treated with 100 µM CoQ0 for 1 h, we detected a slight increase in GSSG, which means ROS-induced GSH oxidation, and a marked decrease in GSH (Figs. 5A, B). However, the concomitant addition of antioxidants, Asc, trolox, and α-Toc, did not suppress CoQ0-induced HeLa cell death (Figs. 2 and S2). Therefore, we concluded that the ROS generated intracellularly were not the main cause of cell death. On the other hand, other antioxidants, NAC and GSH, completely suppressed the CoQ0-induced LDH-release of HeLa cells. This discrepancy in the action of antioxidants may be because NAC and GSH suppress CoQ0-induced cytotoxicity by a mechanism other than antioxidant action. Furthermore, CoQ0-induced cytotoxicity was not suppressed by the addition of an NQO1 inhibitor, dicumarol, or general caspase inhibitor, zVADfmk (Figs. 2 and S2). We reported that CoQ homologues with a shorter isoprenoid side chain than CoQ5 are reduced to a hydroquinone form, CoQH2, by an intracellular quinone-reducing enzyme, NQO1, and induce the apoptosis of HeLa cells.3) Additionally, it has been reported that CoQ0 is a better substrate for NQO1 than CoQ homologues.17) Therefore, these results suggest that the mechanism whereby CoQ0 induces toxicity against HeLa cells is distinct from that of CoQ homologues with an isoprenoid side chain.
In Figs. 2 and S2, antioxidants that suppress CoQ0-induced cytotoxicity had a free SH-group in the structures in common. Therefore, we investigated the effects of compounds with free SH-groups on CoQ0-induced cytotoxicity. As the result, we found that not only antioxidants with free SH-groups, GSH, NAC, and DHLA, but also disulfide bond-reducing reagents with SH-groups, 2-ME and DTT, and other compounds with SH-groups, Cys, CoA, and DMPS, also suppressed CoQ0-induced cytotoxicity (Figs. 3A and S3). Moreover, NAC was effective not only at the concentration usually used as an antioxidant, 10 mM (Figs. 2 and S2), but also at the lower concentration of 100 µM (Figs. 3A and S3). In addition, we did not detect the increase in the endogenous GSSG content commensurate with a decrease in endogenous GSH in CoQ0-treated HeLa cells (Figs. 5A, B). Since the SH-group is nucleophilic, compounds with free SH-groups may conjugate at the free 6-position of the p-benzoquinone ring constituting CoQ0 by Michael addition. It has been reported that quinone compounds with a similar structure to CoQ0, such as p-benzoquinone, 2-hydroxyl-p-benzoquinone, 2-methyl-p-hydroquinone, and menadione, can spontaneously form S-conjugated adducts with GSH by Michael addition and are reduced to hydroquinone forms.13) In addition, it has also been reported that 2-ME, DTT, and DMPS can form S-conjugated adducts with another quinone compound, phenanthraquinone.18) A similar reaction may be occurring between added CoQ0 and exogenous or endogenous SH-group-including compounds.
The Michael addition of compounds with SH-groups to the quinone ring is accompanied by the reduction of quinone to hydroquinone.13) This reduction was observed in our spectrum analysis using CoQ0 and compounds with SH-groups (Fig. 3B). This result suggests that CoQ0 forms S-conjugated adducts with the compounds with SH-groups. However, we found that the spectra of CoQ0 after the addition of DHLA (Fig. 3B-d) or DTT (Fig. 3B-g), distinct from other compounds with SH-groups, are almost the same as that of CoQ0H2. In addition, we found that DHLA and DTT non-enzymatically reduce even CoQ10 with a long isoprenoid side chain at the 6-position to CoQ10H2 (data not shown). Therefore, we could not rule out the possibility that both compounds reduce CoQ0 to form CoQ0H2 that is non-toxic or less toxic to HeLa cells, but do not form S-conjugated adducts with CoQ0.
We next examined the toxic mechanism of CoQ0 against HeLa cells. We found that CoQ0 treatment significantly decreased the intracellular ATP level of HeLa within 1 h (Fig. 5F). It was considered that the decrease was not due to leakage from the cells resulting from collapse of the cell membrane, because we did not detect LDH release, which indicates collapse of the membrane, at the same time (Fig. 1). Therefore, it is suggested that the decrease in the ATP level is a cause of CoQ0-induced cytotoxicity. It has been reported that the intracellular ATP content determines the cell death fate by apoptosis or necrosis, and a decrease in the content particularly induces necrotic cell death.14–16) We found that CoQ0-induced HeLa cell death is not inhibited by the addition of zVADfmk (Figs. 2 and S2). Additionally, we previously reported that CoQ0-induced cytotoxicity was not accompanied by DNA ladder formation, which is one of the typical features of apoptotic cell death, distinct from cytotoxic CoQ homologues.2) These results indicate that CoQ0-induced cell death involves necrosis but not apoptosis. However, necrosis inhibitor, IM-54, also did not suppress CoQ0-induced cytotoxicity (Figs. 2 and S2). It is known that IM-54 can selectively block oxidative stress-induced necrotic cell death without antioxidant action,19) although its inhibition mechanism is not well understood. As mentioned above, since oxidative stress does not participate in the CoQ0-induced cell death, IM-54 may not be able to suppress the CoQ0-induced necrotic cell death.
GAPDH, which is one of the glycolysis-related enzymes, catalyzed the oxidation reaction of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate in the presence of phosphoric acid and NAD+. Since Cys at the 149th position in GAPDH is located in the active site and is a redox-sensitive residue, it has been reported that oxidation of the Cys residue affects the glycolysis of cells through inactivation of the enzyme.20–22) We found that CoQ0-treatment decreased the protein-associated SH-group content without ROS involvement (Figs. 4C, D) and significantly suppressed GAPDH activity of HeLa cells (Fig. 5D). However, the same effect was not found in CoQ1 that conjugated an isoprenoid side chain at the 6-position of the p-benzoquinone ring constituting CoQ0. Therefore, we consider that the SH-group of the Cys residue in GAPDH may be rapidly alkylated by CoQ0, resulting in the suppression of GAPDH activity.
KGDHc, which is one of the Krebs cycle-related enzymes, catalyzes the irreversible decarboxylation reaction of α-ketoglutarate to succinyl CoA in the presence of cofactors, NAD+ and CoA. Furthermore, this enzyme formed a complex consisting of three enzymes: thiamine pyrophosphate-containing oxoglutarate decarboxylase (E1k), LA-containing dihydrolipoyl succinyltransferase (E2k), and FAD-containing dihydrolipoyl dehydrogenase (E3). In the complex, LA, which is an essential cofactor and tightly bound to E2k, participates in the transfer reaction of the succinyl-group to CoA. In a series of decarboxylation reactions, although LA is reduced to DHLA concomitant with the succinyl-group transfer reaction by E2k, DHLA is rapidly oxidized to the original LA by E3, a step before the next succinyl-group transfer reaction. On the other hand, the pyruvate dehydrogenase complex (PDHc), which catalyzed the irreversible decarboxylation reaction of pyruvate to acetyl CoA in the presence of NAD+ and CoA, is an enzyme complex that links glycolysis and the Krebs cycle and consists of three enzymes: thiamine pyrophosphate-containing pyruvate decarboxylase (E1), LA-containing dihydrolipoyl transacetylase (E2), and E3. LA on E2 is also reduced to DHLA concomitant with the acetyl-group transfer reaction in a series of decarboxylation reactions. Therefore, SH-groups of DHLA on both E2k and E2 may be important functional groups for the whole decarboxylation reaction of both enzyme complexes. MacDonald et al. reported that CoQ0 forms adducts with DHLA on the E2k subunit of KGDHc and on E2 of PDHc of rat and human pancreatic islets and INS-1 insulinoma cells.23) Additionally, we detected the significant suppression of KGDHc activities by the treatment of HeLa cells with 100 µM CoQ0 but not CoQ1 (Fig. 5E). This suggests that SH-groups of DHLA on E2k of KGDHc are rapidly alkylated by the addition of CoQ0, resulting in the suppression of KGDHc activity. Moreover, in order to clarify the suppression of PDHc activity, although we tried to measure PDHc activity, we failed to detect any activity in HeLa cells under our experimental conditions (data not shown). Murray et al. reported that the copy number of PDHc in HeLa cells is small and the steady-state ratio of the PDHc and complex III, which is one of the mitochondrial respiratory chain enzyme complexes, is 1 : 100.24) Furthermore, it has been reported that cancer cells almost exclusively derive energy from glycolysis (Warburg effect),25) suggesting the suppression of PDHc activity in such cells, including HeLa cells. On the other hand, intracellular CoA, which is an essential cofactor of KGDHc and PDHc, was completely depleted by the treatment of HeLa cells with CoQ0 but not CoQ1 (Fig. 5C). CoA has an SH-group in its chemical structure. Also, the addition of an excessive quantity of CoA can rescue HeLa cells from CoQ0-induced toxicity (Fig. 3). These results suggest that CoQ0 can alkylate the SH-group of CoA. In a recent study, although we did not detect PDHc activity in CoQ0-treated HeLa cells or control HeLa cells, we strongly consider that not only KGDHc activity but also PDHc activity is diminished in the CoQ0-treated HeLa cells because of the deficiency of the essential cofactor, CoA.
Landi et al. reported that CoQ homologues other than CoQ1 may be present in membranous fractions.26) Saito et al. found that CoQ10 added to rat pheochromocytoma PC12 cells preferentially accumulated in mitochondria.27) Nakamura et al. observed that most exogenous CoQ10 in the rat heart after the intravenous injection of 14C-labeled CoQ10 was present in mitochondria.28) These reports suggest that CoQ homologues with a long isoprenoid side chain exogenously added is preferentially distributed in mitochondria. However, Landi et al. also found that CoQ1 that had the shortest isoprene (C5) unit at the 6-position of the p-benzoquinone ring was not retained in phospholipid bilayers because of its lower hydrophobicity.26) Therefore, it is speculated that CoQ0 without a hydrophobic isoprenoid side chain preferentially distributes in soluble fractions, such as the cytosol and mitochondrial matrix, rather than in the membranous fractions. Indeed, our findings indicated that CoQ0 suppressed the activities of a cytosolic enzyme, GAPDH, and a mitochondrial matrix enzyme, KGDHc, and immediately decreased aqueous SH-group-containing GSH and CoA contents (≤ 1 h) (Fig. 5).
Furthermore, Lenaz et al. reported that CoQ homologues with a short isoprenoid side chain competitively inhibit mitochondrial Complex I activity (NADH oxidation) by the homologues with a long isoprenoid side chain, and the inhibitory effect became stronger as the side chain became shorter.29) Therefore, CoQ0 in mitochondrial inner membranes or cristae may impair the mitochondrial electron transfer pathway and ATP production by oxidative phosphorylation. In any case, as CoQ0 suppresses glycolysis and the Krebs cycle by the inhibition of GAPDH and KGDHc (and possibly also PDHc), intracellular NADH and FADH2 contents decrease, and as a result, ATP production by oxidative phosphorylation is also reduced in HeLa cells.
In summary, we found that CoQ0-induced cytotoxicity is caused by the direct and rapid alkylation of endogenous non-protein and protein-associated SH-groups by the addition of CoQ0, but not by their oxidation by ROS generated by CoQ0-mediated intracellular redox cycling. Based on the results, CoQ0 may suppress several glycolysis- and Krebs cycle-related enzyme activities of HeLa cells, resulting in disruptions of glucose metabolism and associated energy production, and finally induce intracellular ATP depletion followed by necrotic cell death. It has been reported that CoQ0 induces apoptosis of HL-60 leukemia cells via mitochondrial permeability transition pore opening,30,31) and that it has anti-angiogenic and anti-inflammatory properties based on in vitro and in vivo studies.32,33) Although there have been studies reporting the inhibitory effects of compounds with SH-groups, especially antioxidant GSH, on toxicities or functions of CoQ0 and other quinone compounds with a similar structure to CoQ0, we should exercise caution when analyzing the results of such experiments because of the possibility of adduct formation.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.