2018 Volume 41 Issue 2 Pages 208-212
2018 Volume 41 Issue 2 Pages 208-212
Mume fruit, the Japanese apricot (Prunus mume SIEB. et ZUCC.), is popular in Japan and is mostly consumed in the pickled form called umeboshi. This fruit is known to have anti-microbial properties, but the principal constituents responsible for the antimicrobial properties have not yet been elucidated. We investigated the antimicrobial activities of the phenolic compounds in P. mume against enterobacteria. In this study, growth inhibitory activities were measured as an index of the antibacterial activities. The phenolic compounds were prepared from a byproduct of umeboshi called umesu or umezu (often translated as “mume vinegar”). Umesu or umezu phenolics (UP) contain approximately 20% phenolic compounds with p-coumaric acid as a standard and do not contain citric acid. We observed the inhibitory effects of UP against the growth of some enterobacteria, at a relatively high concentration (1250–5000 µg/mL). Alkali hydrolysates of UP (AHUP) exhibited similar antibacterial activities, but at much lower concentrations of 37.5–300 µg/mL. Since AHUP comprises hydroxycinnamic acids such as caffeic acid, p-coumaric acid, and ferulic acid, the antibacterial activities of each of these acids were examined. Our study shows that the phenolic compounds in P. mume other than citric acid contribute to its antimicrobial activity against enterobacteria in the digestive tract.
Mume fruit, the Japanese apricot (Prunus mume SIEB. et ZUCC.) that belongs to the Rosaceae family, is popular in Japan. Unlike many other fruits, most of the harvested mume fruits are processed prior to consumption because of their extreme sourness. The main constituent in the mume fruit is citric acid, and the acid content of the mature fruit may reach up to 6–7% of the flesh weight. Owing to this strong acidity, P. mume is mostly eaten in Japan in the pickled form called umeboshi, which is also used as an effective preservative to retain the freshness of cooked rice.
Other processed mume fruits such as fructus mume (smoked young mume fruits) or bainikuekisu (a concentrated juice of the mume fruit) have been used as folk medicines in east Asian countries.1) For generations, particularly in China, fructus mume has been used as a home remedy or even as a part of Chinese medicine for treatment of cough, ulcers, or digestive function problems. One such noteworthy application of fructus mume is in the treatment of gastrointestinal infections. Chen et al. reported that fructus mume extract showed antimicrobial activity against Streptococcus mutans.2) One study reported that P. mume extract showed antimicrobial activity against pathological oral bacteria, with a minimum inhibitory concentration (MIC) range of 0.15625–0.0003 g/mL.3) In Japan, bainikuekisu is traditionally consumed for alleviating diarrhea and digestive dysfunctions. Bainikuekisu has been recently reported to strongly inhibit human influenza A virus4) and to suppress Helicobacter pylori-induced glandular stomach lesions in Mongolian gerbils.5)
Although many studies have demonstrated antibacterial activity for P. mume-related products, no major study has yet identified the principal constituents responsible for the antibacterial activity. In the case of fructus mume or bainikuekisu, the antibacterial activity can be attributed to the high content of citric acid. Consumption of P. mume-related products is believed to result in a low pH in the digestive tract, thus decreasing the viability of microorganisms. Seneviratne et al. reported that the HPLC profile of P. mume extract showed the presence of citric acid, tartaric acid, and oxalic acid.3) Otsuka et al. reported that the fruit juice concentrate of P. mume contains strong acids, including citric and malic acid.5)
However, we suspected that constituents of the mume fruit other than organic acids might play an active role in its antimicrobial effects. Since the mume fruit also contains considerable amounts of phenolic compounds, we suspected that these substances may be responsible for antimicrobial effects. However, few studies have addressed this hypothesis. Miyazawa et al. reported that (+)-syringaresinol extracted from unripe P. mume inhibited H. pylori motility.6) However, the level of (+)-syringaresinol in raw P. mume is extremely low, on the level of 0.0001%, and thus not sufficient to be responsible for the antibacterial effects of the fruit.
In our previous study, we devised a new procedure for isolating phenolic fractions from a byproduct of umeboshi called umesu or umezu (mume vinegar).7–9) In our study, we found that this phenolic fraction (umesu phenolics: UP) is composed of various hydroxycinnamic acid derivatives. These derivatives had the same characteristics as the phenolic compounds in the flesh of the mume fruit, and UP does not contain free citric acid and sodium chloride.7,8,9) Given these findings, here, we investigated the potential antibacterial actions of the chemical compounds in P. mume against enterobacteria, using UP. We also discuss the effects of citric acid against enterobacteria in vivo.
Caffeic acid, trans-p-coumaric acid, ferulic acid, chlorogenic acid [5-caffenylquinic acid], potassium morpholinopropane, sulfonic sulfate (MOPS) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Diaion HP-20 resin was obtained from Mitsubishi Chemical Co., Ltd., Tokyo, Japan. HPLC-grade methanol, tetrafluoroacetic acid (TFA), and Folin–Ciocalteu reagent were purchased from Nacalai Tesque Inc. (Kyoto, Japan). All other chemicals were of analytical grade.
Determination of Total Phenolics, Sugar, and Moisture ContentThe total phenolic content was determined by the Folin–Ciocalteu method with trans-p-coumaric acid as a standard.10) The total sugar content was assayed by the phenol–sulfuric acid method with glucose as a standard.11) The moisture was expressed as weight loss upon drying at 90°C for 24 h under atmospheric conditions.
SampleA set of UP was prepared by the method reported previously.8,9) Briefly, umesu was loaded onto the Diaion HP-20 resin column and eluted with deionized water to remove the organic acids such as citric acid, sodium chloride and sugars. The column was then eluted with 60% aqueous ethanol (v/v). This fraction, containing UP, was collected, condensed, and lyophilized. UP was stored at −30°C until analysis. UP (lot #12) which was prepared on an industrial scale was obtained from Sunactis Co., Ltd. (Osaka, Japan).
Alkaline Hydrolysates of UP (AHUP)AHUP was obtained as described by Lam et al.12) Freeze-dried UP (50 mg) was suspended in 4 mL of 1 M NaOH that was deoxidized by nitrogen gas bubbling in advance. After 24 h of incubation at 37°C, the suspension was acidified by adding 0.27 mL of phosphoric acid to adjust the pH to 4.5 and extracted with 5 mL of ethyl acetate twice. Each ethyl acetate layer was combined and evaporated to dryness by nitrogen gas bubbling at 60°C. The dried extracts were dissolved in methanol and the concentration of phenolic compounds in the solution was determined by HPLC. Furthermore, AHUP consists of caffeic acid, trans-p-coumaric acid, cis-p-coumaric acid and ferulic acid as aglycone, and also contained several minor phenolic compounds.8,9) The content of each aglycone was 12.1, 20.9, 5.0 and 6.1%, respectively.
HPLC AnalysesA Shimadzu LC-2010 system was used for the HPLC analyses. All samples were injected into a Hydrosphere C18 (ϕ4.6 mm×250 mm) column (YMC, Kyoto, Japan). The column was eluted with mixed solvents A (0.1% TFA in water) : B (methanol) at a flow rate of 1.0 mL/min. The eluent was A : B=80 : 20 for first 10 min, and then the elution followed a linear gradient system ending with 25 : 75 after 80 min. All analyses were performed at 30°C and monitored by UV absorption at 280 nm.
Microorganisms and Culture MediaThe following bacterial strains were obtained from Biological Resource Center, National Institute of Technology and Evaluation (NBRC, NITE): Citrobacter freundii NBRC 12681, Enterobacter aerogenes NBRC 13534, Enterobacter cloacae ssp. cloacae NBRC 13535, Escherichia coli NBRC 15034, Klebsiella oxytoca type NBRC 102593, Proteus hauseri NBRC 3851, Proteus mirabilis type NBRC 105697, and Salmonella enterica ssp. enterica NBRC 105726. The strains kept at −70°C in the Brain Heart Infusion Broth (BHI) with 20% glycerol were activated by transferring into nutrient agar, and by incubation at 36°C for 24 h.
BHI was obtained from Becton Dickinson Company, U.S.A. Nutrient broth (NB) contained 5 g of pancreatic digest of casein (Oriental Yeast, Tokyo, Japan), 2.5 g of yeast extract (Oriental Yeast), and 1 g of glucose per 1 L of deionized water.
For antimicrobial activity assays of alkaline hydrolysates, MOPS was added to NB at 100 mM and the pH was adjusted to 7.0 (MNB).
Determination of in Vitro Antimicrobial Activity against EnterobacteriaIn this study, growth inhibitory activity was measured as an index of the antimicrobial activity. The culture for each bacterial strain was transferred to another nutrient agar plate and incubated at 37°C for 16 h. One loopful of each strain was inoculated in NB using a platinum loop and cultured until the optical density at 560 nm reached 0.5. The culture (500 µL) was distributed into 16.5×1.6-cm pre-warmed test tubes that contained 4.5 mL of NB or MNB, in which UP and AHUP of various concentrations or several drugs had been dissolved and filtered through a Millex-LG filter (0.2 µm, Merck Millipore, Darmstadt, Germany). The culture (100 µL) was then seeded into a 96-well-microplate, which was incubated for 12 h. Finally, the optical density at 630 nm was measured using a plate reader (iMark, BioRad) at regular intervals for 16 h.
To examine the antimicrobial activities of AHUP, AHUP was dissolved in DMSO and diluted in geometric series by DMSO. These solutions were added at 1% to MNB. The total content of phenolic compounds in the medium was expressed as the sum of the contents of trans-p-coumaric acid, caffeic acid, and ferulic acid, which were determined using HPLC. The final concentrations of the phenolic compounds in AHUP were 300, 150, 75, and 37.5 µg/mL, respectively.
As an authentic sample, trans-p-coumaric acid, caffeic acid, and ferulic acid were dissolved in DMSO and the solution was diluted serially in 4 stages by DMSO. These diluted solutions were added to MNB at a concentration of 1%. The final concentration of trans-p-coumaric acid, caffeic acid, and ferulic acid was 1200, 600, and 300 µg/mL, respectively.
The moisture, phenolics, and total sugar contents of UP (lot #12) were 3.25, 19.2, and 57.7%, respectively. UP did not contain any citric acid or sodium chloride, although umesu, which is the raw material for UP, contains 20% sodium chloride and 5% citric acid. We have already reported that UP consists of hydroxycinnamic acid derivatives.8) Chlorogenic acids such as 5-O-caffeoylquinic acid and 3-O-caffeoylquinic acid and prunose II or III-related compounds such as 1,3′,4′,6′-tetra-O-acetyl-6-O-p-coumaroyl-sucrose (prunose II)13) or 4,3′,4′,6′-tetra-O-acetyl-6-O-p-coumaroyl-sucrose (prunose III)14) or their isomers are found in UP (unpublished data).
Antimicrobial Activity of UPThe antimicrobial effects of UP on the enterobacterial strains were determined by microplate growth-inhibition assay. As shown in Fig. 1, a relatively high concentration (1250–5000 µg/mL) of UP only modestly inhibited the growth of all of the enterobacteria, except for Enterobacter cloacae.
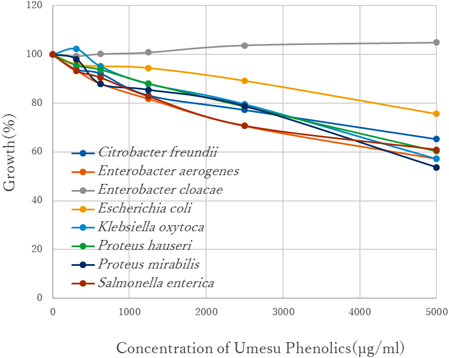
The bacterial growth was described as 100% if the concentration of tested compound was 0 µg/mL.
The antimicrobial effects of AHUP on eight enterobacterial strains are summarized in Fig. 2. The tested AHUP showed strong antimicrobial effects against all of the bacteria selected; its potency was up to about 17 times higher than that of the tested UP. These data suggest that the existence of aglycones is essential for strong antibacterial activity. Given this discovery, we then attempted to identify the aglycones that were the main contributors to the antibacterial effects, using trans-p-coumaric acid, caffeic acid, and ferulic acid as authentic samples.
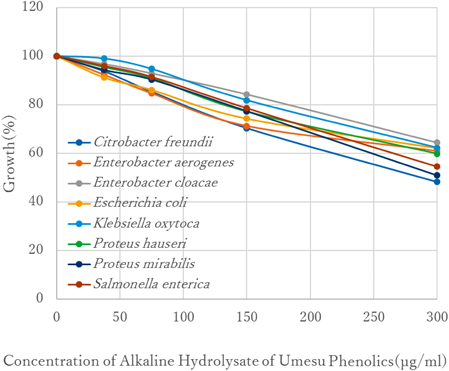
The bacterial growth was described as 100% if the concentration of tested compound was 0 µg/mL.
As shown in Fig. 3, these hydroxycinnamic acids showed similar antibacterial effects against the eight enterobacteria strains. Among them, trans-p-coumaric acid showed the strongest activity, with IC50 ranging from 200 to 400 µg/mL, against the entire group of bacteria. Since AHUP had IC50 values ranging from 300 to 400 µg/mL, it was surmised that trans-p-coumaric acid in the alkaline hydrolysates was the main contributor to the antibacterial activity. Since hydroxycinnamic acids have been shown to exert antibacterial effects,15,16) the presence of these compounds in the AHUP explains their antibacterial capability.

The bacterial growth was described as 100% if the concentration of tested compound was 0 µg/mL.
Recently, we discovered that rat intestinal acetone powder could hydrolyze UP, and the hydroxycinnamic acids, which are one of the main components, were liberated in the form of aglycones (data not shown). Nonspecific esterases may be present in the gastrointestinal tract and intestinal bacteria.17) Therefore, the hydrolysis of UP by certain intestinal esterases inside the gastrointestinal tract may result in decreased viability of various enterobacteria.
Involvement of Citric Acid in Antimicrobial ActivityThe major acid in the P. mume fruit is citric acid. When citric acid was added to NB, the pH level of the NB was significantly reduced. For example, the growth of Escherichia coli NBRC 15034 was inhibited in the presence of only 0.03125% citric acid in NB (Fig. 4a). However, when citric acid with pH adjusted to 7 by NaOH was added to NB, it produced an opposite effect; the higher pH increased the growth rate of the enterobacteria as shown in Fig. 4b. In this case, it was thought that the citric acid was utilized by the intestinal microbes as an additional energy source.
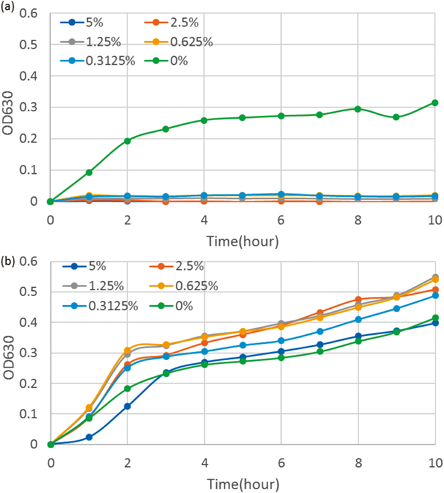
(a) Growth curves of Escherichia coli in NB+citric acid adjusted to pH 7 using NaOH. (b) Growth curves of Escherichia coli in NB+non-pH-adjusted citric acid.
Nanayama also examined the role of citric acid in antimicrobial activity in bainikuekisu and found that citric acid was the prime agent responsible for antibacterial effects.18) Because bainikuekisu is a concentrate of P. mume fruit flesh, high-temperature heating is necessary to produce it. The phenolic substances in P. mume should be decomposed by heat in a high concentration of citric acid. Our preliminary analysis of bainikuekisu showed that it contained approximately 50% citric acid, small amounts of other organic acids and furfural substances, but in contrast to UP, it did not contain phenolic substances (data not shown). Therefore, we concluded that the mechanism underlying the antibacterial activity of UP was quite different from that of bainikuekisu. Furthermore, it is thought that when citric acid derived from P. mume fruit enters into the duodenum from stomach, it is neutralized by bile and pancreatic juice, which are alkaline as a result of the high concentration of bicarbonate ions. The small intestine is alkaline with a pH of approximately 8.5. In these conditions, citric acid could not exert antimicrobial effects.
Seneviratne et al. reported that P. mume aqueous extract has an antimicrobial effect against pathogenic oral bacteria.3) Since an HPLC analysis revealed that the main compounds of P. mume are organic acids, citric acid and other organic acids were suggested to be predominantly involved in the oral antimicrobial effects. However, Seneviratne et al. also suggested that other active components in the P. mume extract also have considerable antibacterial activity, and for clinical application, more molecular studies are needed to elucidate the mechanism and the identity of the other active components involved in the antimicrobial activity of P. mume. It was expected that the phenolic compounds of mume might be still present to a certain degree in fructus mume, although mume fruits were exposed to smoke and temperature to a certain degree.
Thus, our study shows that the phenolic compounds rather than citric acid contribute to the antimicrobial activity of P. mume against enterobacteria in the digestive tract. Since UP is confirmed safe for animals and humans, it should be considered as a good food source that lacks toxicity.19,20)
This work was supported by research Grants from the Regional Innovation Strategy Support Program (Wakayama Kihokukichu Area) of the Ministry of Education, Culture, Sports, Science and Technology of Japan and also by Heisei 27 and 28 year (FY2015 and FY2016) research Grants from the Kishu-Tanabe Ume Promotion Committee. The authors wish to acknowledge Dr. Ryoichi Araki, Associate Professor of Faculty of Education, Wakayama University, for his help in interpreting the significance of the results of this study.
Takahiko Mitani and Kunihiro Kishida have a patent JP5867802 licensed to Kindai University and Wakayama Agricultural Processing Research Corporation.