2018 Volume 41 Issue 4 Pages 470-477
2018 Volume 41 Issue 4 Pages 470-477
The Nardostachys jatamansi DC (NJ) root has been used as a sedative or analgesic to treat neurological symptoms and pain in traditional Korean medicine. Here, we investigate the potential effects of NJ on Alzheimer’s disease (AD) and reveal the molecular mechanism through which NJ exerts its effects. The neuroprotective effect of the NJ root ethanol extract against β amyloid (Aβ) toxicity was examined in vitro using a cell culture system and in vivo using a Drosophila AD model. The NJ extract and chlorogenic acid, a major component of NJ, inhibited Aβ-induced cell death in SH-SY5Y cells. Moreover, the NJ extract rescued the neurological phenotypes of the Aβ42-expressing flies (decreased survival and pupariation rate and a locomotor defect) and suppressed Aβ42-induced cell death in the brain. We also found that NJ extract intake reduced glial cell number, reactive oxygen species level, extracellular-signal-regulated kinase (ERK) phosphorylation, and nitric oxide level in Aβ42-expressing flies, without affecting Aβ accumulation. These data suggest that the neuroprotective activity of NJ might be associated with its antioxidant and anti-inflammatory properties, as well as its inhibitory action against ERK signaling; thus, NJ is a promising medicinal plant for the development of AD treatment.
Alzheimer’s disease (AD) is a neurodegenerative disease that accounts for more than 60% of all cases of dementia.1) The primary cause of AD is largely considered to be the production of β-amyloid (Aβ) by the abnormal cleavage of the amyloid precursor protein in brain tissue.2) Aβ oligomers induce neuronal cell death through various cellular abnormalities, including increased oxidative damage and an increased inflammatory response.1) As there is no effective treatment for AD,3) the development of more fundamental therapies for AD, which can inhibit neuronal cell death, remains an important challenge.
According to Donguibogam, which was published 400 years ago in Korea,4) the root of Nardostachys jatamansi DC (NJ) has been used as a sedative or analgesic to treat neurological symptoms and pain in traditional Korean medicine. Accordingly, the bioactivity of NJ on neurons has been examined in several biomedical studies.5–7) NJ was isolated as a medicinal plant with acetylcholinesterase inhibitory activity in in vitro studies,6,7) and administration of its extract improved learning and memory in mice.5) NJ has also been found to have antioxidant and anti-inflammatory effects. Essential oil from NJ has shown antimicrobial and antioxidant activities.8) An ethyl acetate extract derived from NJ suppresses lipopolysaccharide (LPS)-induced neuro-inflammatory responses in microglial cells.9) In addition, cardioprotective and antiarrhythmic effects of NJ have also been reported in a recent study.10) However, thus far, the in vivo effect of NJ against AD has not been well understood.
Based on its physiological relevance and incorporation of advanced genetic tools, Drosophila has been used as a genetic model system for human diseases, including AD.11–13) The Drosophila genome carries homologs of most AD-related genes such as amyloid precursor protein, tau, and presenilin and its cofactors.14–17) Thus, various AD model flies have been developed, including several human Aβ42-expressing flies,18–21) which exhibit AD-like phenotypes such as neurodegeneration, increased reactive oxygen species (ROS), neuro-inflammation, and learning and memory defects.18–21) Using these AD model flies, many reagents with therapeutic potential, as well as genetic modifiers, have been identified.13,22–26)
In the present study, using a Drosophila system as an in vivo model of AD, in addition to an in vitro mammalian cell culture system, we investigated the neuroprotective effect of NJ on Aβ toxicity. Our data indicate that NJ has neuroprotective properties against Aβ42 toxicity.
The NJ root was purchased from Dong Yang Pharm Co. (Seoul, Korea). The root (100 g; voucher number: CYWDU-KP0004) was pulverized and subjected to the extraction process twice using 30% ethanol at 100°C in a reflux condenser for 3 h. The extract was filtered with a 50 µm filter and concentrated using a lyophilizer at −60°C. The final yield was approximately 12.8 g of dried material. The extract was mixed in fly food at the indicated concentration or stored at −70°C until further use. A representative specimen of NJ was deposited at the Medicinal Herb Garden of Dongguk University for reference purposes on December 11, 2014.
Quantitative Analysis of Chlorogenic AcidChromatographic separation of the analyte was performed using the Acquity ultra-pressure liquid chromatography (UPLC) system with a BEHC18 column (2.1×50 mm, 1.7 µm, Waters, Milford, MA, U.S.A.). An aliquot of the sample (2 µL) was injected into the UPLC system for analysis. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid acetonitrile (B), which was delivered at a flow rate of 0.3 mL/min in the following programmed gradient elution: 3% (B, v/v) isocratic for 10 min, 3→8% (B) in 10 min, 8→100% (B) in 15 min, 100% (B) isocratic for 3 min, 100→3% (B) in 0.5 min, and 3% (B) isocratic for 1.5 min as post-run reconditioning. The column temperature was maintained at 40°C, and a wavelength of 325 nm was used for quantification. The stock solution of chlorogenic acid (CGA) and the extract was prepared by dissolving the reference substances in 50% methanol. For CGA concentrations of 25–250 µg/mL, the linear regression coefficient for CGA was calculated as 1. The percentage concentration of CGA in the extract was 9.04%, which indicates that 100 g NJ root (12.8 g of dried material) contains approximately 1.16 g CGA.
Cell Culture and Viability TestTo investigate the effect of NJ extract on cell viability, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, St. Louis, MO, U.S.A.) assay was performed with SH-SY5Y neuroblastoma cells (American Type Culture Collection, Rockville, MD, U.S.A.), as previously described.27)
Fly StrainsGlass multimer reporter-GAL4 (GMR-GAL4; eye driver), embryonic lethal abnormal vision-GAL4 (elav-GAL4; pan-neuronal driver), Sevenless-GAL4 (sev-GAL4; photoreceptor cells driver), UAS-epidermal growth factor receptor (EGFR), and UAS-33770 (UAS-Aβ42, deposited by Virtuvean) were obtained from the Bloomington Drosophila Stock Center (Bloomington, IN, U.S.A.). UAS-Aβ42 was obtained from Dr. M. Konsolaki (Rutgers University, New Brunswick, NJ, U.S.A.). UAS-hemipterousCA (hepCA, the constitutively active form of Drosophila c-Jun N-terminal kinases kinase (JNKK)) is a gift from Dr. K. Matsumoto (Nagoya University, Nagoya, Japan). The genotypes of the flies used in this study are CTL (w1118), elav>Aβ42 (UAS-Aβ42/UAS-Aβ42; elav-GAL4/elav-GAL4), GMR>33770 (GMR-GAL4, UAS-33770/GMR-GAL4, UAS-33770), sev>hepCA (sev-GAL4/sev-GAL4; UAS-hepCA/MKRS), and GMR>EGFR (GMR-GAL4/UAS-EGFR).
Measurement of the Metabolism of CGA Following Oral Administration to FliesThree to five days-old male flies were used to measure the metabolism of CGA consumed by flies. Fifty male flies per time group, at each of five times (0, 15, 30, 60, 120 min), were starved for 24 h in a plastic vial with only a humidified piece of cotton wool. The flies were fed with 200 µg/mL NJ extract in 5% sugar for 24 h, then transferred to a plastic vial with only a humidified piece of cotton wool. After the indicated time (0–120 min), they were ground in 2 mL of acetone, and the quantity of CGA within the fly bodies was analyzed using UPLC.
Survival and Pupariation AssaysMore than 200 embryos per genotype were raised in standard fly media, with or without the NJ extract, at 25°C. Fifty hatched larvae were maintained per vial. The numbers of pupae and enclosed adult flies were counted every 12 h.
Locomotor ActivityA climbing assay was performed at 25°C as previously described.28) Ten trials, with a total of 100 male flies, were performed for each group. Climbing abilities (ratio of the number of flies that climbed to the top within 8 s against the total number of flies, expressed as a percentage) were recorded for each test and the mean climbing ability for 10 repeated tests was calculated.
Measurement of Cell Death and ROS LevelsFor measuring cell death, acridine orange (AO) staining was performed, as previously described.21) The larval brains were incubated in 1.6 µM AO (Sigma-Aldrich) solution. For measuring the ROS levels, the eye imaginal discs were incubated with 30 µM dihydroethidium (DHE) dye (Sigma-Aldrich) dissolved in Schneider’s medium. ROS levels in SH-SY5Y cells were measured using OxiSelect Intracellular ROS Assay Kit (Cell Biolabs, San Diego, CA, U.S.A.). SH-SY5Y cells were plated into 96-well plates (5×104 cells/well) and incubated overnight at 37°C. Then 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA)/media was added, and the cells were incubated at 37°C for 60 min and treated with NJ or CGA, with or without Aβ25–35 or hydrogen peroxide (H2O2), for 24 h. The fluorescence intensity was measured at 480 nm excitation and 530 nm emission, using a fluorescence microplate reader (SpectraMax Gemini EM, Molecular Device; Sunnyvale, CA, U.S.A.).
Western Blot and Immunohistochemical AnalysesWestern blot and immunohistochemical analyses were performed as previously described.29) Anti-Aβ42 [1 : 2000 in TBST (Tris-buffered saline+0.2% Tween 20), Covance], anti-actin [1 : 2000 in TBST; Developmental Studies Hybridoma Bank (DSHB)], anti-extracellular-signal-regulated kinase (ERK) (1 : 2000 in TBST, Cell Signaling), anti-phospho-ERK (1 : 2000 in TBST, Cell Signaling), anti- c-Jun N-terminal kinase (JNK) (1 : 2000 in TBST, Cell Signaling), anti-phospho-JNK (1 : 2000 in TBST, Cell Signaling), a horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) (1 : 2000 in TBST, Cell Signaling), and a horseradish peroxidase-conjugated anti-mouse IgG (1 : 2000 in TBST, Cell Signaling) antibodies were used for Western blot analysis. For immunohistochemical analyses, mouse anti-Aβ42 [1 : 200 in PBST (phosphate-buffered saline+0.1% Triton X-100), DE2B4, Santa Cruz], anti-reverse polarity (α-Repo; 1 : 10 in PBST, DSHB), and anti-phospho-ERK (1 : 200 in PBST, Cell Signaling) antibodies were used.
Measurement of Nitric Oxide LevelsAt 72 h post-eclosion, 15 heads of male adult flies were prepared in homogenization buffer (0.1 M phosphate buffer at pH 7.4, 25 mM KCl) on ice. The samples were homogenized and centrifuged at 10000×g for 10 min at 4°C. The supernatants were mixed in a 1 : 1 ratio with Greiss reagent (Sigma-Aldrich), then incubated for 15 min at 25°C. Nitrite levels were measured spectrophotometrically, at 550 nm.
StatisticsData in the studies was quantitatively analyzed for statistical significance using a Student’s t-test (two-tailed) for comparisons of two groups. GraphPad Prism, Version 5.0 (GraphPad Software, San Diego, CA, U.S.A.), software was used; p values of <0.05 were considered to be significant. MultiGauge version 3.1 (FUJIFILM, Tokyo, Japan) was used to assess Western blotting data.
We verified the plant used in this study as NJ by identifying CGA, a marker of NJ,30) on chromatographic separation of the ethanol extract (Fig. 1). We then investigated the toxicity of the NJ extract by measuring the viability of SH-SY5Y cells treated with the extract. The NJ extract did not affect cell viability at any concentration up to 100 µg/mL (Fig. 2A).
To investigate the potential neuroprotective functions of NJ in AD, we examined the effect of the NJ extract on Aβ-induced cell death and ROS production. As shown in Fig. 2B, treatment with the extract at low levels suppressed cell death, with maximal effect at 50 µg/mL, similar to treatment with the positive controls donepezil and KSOP1009.31) Moreover, NJ extract suppressed the elevated ROS levels in H2O2 or Aβ-treated cells (Fig. 2C). Similarly, CGA, a major component of NJ, did not show cellular toxicity (Fig. 2D) and inhibited the Aβ-induced cell death (Fig. 2E). These results suggest that NJ and CGA have a protective effect against Aβ neurotoxicity.
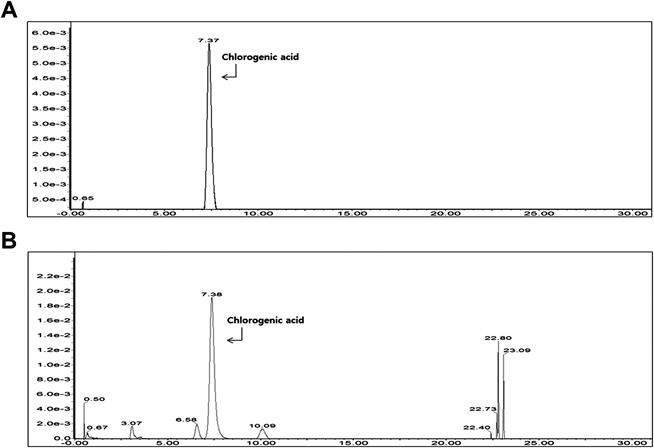
Ultra-pressure liquid chromatograms of CGA (A) and ethanol extract of NJ root (B). The percentage of CGA concentration in the NJ extract was 9.04%.

Effects of the NJ extract (A, B, C) or CGA (C, D, E) on the viability and ROS levels of SH-SY5Y cells with (B, C, E) or without (A, D) Aβ25–35 or H2O2 treatment. All data are expressed as means±standard error (S.E.) (*** p<0.001, ** p<0.01, * p<0.05 vs. 30% ethanol or Aβ 25 µM; ## p<0.01 vs. H2O2 100 µM; Student’s t-test; n=3). -, 30% ethanol; CGA, chlorogenic acid (C, 10 µM); D, 5 µM donepezil; DCF, 2′,7′-dichlorofluorescein; K, 10 µg/mL KSOP1009; NJ, Nardostachys jatamansi (C, 200 µg/mL); NS, not significant; ROS, reactive oxygen species.
To determine the neuroprotective function of NJ in vivo, we tested the effect of NJ extract intake on the development of AD model flies expressing human Aβ42. First, we measured the metabolism of CGA in fly bodies using UPLC, following feeding of NJ to the flies. As shown in Fig. 3A, CGA remained in the fly body for approximately 2 h and was gradually degraded. The NJ extract significantly increased the survival rate of Aβ42-expressing flies (Fig. 3B). The delayed pupariation time and reduced pupariation rate of the AD model flies were also rescued upon NJ intake (Fig. 3C). Furthermore, NJ intake rescued the locomotor impairment in AD model flies (Fig. 3D).

(A) Ultra-pressure liquid chromatograms of the extracts of flies sacrificed at the indicated times after feeding with NJ extract for 24 h. Chlorogenic acid (CGA) was traced to show its metabolism in the fly body. The relative level of CGA at each time point was acquired by comparison with the CGA level at 0 min after oral administration. The arrows indicate peaks corresponding to CGA. (B–D) Effects of NJ intake on survival rate (B), pupariation time (C), and decreased locomotor activity (D) of Aβ42-expressing flies. All data in the graphs are expressed as means±S.E. (*** p<0.001, ** p<0.01, * p<0.05 vs. elav>Aβ42; Student’s t-test; A and B, n≥200; C, n≥50; D, n=100). -, 30% ethanol; AEL, after egg laying; AOA, after oral administration; CGA, chlorogenic acid; CTL, control; D, 5 µM donepezil; K, 10 µg/mL KSOP1009; NJ, Nardostachys jatamansi (C, D, 200 µg/mL); NS, not significant.
We also found that NJ intake reduced the death of neurons in larval brains expressing Aβ42 (Figs. 4A, B), which suggests that the beneficial effects of NJ on Aβ42 flies are due to reduction of Aβ42-induced cell death. Because an increase in ROS caused by Aβ42 is one of the major pathogenic mechanisms of AD,1) we examined whether the NJ extract reduced the increased ROS level in Aβ42-expressing eye imaginal discs. As shown in Figs. 4C and D, the ROS levels were decreased in the eye discs of Drosophila larva following NJ intake.

(A) Dead cells stained by acridine orange (AO). (B) The graph shows the relative number of AO-positive cells per brain. (C) ROS detected using dihydroethidium (DHE) staining in the Aβ42-expressing eye imaginal discs of GMR>33770 larva. (D) The graph shows the relative number of DHE-positive signals. All data in the graphs are expressed as means±S.E. (*** p<0.001, ** p<0.01, * p<0.05 vs. elav>Aβ42 or 30% ethanol; Student’s t-test; B and D, n≥10). -, 30% ethanol; CTL, control; D, 5 µM donepezil; K, 10 µg/mL KSOP1009; NJ, 200 µg/mL Nardostachys jatamansi.
The overactivation of glial cells and the resulting increase in inflammatory responses are other important mechanisms in the AD pathogenesis32); NJ and CGA have an inhibitory effect on neuro-inflammation.9,33) Therefore, we investigated the effect of NJ intake on the hyperproliferation of glial cells in the larval brains of AD model flies. Interestingly, NJ intake restored the number of glial cells to control levels (Figs. 5A, B). Moreover, we found that NJ extract reduced the heightened nitric oxide levels in the heads of adult AD model flies (Fig. 5C). These results suggest that the anti-inflammatory effects of NJ might contribute to its beneficial effects against Aβ42 neurotoxicity.

(A) Representative confocal images of immunostained larval brains with anti-Repo antibody, which shows glial cells. (B) The graph shows the relative number of Repo-positive cells. The number of Repo-positive cells were counted as previously described.29) (C) Comparison of nitric oxide (NO) levels in the adult fly heads. All data in the graph are expressed as means±S.E. (*** p<0.001, ** p<0.01 vs. elav>Aβ42; Student’s t-test; B, n≥10; C, n=15). -, 30% ethanol; CTL, control; NJ, 200 µg/mL Nardostachys jatamansi.
We also tested whether NJ extract affect the level of Aβ42 or AD-associated signaling pathways. In contrast to ROS level or glial cells proliferation, the NJ extract did not change the level of Aβ42 (Figs. 6A, B). Moreover, NJ extract intake did not affect JNK phosphorylation following overexpression of a constitutively active form of JNK kinase in Drosophila eyes. However, interestingly, it reduced the phosphorylation of ERK in EGFR-expressing fly eyes (Fig. 6C). Moreover, the phosphorylation of ERK caused by Aβ42 overexpression in Drosophila brains was decreased by NJ extract intake (Figs. 6D, E). These results suggest that the inhibitory properties of NJ extract on ERK signaling might play a role in its neuroprotective effects against Aβ cytotoxicity.

(A, B) Aβ42 levels in the eye imaginal discs (A) and heads (B) of GMR>33770 flies fed with (NJ) or without (−) 200 µg/mL NJ extract. (A) Images of the eye imaginal discs immunostained with the anti-Aβ42 antibody. (B) Western blot analysis showing Aβ42 protein levels in the heads of GMR>33770 flies. Actin was used as a loading control. (C) Western blot analyses showing the effects of the NJ extract intake on phosphorylation of ERK and JNK in the heads of flies overexpressing EGFR and hepCA, a constitutively active form of JNK kinase, respectively. (D) Western blot analysis showing phosphorylation level of ERK in the heads of elav-GAL4/+ (CTL) and elav>Aβ42 flies fed with (NJ) or without (−) 200 µg/mL NJ extract. (E) Representative confocal images of anti-p-ERK antibody-immunostained adult brains of elav-GAL4/+ (CTL) and elav>Aβ42 flies fed with (NJ) or without (−) 200 µg/mL NJ extract. Magnified view of the central region of each adult brain (dotted rectangle) is presented on the bottom panel. All data in the graphs are expressed as mean±S.E. (*** p<0.001, ** p<0.01 vs. 30% ethanol or elav>Aβ42, Student’s t-test; B, n=3; C, n=10; D, n=6). -, 30% ethanol; CTL, control; NJ, 200 µg/mL Nardostachys jatamansi; NS, not significant.
Here, we demonstrate the neuroprotective function of the NJ extract against Aβ cytotoxicity not only in vitro using human cell lines but also in vivo following oral administration to AD model flies. NJ has been used in traditional Korean medicine to treat neurological symptoms, and the NJ extract has been previously associated with neuronal health.5,34) However, the protective effect of NJ against Aβ42 neurotoxicity is not well known. Moreover, thus far, most of the studies regarding its potential bioactivities associated with AD have been conducted in in vitro systems.6,7,9) Given the fact that orally administered drugs must undergo a complicated process in which they must enter the bloodstream through the digestive tract and pass through the blood–brain barrier without loss of efficacy to show efficacy in the brain,35) it is hard to determine whether NJ has a therapeutic effect on brain diseases when only in vitro experiments are used. In the present study, we, for the first time to our knowledge, demonstrate the neuroprotective effect of NJ on the toxicity of Aβ42 in vivo, as well as in vitro. NJ and CGA, a major component of NJ, decreased neuronal cell death in both mammalian cell culture and Drosophila AD models. Moreover, we found that oral administration of NJ extract reduced ROS levels and glial cell numbers in Drosophila AD models. As neuronal cell death is closely associated with increased ROS and neuro-inflammation in AD, NJ may reduce the Aβ42-induced cell death through its antioxidant and anti-inflammatory activities, as well as its inhibitory action against ERK signaling. Taken together, our data indicate that the NJ extract can protect neurons from Aβ toxicity in vivo, when administrated orally, by reaching the brain without losing its neuroprotective efficacy.
The beneficial effects of NJ on Aβ cytotoxicity are believed to arise from the function of several components of NJ. Indeed, we have confirmed in this study that CGA is a major component of NJ and can inhibit the cytotoxicity of Aβ. Similarly, several previous studies have reported that CGA plays a protective role in various situations associated with AD.36–39) CGA protects neurons against Aβ and glutamate neurotoxicities and scopolamine-induced amnesia.36,37,39) These beneficial effects have been suggested to arise from various properties of CGA, such as antioxidant, anti-acetylcholinesterase, and anti-apoptotic activities.36,37,39) Another NJ-containing component, nardosinone, is also known to exhibit activities associated with neuronal functions.40,41) Nardosinone acts as an enhancer of nerve growth factor during neurite outgrowth from PC12D cells,40) and improves the proliferation, migration, and selective differentiation of mouse embryonic neural stem cells.41) Moreover, several compounds, including nardosinone, that are isolated from NJ exhibit inhibitory activity against LPS-induced nitric oxide production in macrophage cells.42) Given the wide variety and complexity of the onset and pathogenesis of AD, the inhibition of Aβ42 neurotoxicity of NJ, found in the current study, is likely a result of the combined action of various constituents, including CGA and nardosinone. Therefore, in future studies, it will be necessary to isolate CGA and nardosinone from the other effective components of NJ, as well as to study the use of NJ in combination with currently used AD therapies.
In conclusion, we found in this study that the NJ extract exhibits a neuroprotective effect against Aβ42 neurotoxicity in vivo and in vitro. This neuroprotective activity might be associated with its antioxidant and anti-inflammatory properties, as well as its inhibitory action against ERK signaling. Therefore, we propose that NJ may be a promising medicinal plant for further development of AD therapies.
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education [2017R1A2B4004241 (K.S.C.) and 2016R1D1A1B03932530 (B.S.K.)].
The authors declare no conflicts of interest.