2018 Volume 41 Issue 12 Pages 1859-1865
2018 Volume 41 Issue 12 Pages 1859-1865
The lateral hypothalamic area contains neurons expressing neuronal nitric oxide synthase (nNOS), in addition to orexin neurons. Here we examined whether the activity of orexin neurons was regulated by endogenous nitric oxide (NO) in male C57BL/6 mice. Caffeine (30 mg/kg, intraperitoneally (i.p.)) increased the number of orexin neurons positive for c-Fos, a marker of neuronal activity, and also increased the number of NOS/c-Fos-positive cells as identified by reduced nicotinamide adenine dinucleotide phosphate (NADPH) diaphorase histochemistry and c-Fos immunohistochemistry. Diphenhydramine hydrochloride (10 mg/kg. i.p.) decreased c-Fos-positive orexin neurons but had no significant effect on the number of c-Fos-positive NOS neurons. nNOS inhibitor 7-nitroindazole (25 mg/kg, i.p.) alone increased c-Fos-positive orexin neurons, and combined treatment with caffeine and 7-nitroindazole did not show additive effect in the number of c-Fos-positive orexin neurons. In contrast, 7-nitroindazole decreased c-Fos-positive NOS neurons and attenuated caffeine-induced increase in c-Fos-positive NOS neurons. Sleep deprivation increased c-Fos-positive cells in both orexin neurons and NOS neurons, and 7-nitroindazole did not show additive effect with sleep deprivation in the activation of orexin neurons. Together, these results suggest that endogenous NO negatively regulates the activity of a subset of orexin neurons, and this subset of orexin neurons overlaps with that activated by awakening stimuli.
Orexins are neuropeptides produced by a small population of neurons located in the lateral hypothalamic area. Orexin neurons send projections widely throughout the central nervous system, and they play important roles in various physiological processes. For example, orexins are recognized to maintain the arousal state, and the loss of orexin neurons leads to a sleep disorder narcolepsy.1) In addition, orexins may promote feeding behavior, regulate the functions of the autonomic nervous system, and mediate addictive behaviors.2)
A notable feature of the lateral hypothalamic area and its adjacent hypothalamic nuclei is that a substantial number of neurons containing neuronal isoform of nitric oxide synthase (nNOS) are distributed in these regions. These nNOS neurons do not exhibit orexin immunoreactivity, indicating that they constitute a distinct neuronal population from orexin neurons.3) We have previously reported that nitric oxide (NO) produced by adjacent nNOS neurons is involved in selective degeneration of orexin neurons.4) Specifically, sleep deprivation increased the activity of nNOS neurons in the hypothalamus. When sleep deprivation was continued for 7 d, the resultant increase in NO production promoted accumulation of misfolded orexin peptides via inactivation of protein disulfide isomerase, which led to increased endoplasmic reticulum stress and induction of cell death.4) In this scenario, nNOS neurons seem to exert harmful influence on the integrity of the hypothalamus. On the other hand, little information is available on the physiological roles of these nNOS neurons. One of a few examples is that water deprivation increased nNOS expression in the lateral hypothalamic area in rats, although the functional significance of the increased nNOS remain unexplored.5) Kostin et al.6,7) showed that sleep deprivation increased local production of NO in the lateral hypothalamic area. They also showed that NO donor application as well as sleep deprivation inhibited the activity of neurons in this area, but the types of neurons affected by NO have not been specified.8)
In the present study we paid attention to the role of endogenous NO in regulation of the activity of orexin neurons. We examined the activities of orexin neurons and nNOS neurons based on c-Fos immunohistochemistry, and addressed the effects of drugs that affect the arousal level or nNOS activity, as well as the effect of sleep deprivation.
Male C57BL/6 mice (Nihon SLC, Shizuoka, Japan) at 8–10 weeks old were used in the present study. Mice were maintained at 22–24°C with food and water available ad libitum, under 12-h light/dark cycle. Zeitgeber time (ZT) 0:00 corresponded to 8:00 a.m. All experimental procedures using animals were approved by Kumamoto University ethics committee on animal experiments, and the experiments were conformed to the guidelines for animal experiments issued by Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Drug AdministrationCaffeine (30 mg/kg, Sigma-Aldrich Chemicals, St. Louis, MO, U.S.A.) and diphenhydramine HCl (10 mg/kg; Tokyo Kasei Co., Ltd., Tokyo, Japan) were dissolved in physiological saline and administered to mice via intraperitoneal injection at 10 µL/g body weight. A selective nNOS inhibitor 7-nitroindazole (7-NI; Tokyo Kasei Co., Ltd.) was dissolved in physiological saline containing 5% Tween 80 (Nacalai Tesque, Kyoto, Japan) and intraperitoneally administered at 25 mg/kg. In the case of combined treatment, caffeine was administered 30 min after 7-NI administration. Caffeine and diphenhydramine HCl were administered between ZT 4:00 to ZT 5:00 during the light phase. A group of mice received caffeine administration between ZT 12:00 and ZT 13:00 during the dark phase.
Sleep DeprivationMice were individually housed in a cage from two days before the sessions of sleep deprivation. We used two different procedures for sleep deprivation. “Forced” sleep deprivation employed a conventional method of sleep deprivation, where 5-min gentle handling was given to mice every 1 h.4) In the present study, we performed gentle handling of mice from ZT 1:00 to ZT 9:00 during the light phase. “Voluntary” sleep deprivation was performed essentially according to the methods described by Suzuki et al.,9) where the mouse in a cage was transferred to another cage alternatively at 1-h intervals from ZT 1:00 to ZT 9:00. 7-NI (10 mg/kg) was administered just before the first session of forced sleep deprivation.
Assessment of the Arousal LevelThe arousal level of mice was estimated by the sensitivity of the mouse to a general anesthetic pentobarbital. At 90 min after administration of drugs (caffeine or diphenhydramine HCl) or after the completion of sleep deprivation session, pentobarbital sodium (Nacalai Tesque) was intraperitoneally administered at 50 mg/kg. Disappearance of limb tremors in addition to the loss of righting reflex was judged as signs of complete anesthesia, and the time required for obtaining complete anesthesia was measured as the parameter reflecting the arousal level of animals.
Histochemical ExaminationsProcedures for histochemical examinations followed essentially those described in our previous studies.4,10) Mice were anesthetized with pentobarbital sodium and perfused transcardially with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (Nacalai Tesque) in phosphate buffer. Then the brain was isolated and post-fixed overnight with 4% paraformaldehyde, then immersed in 15% sucrose. After freezing, coronal sections of the hypothalamus of 30 µm thickness were obtained every 150 µm and mounted onto slides. Brain sections were washed with Tris-buffered saline (TBS) containing 0.1% Triton X-100 (MP Bio Japan K.K., Tokyo, Japan) and then incubated in 10 mM sodium citrate buffer (pH 8.5) for 30 min at 80°C for antigen retrieval. After cooling they were again washed with TBS/Triton X-100, and blocked with 3% normal donkey serum (Thermo Fisher Scientific K.K., Tokyo, Japan) in TBS/Triton X-100 for 1 h. Then, sections were incubated at 4°C for 72 h in a primary antibody solution consisting of goat polyclonal anti-orexin A (C-19) antibody (1 : 300; sc-8070, Santa Cruz Biotechnology, Dallas, TX, U.S.A.) and rabbit anti-c-Fos (1 : 3,500; sc-52, Santa Cruz Biotechnology) in TBS/Triton X-100 with 3% normal donkey serum. After wash with TBS/Triton X-100, sections were incubated in a secondary antibody solution for 2 h at 22–25°C. Secondary antibodies were Alexa Fluor 488-labeled donkey polyclonal anti-goat immunoglobulin G (IgG) (H+L) (1 : 200; A-11055, Thermo Fisher Scientific K.K.) and Alexa Fluor 555-labeled donkey polyclonal anti-rabbit IgG (H+L) (1 : 500; A-31572, Thermo Fisher Scientific K.K.). Immunoreactive neurons in individual sections were examined under an epifluorescence microscope. The percentage of c-Fos-positive cells in orexin neuronal population was obtained from areas of 360×270 µm2 in the lateral hypothalamic area in four sections derived from individual mice encompassing the posterior hypothalamus.
Nicotinamide adenine dinucleotide hydrogen phosphate (NADPH) diaphorase histochemistry can be used to stain nNOS-containing neurons in the central nervous system.10,11) The reaction of NADPH diaphorase was carried out at 37°C for 90 min in a solution containing 0.5 mg/mL β-NADPH (Nacalai Tesque), 0.2 mg/mL nitroblue tetrazolium (Nacalai Tesque) and 0.3% Triton X-100 in 0.1 M PBS (pH 7.4). After NADPH diaphorase staining, sections were processed for c-Fos immunohistochemistry using rabbit anti-c-Fos (1 : 1500) in PBS containing 0.3% Triton X-100 and 3% normal goat serum. After incubation with biotinylated goat anti-rabbit IgG (1 : 200, Vector Laboratories, Burlingame, CA, U.S.A.), specimens were treated with avidin–biotinylated horseradish peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories), and then peroxidase was visualized with diaminobenzidine and H2O2. The percentage of c-Fos-positive cells in NADPH diaphorase-positive neurons was obtained from areas of 360×270 µm2 in the lateral hypothalamic area as in the case with c-Fos-positive orexin neurons. Data sets shown in individual graphs were obtained from the same series of histochemical examinations to avoid potential influences of the variability of staining intensities among different sets of experiments.
Statistical AnalysisNumerical data were expressed as means±standard error of the mean (S.E.M.). Differences of values between two groups were analyzed by Student’s t-test. When comparison was made among more than two groups, one-way ANOVA followed by post-hoc comparisons by Tukey’s test was used. Two-tailed probability values less than 5% were considered significant.
We utilized c-Fos expression as a marker of neuronal activity, and c-Fos expression in orexin neurons was addressed by double immunofluorescence histochemistry using anti-orexin A and anti-c-Fos antibodies. On the other hand, NADPH diaphorase histochemistry was combined with c-Fos immunohistochemistry to identify active NOS neurons. The percentage of c-Fos-positive orexin neurons was higher during the dark phase than during the light phase. Administration of caffeine (30 mg/kg, intraperitoneally (i.p.)) significantly increased the percentage of c-Fos-positive neurons within orexin neuronal population, during both the light phase and the dark phase (Figs. 1A, C).
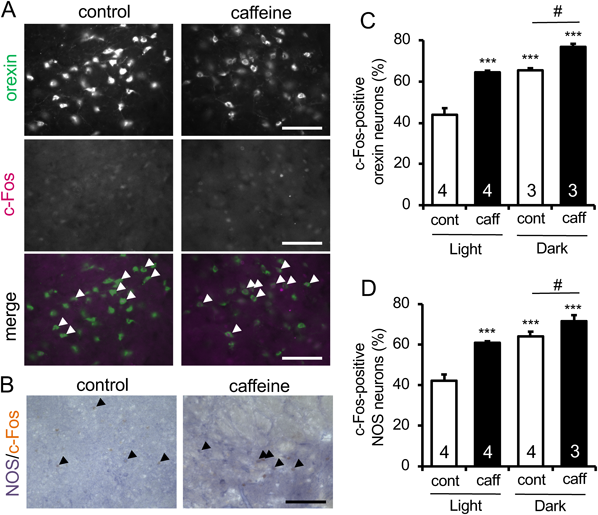
Histochemical examinations were performed at 90 min after administration of vehicle or caffeine (caff; 30 mg/kg) during the light phase and the dark phase. (A) Representative photomicrographs showing the results of double immunofluorescence histochemistry for orexin-A and c-Fos with and without caffeine administration during the dark phase. Scale bars=100 µm. Arrowheads indicate doubly positive cells. (B) Representative results of NADPH diaphorase staining combined with c-Fos immunohistochemistry on sections obtained from mice with and without caffeine administration during the dark phase. Scale bar=100 µm. Arrowheads indicate doubly positive cells. (C, D) Quantitative results on the percentage of c-Fos-positive cells in orexin neurons (C) and in NOS neurons (D). The numbers of animals examined under each condition is given in each column. *** p<0.001 vs. control in the light phase; # p<0.05. (Color figure can be accessed in the online version.)
As shown in Figs. 1B and D, the neuronal activity of NOS neurons in the lateral hypothalamic area exhibited similar profiles to that of orexin neurons. Namely, the percentage of active NOS neurons was higher during the dark phase than during the light phase. In addition, caffeine significantly increased the percentage of c-Fos-positive NOS neurons (Fig. 1D).
Diphenhydramine Inhibits Orexin Neurons but Does Not Affect the Activity of NOS NeuronsIn all the following sets of experiments, we addressed the activities of orexin neurons and NOS neurons during the light phase. In contrast to caffeine, histamine H1 receptor antagonist diphenhydramine is known to decrease the arousal level and promote sleep.12) We observed that c-Fos-positive orexin neurons decreased significantly at 90 min after administration of 10 mg/kg diphenhydramine HCl (Figs. 2A and S1A), while the percentage of c-Fos-positive cells within NOS-positive neuronal population was not changed significantly (Figs. 2B and S1B). Consistent with the decreased arousal level, diphenhydramine HCl significantly shortened the latency for pentobarbital anesthesia (Fig. 2C).

Histochemical examinations were performed at 90 min after administration of vehicle or diphenhydramine HCl (DPH; 10 mg/kg) during the light phase. (A, B) Quantitative results on the percentage of c-Fos-positive cells in orexin neurons (A) and in nNOS neurons (B). (C) Effect of diphenhydramine on the arousal level of mice. The duration required for the disappearance of limb tremors as well as the righting reflex after administration of pentobarbital sodium (50 mg/kg) was measured. The numbers of animals examined is given in each column. ** p<0.01, *** p<0.001 vs. control.
To address whether NO produced by nNOS neurons modulated the activity of orexin neurons, we examined the effect of 7-NI on c-Fos expression. Notably, administration of 7-NI (25 mg/kg) alone significantly increased the percentage of c-Fos-positive cells within orexin neuronal population (Figs. 3A, C). Here we confirmed again that caffeine alone increased the activity of orexin neurons, and we additionally observed that the effect of caffeine and 7-NI was not additive. That is, caffeine did not further increase the number of c-Fos-positive orexin neurons in the presence of 7-NI (Figs. 3A, C). In contrast, 7-NI alone markedly decreased the percentage of c-Fos-positive cells in NOS neurons and attenuated caffeine-induced increase in c-Fos-positive NOS neurons (Figs. 3B, D).

Histochemical examinations were performed at 90 min after administration of vehicle or caffeine (caff; 30 mg/kg) during the light phase. Administration of 7-NI (25 mg/kg) preceded 30 min from caffeine administration. (A, B) Representative results of orexin-A/c-Fos immunofluorescence histochemistry (A) and NADPH diaphorase/c-Fos histochemistry (B) on sections obtained from mice with and without 7-NI administration during the light phase. Scale bars=100 µm. Arrowheads indicate doubly positive cells. (C, D) Quantitative results on the percentage of c-Fos-positive cells in orexin neurons (C) and in nNOS neurons (D). (E) Shown are the durations required for the disappearance of limb tremors as well as the righting reflex after administration of pentobarbital sodium (50 mg/kg). The number of animals examined under each condition is given in each column. * p<0.05, ** p<0.01, *** p<0.001 vs. control; ### p<0.001. (Color figure can be accessed in the online version.)
We also examined the effect of drugs on the arousal level of mice based on the latency for pentobarbital anesthesia (Fig. 3E). Caffeine (30 mg/kg) prolonged the latency for pentobarbital to produce anesthetic effect, which reflected the arousal-promoting effect of caffeine. In addition, tendency for prolongation of the latency for pentobarbital anesthesia was observed at 120 min after administration of 7-NI (10 mg/kg), although the effect did not reach statistical significance. Combined treatments with caffeine and 7-NI gave comparable results with caffeine alone, suggesting no additive effect between caffeine and 7-NI (Fig. 3E).
Sleep Deprivation Increases the Activities of Orexin Neurons and NOS Neurons That Are Differentially Affected by nNOS InhibitionNext we examined the effect of sleep deprivation on the activities of orexin neurons and NOS neurons, using two different procedures for sleep deprivation. One is a forced sleep deprivation, where gentle handling was given to the mouse every 1 h during the light phase.4) The other is a voluntary sleep deprivation, where the mouse in a cage was transferred to another cage at 1 h intervals.9) In the latter case, mice exhibited spontaneous exploratory behavior and stayed awake. We found that forced sleep deprivation significantly increased the percentage of c-Fos-positive orexin neurons as compared to control (Figs. 4A and S2A). Voluntary sleep deprivation was also accompanied by a modest but insignificant increase in c-Fos-positive orexin neurons. In the case of NOS neurons, forced but not voluntary sleep deprivation induced a marked and significant increase in c-Fos-positive cells (Figs. 4B and S2B).

Mice underwent forced SD (intermittent handling) or voluntary SD (vol.; intermittent exchange of cage) during the light phase. Shown are quantitative results on the percentage of c-Fos-positive cells in orexin neurons (A) and in nNOS neurons (B). The number of animals examined under each condition is given in each column. ** p<0.01, *** p<0.001 vs. control.
Finally, the effect of combination of sleep deprivation and 7-NI administration was addressed. In this set of experiments, 7-NI (10 mg/kg) was administered just before the initiation of 8-h session of forced sleep deprivation. Similar to the case with caffeine, the effect of sleep deprivation on the percentage of c-Fos-positive orexin neurons was not additive to that of 7-NI (Figs. 5A and S3A). On the other hand, the increase of c-Fos-positive NOS neurons by sleep deprivation was counteracted by 7-NI-induced decrease, although sleep deprivation still increased c-Fos-positive NOS neurons significantly in the presence of 7-NI (Figs. 5B and S3B). In these experimental settings, sleep deprivation tended to decrease, whereas 7-NI significantly increased, the arousal level of mice. Combined treatment apparently cancelled the effect of each other (Fig. 5C).

7-NI (25 mg/kg) was administered just before the initiation of forced SD. (A, B) Quantitative results on the percentage of c-Fos-positive cells in orexin neurons (A) and in nNOS neurons (B). (C) Comparisons of the durations required for the disappearance of limb tremors as well as the righting reflex after administration of pentobarbital sodium (50 mg/kg). The number of animals examined under each condition is given in each column. * p<0.05, ** p<0.01, *** p<0.001 vs. control; ## p<0.01.
In the present study we addressed the activities of orexin neurons and nNOS neurons in the hypothalamus under various conditions, to obtain insights into the physiological role of endogenous NO, particularly in the regulation of orexin neurons. Besides, the present study provides the first evidence that nNOS neurons in the hypothalamus respond to specific stimuli. That is, caffeine increased the percentage of active nNOS neurons as well as of active orexin neurons. By contrast, diphenhydramine had no significant effect on the activities of nNOS neurons while the drug decreased the activities of orexin neurons. The stimulatory effect of caffeine on orexin neurons has been reported in several previous studies,13,14) but the effect of caffeine on nNOS neurons as well as the effects of diphenhydramine on orexin and nNOS neurons has not been addressed so far. In the present study we set the dose of caffeine at 30 mg/kg, i.p. because previous studies reporting the effect of caffeine on orexin neurons in rats employed 10, 30 and 75 mg/kg i.p.13) or 10 and 30 mg/kg i.p.14) Considering that 100 mL of coffee contains approximately 60 mg of caffeine, we should note that caffeine at 30 mg/kg may be higher than the dose expected from daily consumption of coffee and other caffeine-containing beverages. Caffeine is well known as a central nervous system stimulant, whereas diphenhydramine promotes sleep induction via blocking histamine H1 receptors,12) and indeed, we confirmed that the arousal level of mice was changed by these drugs. The changes in the activities of orexin neurons in response to these drugs are consistent with the effects of these drugs on the arousal level of mice because orexins are considered to play an important role in maintaining the arousal state.2) On the other hand, nNOS neurons may respond preferentially to the awakening stimuli, because caffeine but not diphenhydramine produced a significant effect on the activity of nNOS neurons. An alternative (but not mutually exclusive) interpretation is that nNOS neurons may be activated secondarily following the activation of orexin neurons. Consistent with the latter proposal, we found that the activities of nNOS neurons increased during the dark phase of diurnal cycle when active orexin neurons were increased as compared to the light phase. Moreover, increased activities of nNOS neurons were observed after forced sleep deprivation which was accompanied by increased activities of orexin neurons, whereas voluntary sleep deprivation did not significantly increase the activities of orexin neurons and failed to activate nNOS neurons. Indeed, expression of orexin receptors 1 and 2 is detected in the lateral hypothalamic area,15) although it is unknown whether nNOS neurons in this brain region possess these receptors and receive direct inputs from orexin neurons. In this context, the activity of orexin neurons has been reported to change in response to various behavioral states as well as the diurnal cycle.16,17) Potential correlation between the activity of nNOS neurons and that of orexin neurons may be addressed in further investigations with these behavioral states taken into consideration.
To address the role of endogenous NO, we examined the effect of 7-NI, a selective nNOS inhibitor, alone or in combination with caffeine. An unexpected finding was that 7-NI alone was sufficient to increase active orexin neurons significantly, suggesting that the ambient level of NO is a limiting factor for activation of orexin neurons. Interestingly, the effects of caffeine and 7-NI on the activation of orexin neurons were not additive, and caffeine no longer increased active orexin neurons under nNOS inhibition. We should note that a substantial portion of orexin neurons was inactive even after treatment with caffeine, 7-NI or both, which suggests that there exist subpopulations of orexin neurons distinguished by sensitivities to caffeine or NO. Additionally, the occlusive effects of caffeine and 7-NI on the activation of orexin neurons are consistent with the idea that the population of caffeine-sensitive orexin neurons overlaps with that of NO-sensitive orexin neurons.
We did not address the detailed cellular mechanisms of inhibition of orexin neuron activity by NO, but the findings reported in a precedent study indicate that NO plays an inhibitory role on neuronal excitation via multiple mechanisms. That is, Kostin et al.8) demonstrated that exogenous application of NO donor into the perifornical and lateral hypothalamic areas of rats inhibited the activities of neurons recorded extracellularly in these areas. They also found that NO-induced inhibition of neuronal activities was diminished by blockade of adenosine A1 receptors and γ-aminobutyric acid (GABA)A receptors.8) Unfortunately, the cell types affected by NO were not identified in their study, but these mechanisms of action may be responsible at least in part for the inhibitory effect of ambient NO on orexin neurons observed in the present study.
Another unexpected finding was that 7-NI substantially inhibited the activities of nNOS neurons in the lateral hypothalamic area. The straightforward interpretation of these data is that ambient or stimulated production of NO by nNOS neurons activates nNOS neurons themselves in a feed-forward manner. At present, we cannot make logical explanations on the mechanism of this action of endogenous NO. But NO may modulate neuronal excitability in a context-dependent manner via regulation of voltage-gated K+ channels.18) We may also consider a possibility that the effect of NO on the activity of nNOS neurons emerges indirectly, for example, via inhibition of the activity of local GABAergic interneurons sending inhibitory inputs to nNOS neurons. Details should be addressed in further investigations.
In the present study we addressed the effect of two kinds of sleep deprivation, that is, “forced” and “voluntary” sleep deprivation. Forced sleep deprivation by intermittent gentle handling for 6 h has been reported to show no effect on plasma corticosterone level,19) suggesting that general stress response is minimally induced by this method. Voluntary sleep deprivation induces a similar level of sleep shortage to that induced by gentle handling as assessed by electroencephalogram.9) However, the latency to sleep in mice after voluntary sleep deprivation is longer than that in mice after forced sleep deprivation, indicating that voluntary sleep deprivation produced milder effect than forced sleep deprivation in lowering the arousal level.9) This difference may be relevant to the fact that forced sleep deprivation produced more prominent effect on the activities of orexin neurons and nNOS neurons.
Our results indicated that forced sleep deprivation promoted activation of orexin neurons, which was consistent with previous observations by others.15,20) In addition, activation of nNOS neurons in the lateral hypothalamic area by sleep deprivation was consistent with our previous observations using a longer term (12-h) of forced sleep deprivation.4) Similar to the cases with caffeine administration, the effect of sleep deprivation on the activation of orexin neurons was not additive to that of 7-NI, suggesting that the same population of orexin neurons respond to both sleep deprivation-induced activation and NO-induced inhibition. Notably, sleep deprivation was not accompanied by the increase in the arousal level of mice, although the activity of orexin neurons was increased after sleep deprivation. This apparent discrepancy might be attributable to the dysfunction of orexin neurons. Indeed, our previous studies suggest that sleep deprivation results in production of excess amount of NO, and inactivation of protein disulfide isomerase by NO promotes accumulation of misfolded orexin peptides4) and compromised release of mature orexin.21) That means, even if orexin neurons are electrically active, they may fail to release orexins from their nerve terminals. However, one of our observations contradicting with this hypothesis is that sleep deprivation did not increase the arousal level even after 7-NI treatment (see Fig. 5). The causes of dissociation between the activity of orexin neurons and the arousal level after sleep deprivation may have to be explained by orexin-independent mechanisms.
High concentrations of NO are generally considered to produce toxic effects on neurons and other cell populations. We have not directly examined NO concentration in the present study, but several precedent studies may provide relevant information. Kostin et al.7) reported that extracellular NO levels before and after sleep deprivation for 3 h were around 0.4 and 0.6 µM, respectively. In addition, results in our previous study4) suggest that sleep deprivation for 12 h was accompanied by increased NO production as indicated by increased protein S-nitrosation in the hypothalamus. In the same study, severe conditions of sleep deprivation (during the entire light phase of 12 h for consecutive 7 d) resulted in a significant decrease in the number of orexin neurons, which reflected the toxic effect of NO.4) On the other hand, in the present study, we did not observe any changes in the number of orexin neurons after a shorter period (8 h) of sleep deprivation or after caffeine administration (data not shown). Therefore, the concentrations of endogenous NO produced under the present experimental conditions are considered to be lower than toxic levels.
Overall, the present results indicate that the ambient level of NO produced by local nNOS neurons may be sufficient for restricting the activity of a subset of orexin neurons. In addition, NO-sensitive orexin neurons may correspond to orexin neurons that are active in response to awakening stimuli such as caffeine administration and sleep deprivation. Moreover, the activity of nNOS neurons in the lateral hypothalamic area may be elevated when orexin neurons are active, and the detailed physiological significance of the elevated activity of nNOS neurons may deserve further investigations.
This work was supported by The Smoking Research Foundation; JSPS KAKENHI, MEXT, Japan [Grants 16H04673, 16K15204]; and Program for Leading Graduate Schools “HIGO (Health life science: Interdisciplinary and Glocal Oriented),” MEXT, Japan.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.