Abstract
Neck and shoulder stiffness is a typical subjective symptom in developed countries. This stiffness is caused by factors such as muscle tension and poor blood flow, leading to reduce work efficiency and diminish QOL. NKCP®, a natto-derived dietary food supplement whose main component is bacillopeptidase F, has antithrombotic, fibrinolytic, and blood viscosity-lowering effects. Here, we investigated the effect of NKCP® on neck and shoulder stiffness in a double-blind placebo-controlled randomized crossover study. Thirty subjects with neck and shoulder stiffness were randomly divided into 2 groups and ingested 250 mg of NKCP® or placebo daily for 4 weeks. Headache score significantly improved in the NKCP® group compared to the placebo group. Moreover, NKCP® significantly improved the score of visual analogue scale for neck and shoulder stiffness and pain, reduced muscle stiffness of the neck, and increased the skin surface temperature of neck and shoulders, compared to before ingestion. No adverse effects were observed during this study. These results suggest that NKCP® may alleviate headaches and chronic neck and shoulder stiffness and pain.
Neck and shoulder stiffness is a typical subjective symptom felt by people in developed countries when under physical strain. This stiffness is caused by factors such as increased use of visual display terminals (VDTs) to perform routine tasks, lack of exercise, and physical stress. These factors increase muscle tension and reduce blood flow around the neck and shoulders.1–4) Because chronic neck and shoulder discomfort can lead to a decrease in work efficiency and diminished QOL, the alleviation of this discomfort is highly desirable in developed countries.
Natto is a traditional food made from fermented soybeans that has long been eaten in Japan. It has a balanced nutritional value and contains proteases that lower cholesterol, dissolve thrombi, and improve blood flow. Thus, it is an exceptional food that can help to improve public health.5–7) However, natto is sticky and highly pungent, so some individuals avoid eating it. Bacillus subtilis var. natto is a bacterium that is used to ferment soybeans to produce natto. NKCP® is as a functional supplement that contains bacillopeptidase F, a protease that is isolated and purified from the culture of B. subtilis var. natto.8) B. subtilis var. natto is completely removed from NKCP® to overcome difficulties related to ingestion, such as the distinctive odor of natto. Previous in vitro, animal, and human clinical studies have indicated that NKCP® inhibits coagulation, reduces the viscosity of blood, and dissolves thrombi.8–10) Hitosugi et al. reported that ingesting NKCP® 250 mg/d for 4 weeks alleviated neck and shoulder stiffness and sensitivity to cold in a crossover study involving patients with lifestyle-related diseases such as hypertension, hyperlipidemia, and diabetes mellitus.11) However, that study was based on a small sample size, so it failed to show statistically significant improvement as a result of ingesting NKCP® in comparison to a placebo. Moreover, the study measured only subjective symptoms. The use of more objective measures would provide greater clinical insight. In addition, as the study involved patients with lifestyle-related diseases, it remains unclear whether or not NKCP® is effective at alleviating neck and shoulder symptoms in healthy individuals.
In the current study, we performed a double-blind cross-over study to examine the effects of NKCP® on healthy subjects with neck and shoulder stiffness and pain.
MATERIALS AND METHODS
SubjectsThis study was approved by the clinical research ethics committees of the University of Shizuoka and the Seirei Health Care Division in Japan. This study was conducted in accordance with ethical principles based on the Helsinki Declaration and registered UMIN Clinical Trials Registry (UMIN000019824). The study was performed from November 2015 to February 2016 at the University of Shizuoka and the Seirei Healthcare Support Center Shizuoka in Japan.
The details of the study were explained to potential subjects verbally and in writing prior to the start of the study. Consent for study participation was obtained in writing. Inclusion criteria were subjects (men or women aged 20–80 years) with the physical symptoms of neck and shoulder stiffness and pain who routinely worked at a desk. Exclusion criteria were subjects with an underlying illness, pregnant or nursing, dementia, taking pharmaceuticals or supplements or consuming foods that might affect this study, and participating in other clinical studies. No specific restrictions were placed on receiving massages or on routinely applying a poultice to the neck and shoulders.
Power AnalysisPower analysis was performed using G*Power 3.1.9.2 software. A previous cross-over study examined the effects of NKCP® in 17 patients (dropout rate: 0%) with lifestyle-related diseases (Hitosugi et al.). Based on the results of that study, a power analysis was performed with an effective dose of 0.5, a power of 0.7, and a significance level of 0.05. Assuming a dropout rate of 5%, the sample size for the current study was determined to be 30 subjects.
Test SupplementsNKCP® and placebo were obtained from Daiwa Pharmaceutical Co., Ltd. The components of NKCP® and placebo are shown in Table 1. The placebo was filled with dextrin in place of NKCP®. Representative photo images of packed and tablets are indicated in Fig. 1. The two were identical in packaging and appearance, so they could not be distinguished from each other.
Table 1. The Component of NKCP
®| Content of NKCP® | Component |
|---|
| 125 mg/tablet | Digestion resistant dextrin, Microcrystalline cellulose, Sucrose, Fatty acid ester, Silicon dioxide, Shellac |
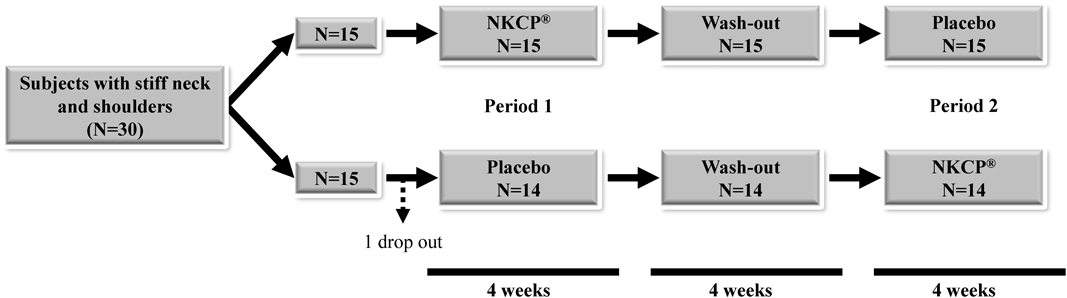
The study was a double-blind placebo-controlled crossover comparative study (Fig. 2). Subjects were divided into 2 groups, in which they were equally distributed by age and sex. Subjects ingested 250 mg of NKCP® or a placebo once a day before bedtime. After ingesting one test supplement for 4 weeks, subjects had a 4-week washout period, after which they ingested the other test supplement for 4 weeks. Neck and shoulder stiffness was assessed prior to ingestion, at 4 weeks (after the ingestion of the first test supplement), at 8 weeks (prior to the ingestion of the second test supplement), and at 12 weeks (after the ingestion of the second test supplement). During the study period, subjects continued with their usual activities and lifestyle.
Primary EndpointsVisual Analogue Scale Score for Neck and Shoulder Stiffness and PainNeck and shoulder stiffness and pain were evaluated using a visual analogue scale (VAS) with a 10-cm line ranging from “no pain” or “no stiffness” on the left to “worst pain” or “worst stiffness” (any more would be intolerable) on the right.
Subjective SymptomsHeadaches, lower back pain, cold hands and feet, eyestrain, and dry eye were evaluated as subjective symptoms on a 5-point scale.12) On the scale, 1 point indicated that there was “no feeling” of the symptom, 2 points indicated “feeling a little bit,” 3 points indicated “moderate feeling,” 4 points indicated “severe feeling,” and 5 points indicated “unbearable feeling.”
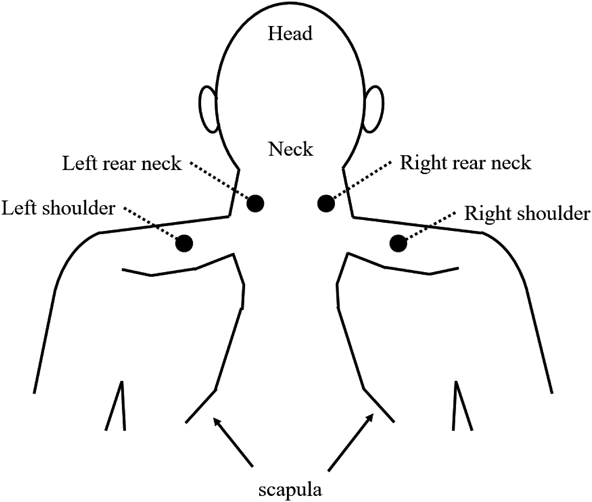
Muscle StiffnessA muscle hardness meter, PEK-MP (Imoto Machinery, Tokyo, Japan), was used to measure muscle stiffness. During measurement, subjects sat on a chair with a back but no elbow rests and kept their backs straight. The positions on the neck and shoulders where subjects felt stiffness (the left and right side of the neck and left and right shoulders) were recorded as shown in Fig. 3. At the initial measurement, the locations where measurements were carried out were photographed, and all subsequent measurements were carried out at the same location. The muscle hardness meter was placed perpendicular to the spot, and then the measurement was carried out. An average was calculated based on three measurements.
Secondary EndpointsSurface Temperature of the Skin of the Neck and ShouldersA FLIR C2 thermal imaging system (FLIR Systems, U.S.A.) was used to measure skin surface temperature. Subjects first waited in a room with a temperature of 22±2°C, then sat in a chair, where the imaging system was used to measure skin surface temperature at 4 locations, as shown in Fig. 3.
Blood Flow Velocity at the Neck, Shoulders, and FingertipsA LDF MBF-11A blood flow meter (JMS Co., Hiroshima, Japan) was used to measure blood flow velocity. Subjects sat in a chair, and blood flow velocity was measured at 4 spots, as shown in Fig. 3, and at the tip of both index fingers. Measurements were taken 3 times, and then the average blood flow velocity at the neck, shoulders, and tips of the index fingers was calculated.
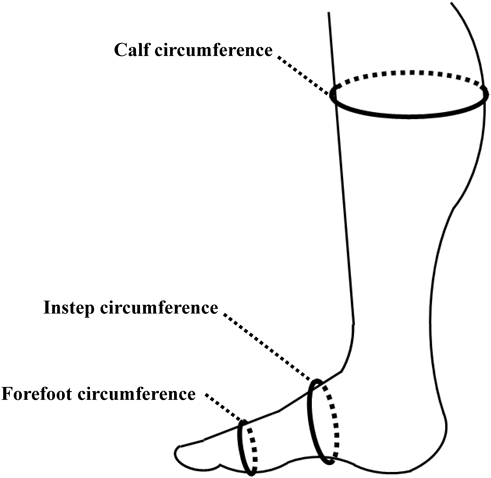
Swelling of the FeetTo assess swelling of the feet, subjects sat in a chair, and calf circumference, instep circumference, and forefoot circumference were measured (Fig. 4). Instep circumference was defined as the circumference of the foot from the highest point of the instep to the dorsum of the foot. Forefoot circumference was defined as the circumference of the foot at a line connecting the hallux and the fifth toe.
Statistical AnalysisMeasurements are expressed as the mean±standard deviation (S.D.). A paired t-test was used to statistically analyze measurements and the extent of changes in those measurements. The level of significance was set to p<0.05.
RESULTS
SubjectsThirty healthy volunteers with neck and shoulder stiffness and pain were enrolled in this study. One subject withdrew consent. The results for 29 subjects were analyzed (Fig. 1). The characteristics of the 29 subjects prior to the study are shown in Table 2. The subjects consisted of 8 males and 21 females with an age of 39.0±7.7 years, a body mass index (BMI) of 20.6±2.4, a systolic blood pressure (SBP) of 117±18 mmHg, a diastolic blood pressure (DBP) of 73±11 mmHg, and a pulse rate (PR) of 73±10 bpm. Adverse effects were not observed during the study period. Changes in SBP, DBP, and PR were not observed during the study period (data not shown).
Table 2. Baseline of the Healthy Volunteers in This Study
| n | 29 |
|---|
| Male/female | 8/21 |
| Age | 39.0±7.7 |
| Height (cm) | 163±7 |
| Body weight (kg) | 55.1±9.0 |
| BMI (kg/m2) | 20.6±2.4 |
| SBP (mmHg) | 117±18 |
| DBP (mmHg) | 73±11 |
| PR (bpm) | 73±10 |
BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, PR: Pulse rate.
The results for the primary endpoints are shown in Table 3. Changes in the VAS score for neck and shoulder stiffness and pain did not differ between the NKCP® group and the placebo group. NKCP® group had a significantly lower VAS score for neck and shoulder stiffness (3.95±2.27, p=0.008) and pain (2.38±1.75, p=0.004) compared to the VAS score prior to ingestion (4.70±2.10, 3.16±2.44). Significant changes were not observed in the VAS score after the ingestion of the placebo.
Table 3. The Results of Primary Endpoint
| Placebo | NKCP® | p-Value placebo vs. NKCP® |
|---|
| Before | After | p-Value before vs. after | Before | After | p-Value before vs. after |
|---|
| VAS | Neck and shoulder stiffness | 4.51±2.26 | 4.06±2.46 | 0.177 | 4.70±2.10 | 3.95±2.27 | 0.008 | 0.540 |
| (cm) | Neck and shoulder pain | 3.08±2.28 | 2.78±2.42 | 0.300 | 3.16±2.44 | 2.38±1.75 | 0.004 | 0.164 |
| Five-grade evaluation of physical symptom | Headache | 2.00±1.31 | 2.24±1.35 | 0.270 | 2.21±1.18 | 1.86±1.19 | 0.096 | 0.041 |
| Low back pain | 2.41±1.24 | 2.48±1.30 | 0.752 | 2.38±1.35 | 2.52±1.40 | 0.502 | 0.825 |
| Cold hands and feet | 2.55±1.27 | 2.72±1.41 | 0.445 | 2.72±1.44 | 2.66±1.34 | 0.738 | 0.387 |
| Eyestrain | 2.93±1.16 | 3.00±1.10 | 0.769 | 3.07±1.10 | 2.83±1.00 | 0.199 | 0.320 |
| Dryeye | 2.34±1.17 | 2.55±1.18 | 0.375 | 2.55±1.35 | 2.34±1.14 | 0.352 | 0.201 |
| Muscle stiffness | Neck | 6.39±0.89 | 6.16±0.69 | 0.288 | 6.45±0.82 | 6.15±0.81 | 0.009 | 0.754 |
| Shoulders | 7.16±1.62 | 7.07±0.43 | 0.781 | 7.23±0.54 | 7.05±0.55 | 0.142 | 0.750 |
The headache score for the NKCP® group was significantly decreased in comparison to that for the placebo group (p=0.041). Significant changes were not observed in the scores for lower back pain, cold hands and feet, eyestrain, and dry eye.
Measurement of Muscle Stiffness in the Neck and ShouldersChanges in the stiffness of muscles in the neck and shoulders did not differ between the NKCP® group and the placebo group. Muscle stiffness in the neck was significantly lower in the NKCP® group after ingestion (before ingestion: 6.45±0.82; after ingestion: 6.15±0.81, p=0.009). This change was not observed in the ingestion of the placebo.
Secondary EndpointsSkin Surface Temperature of the Neck and ShouldersThe results for the secondary endpoints are shown in Table 4. Changes in the skin surface temperature of the neck and shoulders did not differ between the NKCP® group and the placebo group. The skin surface temperature of the neck and shoulders was significantly higher (35.7±0.8, p<0.001, 35.4±0.8, p=0.001) after the ingestion of NKCP® than prior to ingestion (35.0±1.1, 34.6±1.2). These changes were not observed in the ingestion of the placebo.
Table 4. The Results of Secondary Endpoint
| Placebo | NKCP® | p-Value placebo vs. NKCP® |
|---|
| Before | After | p-Value before vs. after | Before | After | p-Value before vs. after |
|---|
| Skin surface temperature | Neck | 35.3±0.9 | 35.6±0.9 | 0.133 | 35.0±1.1 | 35.7±0.8 | <0.001 | 0.124 |
| (°C) | Shoulders | 34.8±1.3 | 35.3±0.8 | 0.057 | 34.6±1.2 | 35.4±0.8 | 0.001 | 0.391 |
| Blood flow (mL/min) | Neck | 14.4±11.0 | 14.1±5.7 | 0.865 | 16.4±10.8 | 14.3±8.6 | 0.143 | 0.440 |
| Shoulders | 12.3±8.1 | 11.7±5.1 | 0.540 | 12.3±7.9 | 13.7±11.7 | 0.542 | 0.447 |
| Index finger | 37.2±20.8 | 38.0±20.8 | 0.830 | 33.1±19.0 | 37.9±21.7 | 0.186 | 0.477 |
| Circumference (cm) | Calf | 35.2±2.6 | 35.6±2.73 | 0.011 | 35.6±2.5 | 35.6±2.65 | 0.823 | 0.123 |
| Instep | 22.4±1.5 | 22.6±1.42 | 0.187 | 22.5±1.5 | 22.6±1.54 | 0.399 | 0.706 |
| Forefoot | 21.7±1.0 | 22.1±1.20 | 0.018 | 21.8±1.2 | 22.1±1.39 | 0.146 | 0.624 |
Significant changes were not observed in blood flow velocity at the neck, shoulders, or tips of the index fingers after taking NKCP® or the placebo.
Swelling of the FeetSignificant differences in changes in the swelling of the feet were not observed in the NKCP® group or the placebo group. Significant increases were found in calf and forefoot circumference after ingestion of the placebo (35.6±2.6, p=0.011, and 22.1±1.20, p=0.018, respectively) compared to prior to ingestion (35.2±2.6, and 21.7±1.0, respectively). Increased foot swelling was not observed after the ingestion of NKCP®.
DISCUSSION
In order to examine whether or not the natto-derived dietary food supplement NKCP® alleviates symptoms such as neck and shoulder stiffness, a double-blind placebo-controlled randomized crossover study was performed. The subjects were healthy volunteers suffering from neck and shoulder stiffness and pain. The results showed that NKCP® significantly alleviated headaches in comparison to placebo although significant differences in VAS score for neck and shoulder stiffness and pain, muscle stiffness, and skin surface temperature were not observed between the NKCP® group and the placebo group. Moreover, shoulder disorders, including the VAS score of neck and shoulder stiffness and pain, muscle stiffness in the neck, and skin surface temperature of the shoulders and neck, were significantly improved after NKCP® ingestion compared with prior to ingestion. These results suggest that NKCP® alleviated the symptom of neck and shoulder stiffness.
In developed countries, subjective symptoms such as neck and shoulder stiffness and pain have become more prevalent with the increased use of VDTs to perform routine tasks, along with chronic lack of exercise.1) However, neck and shoulder stiffness is difficult to define, and it is often determined subjectively, so assessing its severity has been difficult. Although the Visual Analogue Scale is commonly used for subjective assessment, it is not appropriate for comparing subjects. Accordingly, the VAS needs to be combined with some objective assessment methods to accurately assess the effects of treatment on neck and shoulder stiffness.
Recently, convenient muscle hardness meters have been developed, and these devices are widely used in fields such as sports medicine and rehabilitation.13,14) Hardness meters allow simple and easy measurement of muscle stiffness at various sites, and therefore allow quantitative assessment of changes in the stiffness of muscles in the shoulders as a result of treatment.15,16) Thus, in the present study, muscle stiffness as measured by muscle hardness meter was a primary endpoint, along with the VAS score for neck and shoulder stiffness and pain. The results indicate that ingestion of NKCP® reduced the VAS score for neck and shoulder stiffness and pain, as well as neck muscle stiffness, compared to prior to ingestion. Using the VAS score as a subjective assessment and muscle stiffness as an objective assessment, the current study has confirmed that NKCP® alleviated neck and shoulder stiffness. Other studies have reported that massage, acupuncture, and moxibustion alter muscle stiffness.15) However, this study is the first to report that intake of a functional food alters muscle stiffness. NKCP® may help to alleviate symptoms and improve QOL for individuals suffering from neck and shoulder stiffness and pain.
In neck and shoulder stiffness, the muscle that stiffens is primarily the trapezius, which runs from the occipital region to the scapula. Veins running beneath the trapezius do not have accompanying arteries, and the trapezius has 1.5 times as many venous junctions as it has arterial bifurcations.17) Unlike veins around other muscles, the veins around the trapezius lack valves to prevent the backward flow of blood and congestion. These factors explain why the underside of the trapezius is readily susceptible to congestion. In addition, poor posture and sitting for a prolonged period can cause excessive tension in the shoulders and neck. This tension induces local congestion and causes the accumulation of waste products and endogenous algesic substances. Thus, it has been reported that excessive tension in the shoulders and neck is significantly related to the onset of shoulder stiffness and tension headaches.18) Thus, NKCP® may have alleviated muscle stiffness in the neck and headache score because NKCP® improved peripheral circulation around the trapezius.
Skin surface temperature and blood flow are also used to assess neck and shoulder pain and dysfunction.19–23) In order to assess blood flow near the shoulders and neck, the current study also measured skin surface temperature and blood flow. NKCP® did not increase blood flow in the shoulders and neck, but it increased skin surface temperature in those areas. These results suggest that muscle stiffness in the neck was reduced due to an increase in skin surface temperature resulting from improved peripheral circulation. Moreover, they suggest that congestion in the neck and shoulders was reduced, and the amount of waste products and endogenous algesic substances in the neck and shoulders also decreased as a result of improved peripheral circulation. This improvement in peripheral circulation and its benefits presumably led to a lower headache score.
In this study, calf circumference and forefoot circumference increased and foot swelling increased in the placebo group. However, in the NKCP® group, foot swelling did not increase. Swelling of the feet is often found in people who work in front of a VDT for a prolonged period, indicating poor peripheral circulation.24) The present study suggests that ingestion of NKCP® increased the skin surface temperature, thus improving peripheral blood flow in the shoulders and neck as well as in the lower extremities. Thus, NKCP® may prevent swelling of the feet in addition to improving neck and shoulder stiffness.
NKCP® is a functional food supplement containing bacillopeptidase F as a major component. The amount of bacillopeptidase F in 250 mg of NKCP® is 37.9±1.1 µg and that of natto in one package is 22.2±9.4 µg of bacillopeptidase F. Thus, about two packages of natto contain the same amount of bacillopeptidase F as 1 tablet of NKCP®. Although two packages of natto can be ingested on a daily basis, it is doubtful whether sufficient amount of bacillopeptidase F can be obtained from natto since its amount contained in natto differs depending on the product. In addition, as there are also many people who are difficult to take natto for their strong smell, it might to be possible for these people to ingest its sufficient amount using tablets. NKCP® is available commercially and is commonly taken, and serious adverse reactions to NKCP® have not been observed. In order to determine the safety of NKCP®, the subjects in the current study kept a diary during the study period, and blood pressure measurements were conducted. In addition, participants were asked about their condition before and after ingestion. No adverse events were observed. These findings suggest that NKCP® poses little safety risk. However, the safety of NKCP®, such as in its interaction with drugs, needs to be examined further in the future.
The current study had several limitations. NKCP® could not show significantly improve the neck and shoulder stiffness except headache score compared to the placebo group. One reason for this is that the symptoms of neck and shoulder stiffness can vary widely between individuals as they are subjective symptoms. Moreover, sample size of this study is too small. Future studies of efficacy should use a larger sample size to assess whether NKCP® can induce a significant improvement of shoulder disorders compared to placebo. NKCP® could not show the improvement of blood flow by objective assessment in this study although NKCP® possesses favorable effects, such as the inhibition of coagulation, the decrease in viscosity of blood, and the dissolution of thrombus, resulting in the improvement of blood flow disturbances.8–10) In addition, the improvement of blood flow disturbance is supported by the evidence that NKCP® ingestion increased skin surface temperature of neck and shoulder. Unfortunately, the rheometer used in this study may have indicated only the blood flow velocity at the surface of the skin and not in the veins beneath the trapezius. A rheometer that can measure blood flow deep within muscles is required to accurately assess blood flow velocity at sites of neck and shoulder stiffness. In the future, further studies are needed to address these points in order to ascertain the way in which NKCP® affects blood flow and shoulder disorders.
In this study, NKCP® ingestion was significantly correlated with improved headache score in comparison to a placebo. It was also correlated with decreased VAS score for neck and shoulder stiffness and pain, reduced neck stiffness, and increased skin surface temperature of the neck and shoulders, which appears to have alleviated shoulder and neck stiffness, compared to before treatment. This indicates that NKCP® has the potential to serve as a functional food supplement that can alleviate subjective and objective symptoms of neck and shoulder stiffness. NKCP® may be a useful functional food supplement for individuals with those symptoms. Therefore, consuming NKCP® may increase QOL and work efficiency in developed countries.
Acknowledgments
The authors wish to thank Ms. Hiroko Mochizuki, Ms. Natsumi Yorimitsu, Ms. Nana Amano, and Ms. Haruka Ebi of the University of Shizuoka for their technical assistance. The authors are grateful to Mr. Katsuhiko Shimizu, Mr. Shigenori Hosota, and Mr. Noriyoshi Wakano of the Seirei Healthcare Support Center Shizuoka in Shizuoka for their assistance. The authors also thank Mr. Philip Hawke of the University of Shizuoka Scientific English Program for proofreading the English in this manuscript.
Conflict of Interest
Yoichi Sunagawa, Yasufumi Katanasaka, and Tatsuya Morimoto received a trust research Grant from Daiwa Pharmaceutical Co., Ltd. The other authors declare no conflicts of interest. Daiwa Pharmaceutical Co., Ltd. had no role in the design; collection, analysis, and interpretation of the data; manuscript drafting; or in the decision to submit the manuscript for publication.
REFERENCES
- 1) Sillanpää J, Huikko S, Nyberg M, Kivi P, Laippala P, Uitti J. Effect of work with visual display units on musculo-skeletal disorders in the office environment. Occup. Med., 53, 443–451 (2003).
- 2) Lundberg U. Stress responses in low-status jobs and their relationship to health risks: musculoskeletal disorders. Ann. N. Y. Acad. Sci., 896, 162–172 (1999).
- 3) Lundberg U. Psychological stress and musculoskeletal disorders: psychobiological mechanisms. Lack of rest and recovery greater problem than workload. Lakartidningen, 100, 1892–1895 (2003).
- 4) Faucett J, Rempel D. VDT-related musculoskeletal symptoms: interactions between work posture and psychosocial work factors. Am. J. Ind. Med., 26, 597–612 (1994).
- 5) Sumi H, Hamada H, Tsushima H, Mihara H, Muraki H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia, 43, 1110–1111 (1987).
- 6) Suzuki Y, Kondo K, Ichise H, Tsukamoto Y, Urano T, Umemura K. Dietary supplementation with fermented soybeans suppresses intimal thickening. Nutrition, 19, 261–264 (2003).
- 7) Dabbagh F, Negahdaripour M, Berenjian A, Behfar A, Mohammadi F, Zamani M, Irajie C, Ghasemi Y. Nattokinase: production and application. Appl. Microbiol. Biotechnol., 98, 9199–9206 (2014).
- 8) Omura K, Hitosugi M, Kaketani K, Zhu X, Maeda H, Tokudome S. Fibrinolytic and anti-thrombotic effect of NKCP, the protein layer from Bacillus subtilis (natto). Biofactors, 22, 185–187 (2004).
- 9) Omura K, Hitosugi M, Zhu X, Ikeda M, Maeda H, Tokudome S. A newly derived protein from Bacillus subtilis natto with both antithrombotic and fibrinolytic effects. J. Pharmacol. Sci., 99, 247–251 (2005).
- 10) Hitosugi M, Ikeda M, Zhu X, Kato H, Omura K, Nagai T, Tokudome S. Anticoagulant and fibrinolytic effects of functional food materials produced by Bacillus subtilis natto. J. Japan Soc. Biorheol., 21, 35–40 (2007).
- 11) Hitosugi M, Hamada K, Misaka K. Effects of Bacillus subtilis var. natto products on symptoms caused by blood flow disturbance in female patients with lifestyle diseases. Int. J. Gen. Med., 8, 41–46 (2015).
- 12) Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: a new measure of pruritus. Br. J. Dermatol., 162, 587–593 (2010).
- 13) Kinoshita H, Miyakawa S, Mukai N, Kono I. Measurement of tissue hardness for evaluating flexibility of the knee extensor mechanism. Football Science, 4, 15–20 (2006).
- 14) Tsuda Y, Uchida S, Kuramoto I, Sugano H, Nitta K. An examination for measuring the softness of human shoulders. J. Int. Soc. Life Inf. Sci., 23, 332–336 (2005).
- 15) Uchida S, Tsuda Y, Kimura T, Yamaoka K, Nitta K, Sugano H. Assessing perceived shoulder stiffness using hardness meters. Jpn. J. Psychosom. Med., 51, 1120–1132 (2011).
- 16) Okuno H, Takeda T, Sasaoka T, Fukuda F, Ishizaki N, Kitakoji H, Yano T, Yamamura Y. Relationship between katakori (shoulder stiffness) and shoulder hardness. JJSAM, 59, 30–38 (2009).
- 17) Nakamura T, Murakami G, Noriyasu S, Yoshio M, Sato I, Uchiyama E. Morphometrical study of arteries and veins in the human sheet-like muscles (pectoralis major, latissimus dorsi, gluteus maximus and trapezius) with special reference to a paradoxical venous merging pattern of the trapezius. Ann. Anat., 188, 243–253 (2006).
- 18) Fernández-de-Las-Peñas C, Ge HY, Alonso-Blanco C, González-Iglesias J, Arendt-Nielsen L. Referred pain areas of active myofascial trigger points in head, neck, and shoulder muscles, in chronic tension type headache. J. Bodyw. Mov. Ther., 14, 391–396 (2010).
- 19) Bornmyr S, Svensson H, Lilja B, Sundkvist G. Skin temperature changes and changes in skin blood flow monitored with laser Doppler flowmetry and imaging: A methodological study in normal humans. Clin. Physiol., 17, 71–81 (1997).
- 20) Yakubo S, Komaki K, Yagi H, Kanmatsuse K. The effect of kakkon-to on shoulder stiffness and neck body surface temperature. Kampo Medicine, 47, 795–802 (1997).
- 21) Kanai S, Taniguchi N, Okano H. Effect of magnetotherapeutic device on pain associated with neck and shoulder stiffness. Altern. Ther. Health Med., 17, 44–48 (2011).
- 22) Park JY, Hyun JK, Seo JB. The effectiveness of digital infrared thermographic imaging in patients with shoulder impingement syndrome. J. Shoulder Elbow Surg., 16, 548–554 (2007).
- 23) Tsukahara H, Koikeda T, Arai T, Hayashi H, Ohno S, Suzuki N. Supplementation effect of astaxanthin on blood flow and shoulder stiffness: a preliminary pilot study. Jpn. J. Compl. Alternative Med., 5, 49–56 (2008).
- 24) Mitsuya R, Ebine Y, Nozaki M, Noro K. Prevention of deep vein thrombosis in VDU work. Int. J. Occup. Saf. Ergon., 9, 393–403 (2003).