Abstract
Psoriasis is a chronic inflammatory skin disease characterized by red, scaly and raised plaques. Thus far, T-cell infiltration is one of the most prominent pathogenic triggers, however, the exact molecular mechanisms underlying psoriasis have not been clearly established. Sphingolipid sphingosine-1-phosphate (S1P) is a lysophospholipid regulator modulating a variety of immune cell trafficking via interactions with its cognate receptors, S1P1–5. Activation of S1P signaling has recently emerged as a novel therapeutic avenue for psoriasis treatment. Here, we test a newly developed selective S1P1 modulator, Syl930, in four different psoriasis animal models. Our data reveals that oral administration of Syl930 can induce strong anti-proliferative and anti-inflammatory effects. Specifically, Syl930 decreases the pathological thickening of back skin induced by sodium lauryl sulfate (SLS), inhibits the proliferation of basal cells in a vaginal epithelium model and increases the granular layer scales in a mouse tail assay. Moreover, Syl930 can ameliorate the parakeratosis and acanthosis as well as improve granular layer composition and decrease the thickening of epidermis in a propranolol-induced guinea pig psoriasis model. Therefore, we demonstrate that Syl930 is a promising candidate for psoriasis therapy in clinical.
Psoriasis is a chronic inflammatory skin disease characterized by red, scaly and raised plaques.1) It affects approximately 2% of the population. The pathological alterations of psoriasis include profound acanthosis with elongation of epidermal rete ridges, increasing hyperkeratosis, loss of the granular layer, as well as parakeratosis. Although psoriasis is not life threatening, it can profoundly impact QOL, causing impairment skin to other major diseases including type 2 diabetes, myocardial infarction, and arthritis.2)
The molecular mechanisms underlying psoriasis are poorly understood. In addition to genetic susceptibility to psoriasis, infection, physical trauma, drugs as well as environmental factors have been recognized as triggers of psoriasis. Pathogenically, T-cell infiltration and associated elevated cytokine levels drive epidermal hyperplasia, resulting in the psoriatic phenotype.3) Focusing on pathogenic mechanisms, five therapeutic strategies have been developed, including inhibition of T cell activation, depletion of pathogenic T cells, blocking of leukocyte recruitment, inhibition of cytokines release, and immune regulation.1,4,5)
Sphingolipid sphingosine-1-phosphate (S1P) is a lysophospholipid, which regulates a variety of immune cell trafficking. S1P acts via interaction with five G protein-coupled S1P receptors (S1P1–5) and regulates a series of downstream activities such as blood vessel constriction, heart rate modulation and lymphocyte trafficking.6,7) Activation of S1P1 recirculates lymphocytes from peripheral to secondary lymphoid organs thus allowing them to contribute to an immune reaction. It has been reported that S1P inhibits cell growth of epidermal cells, induces differentiation of keratinocytes and demonstrates anti-proliferative and anti-inflammatory effects in mouse models of psoriasis.8–11) Thus, targeting S1P signaling may represent an emerging therapeutic avenue for psoriasis treatment.
Syl930 is a selective S1P1 receptor modulator similar to Fingolimod (FTY720), which is the first approved S1P1 immunomodulatory drug for the treatment of multiple sclerosis.12,13) Sy1930 has already been proved to exhibit profound efficacy in a rat experimental autoimmune encephalomyelitis (EAE) model. Currently, it is in phase I clinical trial in China designed for rheumatoid arthritis therapy. However, its effect in psoriasis animal models is still unknown. Hence, we aimed to investigate the potential effects of Syl930 in various animal models of inflammatory skin diseases including the sodium lauryl sulfate (SLS)-induced mouse skin irritation model, the diethylstilbestrol-induced mouse psoriasis model (vaginal epithelium model), the mouse tail assay, and the propranolol-induced guinea pig psoriasis model, respectively.
MATERIALS AND METHODS
ReagentsSyl930 and FTY720 were synthesized according to previously described methods.14) Methotrexate (MTX) were obtained from Shanghai Pharma (Shanghai, China). For animal studies, Syl930, MTX and FTY720 were dissolved in distilled water. Diethylstilbestrol was purchased from Melonepharma (Dalian, China). SLS was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Propranolol was obtained from Prosperity Galaxy Chemical (Hubei, China).
AnimalsFemale KM mice specific-pathogen-free (SPF) were purchased from Vital River (Beijing, China). The mice were 6–8 weeks old and housed under standardized light in climate controlled conditions. Female guinea pigs were purchased from National Institute of Food and Drug Center (Beijing, China). All animal experiments were carried out in accordance with the guidelines of the Committee on Animals of the Institute of Materia Medica, Chinese Academy of Medical Science & Peking Union Medical College (Permission Number: 00000189).
SLS-Induced Mouse Skin Irritation ModelKM mice were treated with 15% SLS solution daily on the shaved back skin for 7 consecutive days, for the negative control group, the KM mice were treated with distilled water. The SLS treated mice were grouped randomly and administrated orally with FTY720 (0.3 mg/kg) or different doses of Syl930 once a day for 10 d. For vehicle groups, animals orally received distilled water only. All of the mice were sacrificed by cervical dislocation and back skin samples were collected for evaluation.
Diethylstilbestrol-Induced Mouse Psoriasis Model (Vaginal Epithelium Model)After female KM mice were injected intraperitoneally with diethylstilbestrol (0.2 mg) daily for 3 d, the mice were grouped randomly and treated orally with MTX (2.0 mg/kg, q.d.) or Sly930 (1.0 mg/kg, q.d.) for 12 consecutive days. On the day 12, the mice were injected intraperitoneally with colchicin (2 mg/kg) to induce mitosis arrest. Six hours later the mice were sacrificed by cervical dislocation and vaginal samples were collected for histological evaluation. Skin samples were embedded in paraffin and 5 µm thick sections were prepared. The sections were then stained with hematoxylin/eosin. The mitotic cell numbers were determined among 300 randomly chosen basal cells. The mitotic index was calculated as (mitotic cell numbers/300)×100%.
Mouse Tail AssayThe mouse tail assay was performed as previously described.11,15) Briefly KM mice were grouped and administrated orally with MTX (2.0 mg/kg), FTY720 (1.0 mg/kg) or Syl930 (1.0, 3.0 mg/kg) daily for 12 d. Two hours after the last administration, the mice were sacrificed by cervical dislocation and tail samples were collected for histological examination. The presence of a modified granular layer was evaluated as previously described.15) In total 100 scales per animal were examined. The number of the modified granular layer scales (A) and the number of total scales (B) were counted for each mouse and the percentage of granular layer scale was calculated as follows: (A/B)×100%.
Propranolol-Induced Guinea Pig Psoriasis ModelThe propranolol-induced guinea pig psoriasis model was carried out as previously described.16) Briefly, guinea pigs were treated with 5% (m/v) propranolol ethanol solution containing azone and propanediol (1 : 3) on both ears twice a day to induce psoriasis. Three weeks later, guinea pigs were administrated orally with MTX (1.0 mg/kg) or Syl930 (0.5, 1.0 mg/kg) daily for a period of 14 d, respectively. The animals were sacrificed by cervical dislocation and ear samples were collected for histological evaluation and scoring.
HistologySkin samples were embedded in paraffin and 5 µm thick sections were prepared. The sections were then stained with hematoxylin/eosin and evaluated in a double-blinded manner. The pathology score was assessed in respect to epidermal thickening, fibroplasia, inflammatory cell infiltration and vasodilatation by a semi-quantitative examination (epidermal thickening: 0=no thickening, 1=slightly thickening of 5–7 layers, 2=moderate thickening of 8–10 layers, 3=severe thickening of more than 10 layers; fibroplasia: 0=no fibroplasia, 1=mild fibroplasia of less than 1/3 dermis thickness, 2=moderate fibroplasia of 1/3–2/3 dermis thickness, 3=severe fibroplasia of more than 2/3 dermis thickness; inflammatory cell infiltration: 0=no inflammatory cell infiltration, 1=mild inflammatory cell infiltration, 2=moderate inflammatory cell infiltration, 3=severe inflammatory cell infiltration with diffuse lesions; vasodilatation: 0=no vasodilatation in dermis, 1=mild vasodilatation in dermis, 2=moderate vasodilatation in dermis with limited area, 3=severe and diffuse vasodilatation in dermis). For the propranolol-induced guinea pig psoriasis model, the pathology score was evaluated in 3 different aspects including corneous layer, epidermis and dermis. The score index for corneous layer were Munro abscess, 1.5, hyperkeratosis, 0.5 and parakeratosis, 0.5–1.5. Epidermis was semi-quantitatively evaluated as lengthening of rete ridges, 0.5–1.5; lack of granular layer, 1; acanthosis, 1. The scores for dermis were determined as inflammatory cell infiltration, 0.5–1.5; papillary papillae congestion, 1; thinning above papillae, 1.
Statistical AnalysisUnless otherwise specified, data are presented as the mean±standard error of the mean (S.E.M.). The statistical tests used and p values are indicated in each figure legend. Normally distributed data was statistically analyzed using unpaired two-tailed t-test for single comparisons, and two-way ANOVA for multiple comparisons. ANOVA analyses were followed by Bonferroni’s post hoc tests. p<0.05 was considered to indicate statistical significance. Numbers of mice per group used in each experiment are annotated in the corresponding figure legends as n. Statistical analysis was performed using Student’s t-test by Graphpad Prism 7.
RESULTS
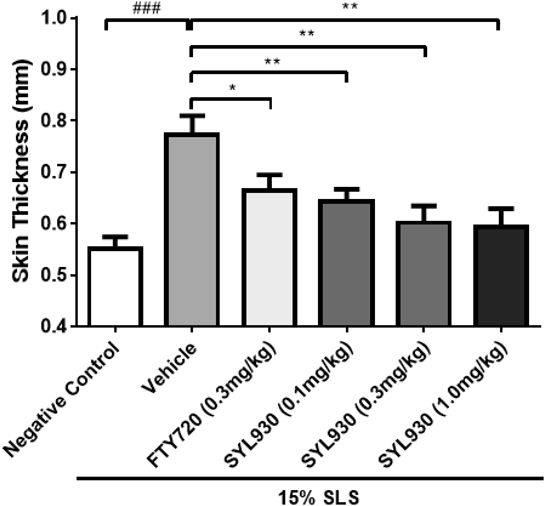
SLS-Induced Mouse Skin Irritation ModelTo explore the potential effect of Syl930 on skin inflammation, we firstly employed an SLS-induced mouse skin irritation model which could mimic the early skin symptom of psoriasis. Topical application of SLS to the shaved back of KM mice lead to inflammation characterized by a dramatic increase in the thickness of the skin. Our data indicated that oral administration of the positive control, FTY720, could decrease the thickening. Importantly, Syl930 also significantly attenuated the thickness of back skin induced by SLS in a dose-dependent manner (Fig. 1, Table 1), strongly supporting that Syl930 could inhibit the skin inflammation induced by SLS.
Table 1. Skin Thickness in SLS-Induced Mouse Skin Irritation Model
| Group | Dose (mg/kg) | Number | Skin thickness (mm) |
|---|
| Negative control | — | 11 | 0.55±0.08 |
| Vehicle | — | 11 | 0.77±0.12### |
| FTY720 | 0.3 | 11 | 0.66±0.11* |
| SYL930 | 0.1 | 11 | 0.64±0.08** |
| SYL930 | 0.3 | 11 | 0.60±0.11** |
| SYL930 | 1.0 | 11 | 0.57±0.10** |
Compared to negative control group, ### p<0.001; compared tovehicle group, * p<0.05, ** p<0.01. Data are indicated as mean±S.E.M.
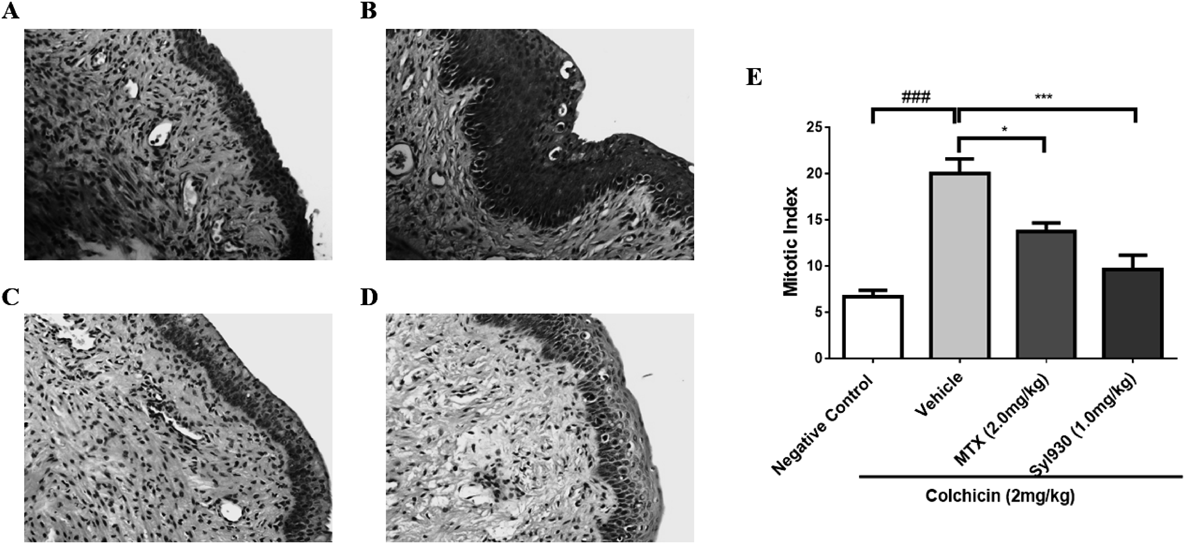
As diethylstilbestrol has been reported to induce mitotic drive of basal cell vaginal epithelium in female mice, it has been widely used as an indirect method to evaluate therapeutic potential for psoriasis. As shown in Fig. 2, administration of Syl930 at the dose of 1.0 mg/kg could significantly reduce the mitotic index of basal cells compared to thevehicle group, and more efficient than the positive control, MTX at the dose of 2 mg/kg, which is a widely used immunosuppressant agent in the treatment of autoimmune diseases.17–19) Furthermore, Syl930 did not affect body weight during the treatment, while MTX decreased the body weight significantly (Table 2), thus highlighting that Syl930 could induce less toxicity.
Table 2. Body Weight and Mitotic Index in Vaginal Epithelium Model
| Group | Dose (mg/kg) | Body weight (g) | Mitotic index |
|---|
| Start | End |
|---|
| Negative control | — | 20.1±1.6 | 27.2±2.3 | 6.7±1.7 |
| Vehicle | — | 20.5±1.5 | 27.0±1.9 | 20.0±4.2### |
| MTX | 2.0 | 20.2±0.9 | 23.0±4.4* | 13.6±2.7* |
| SYL930 | 1.0 | 20.7±2.1 | 26.0±2.7 | 9.6±4.3*** |
Compared to negative control group, ### p<0.001; compared to vehicle group, * p<0.05, *** p<0.001. Data are indicated as mean±S.E.M.
Psoriatic skin exhibits pathological changes including marked hyperkeratosis and loss of the granular layer.3) The mouse tail test was a useful assay for studying dermatological therapeutics that influence orthokeratosis and granular layer.15) Thus, we applied this assay to evaluate the effect of Syl930. The number of granular layer scales was used to investigate the increase of orthokeratosis directly. As shown in Fig. 3, Syl930 at 3 mg/kg could increase the granular layer scales compared to control group while FTY720 had no impact on orthokeratosis induction. Of note, as a positive control, MTX at 2 mg/kg significantly induced orthokeratotic cell differentiation in the epidermal scales, which have been proved in rat tail model.19)
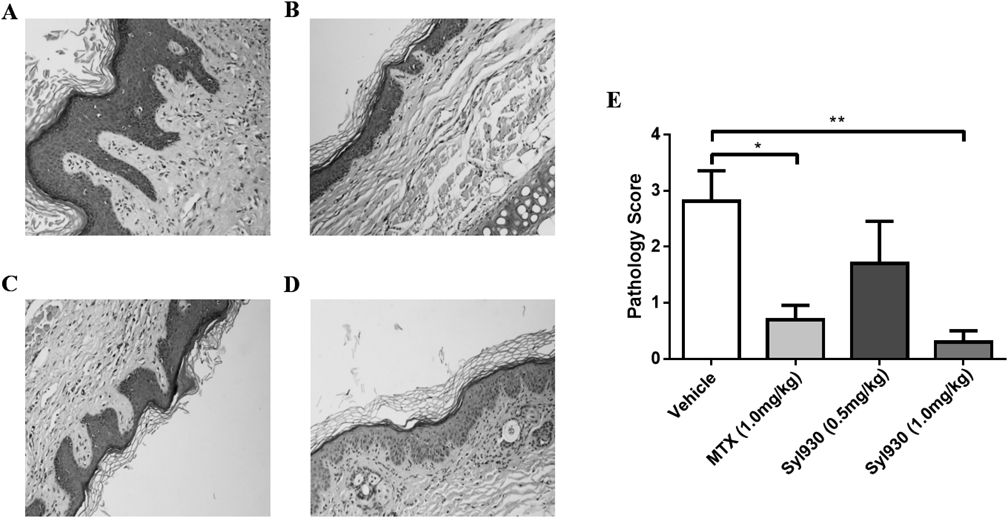
Propranolol-Induced Guinea Pig Psoriasis ModelFinally, we used propranolol-induced guinea pig psoriasis model to assess the effect of Syl930 on psoriasis in an additional relevant model organism. The body weights showed in Table 3. Histological examination revealed an increase of parakeratosis, acanthosis, diminished granular layer and elongation of rete ridges, as well as dilated capillaries in propranolol-treated ears. Interestingly, Syl930 at the dose of 1.0 mg/kg reduced the parakeratosis and acanthosis, increased the granular layer, and decreased the thickening of epidermis (Figs. 4A–D). Similar results were observed in the positive control MTX treated group. Pathological scores revealed that Syl930 at the dose of 1.0 mg/kg could significantly improve the inflammatory parameters (Fig. 4E).
Table 3. Body Weight in Propranolol-Induced Guinea Pig Psoriasis Model
| Group | Dose (mg/kg) | Body weight (g) |
|---|
| Start | End |
|---|
| Vehicle | — | 570±39 | 618±41 |
| MTX | 1.0 | 559±46 | 586±70 |
| Syl930 | 0.5 | 578±19 | 601±13 |
| Syl930 | 1.0 | 581±81 | 593±71 |
Data are indicated as mean±S.E.M.
DISCUSSION
The molecular mechanisms underlying psoriasis remain unclear. T cell infiltration and associated elevated cytokines level are considered important pathogenic triggers which initiate keratinocyte cell growth. Inhibition of cytokine release and their downstream signaling pathways, blocking of T cell infiltration, and immune regulation are major strategies for psoriasis therapy.3,4) For example, targeting interleukin (IL)-17 and its receptor show promising therapeutic effects for psoriasis treatment.20,21) JAK3 inhibitor also achieved good efficacy in phase III clinical trials. Considering the role of S1P in lymphocyte trafficking, it is conceivable that activation of S1P signaling could decrease the infiltrated T cell and thus represent another potential strategy for the treatment of psoriasis.
So far, fingolimod is the first and only approved S1P1 modulator without S1P3 selectivity.12) The next generation S1P1 modulators with S1P3 selectivity, such as siponimod, ponesimod, oxanimod, are under clinical development for treatment of autoimmune diseases including multiple sclerosis, psoriasis and ulcerative colitis.22) It has been reported that ponesimod treatment in patients with chronic plaque psoriasis achieved significant clinical benefits in a phase II study.23) Syl930, as a novel selective S1P1 modulator, is now in phase I trial in China indicated for rheumatoid arthritis. Despite the effects of Syl930 on encephalomyelitis and rheumatoid arthritis have been tested in animal models, the effect of Syl930 on psoriasis is still unknown.
To explore the potential efficacy of Syl930 on psoriasis treatment, four animal models had been investigated. Considering psoriasis is in essence a skin disease, we firstly used SLS to induce skin irritation in mice. Both Syl930 and FTY720 diminished the irritation reaction characterized by thickening of the skin. The results indicated that this model is suitable for evaluating S1P1 modulators and that Syl930 may have potential therapeutic effects on psoriasis. Encouraged by the results of the SLS experiment, we explored three classic psoriasis animal models to assess the effect of Syl930. Diethylstilbestrol-induced mouse psoriasis model and mouse tail assay are widely used screening models for anti-psoriasis drugs, which mimic uncontrolled proliferation of keratocytes and orthokeratosis in psoriasis patients. In line with SLS model, Syl930 exhibited significant therapeutic effects. It inhibits the proliferation of basal cells of vaginal epithelium and increases the orthokeratosis, which further highlights its potential use in psoriasis therapy. Finally, we used the established propranolol-induced guinea pig model to assess the anti-psoriasis effect of Syl930. As expected, it ameliorates the pathological damage of the skin in a dose-dependent manner. As the first-line therapeutic drug for psoriasis, MTX undoubtedly displays good effects. However, since MTX is an immunosuppressive drug, we observed severe the body weights reduction in the MTX treated group compared to control group, while it was not observed in the Syl930 group. Taken together, these results show that Syl930 is an eligible immunomodulator for psoriasis with a good safety profile.
Due to the differences in pathogenesis of psoriasis between human patients and rodents, these models might not reflect the physiological characteristics of psoriasis in patients and additional animal models should be applied and investigated. In the future, we will employ other animal models such as the imiquimod-induced mouse model and severe combined immunodeficient mouse models to further explore Syl930’s function.
Taken together, based on the analysis with different animal model, we propose that oral administration of Syl930 exhibit potential therapeutic effects in these animal models. These results will be useful to evaluate preclinical effect of the S1P1 selective modulator and predict its efficacy clinically.
Acknowledgments
This work is supported by National Natural Science Foundation of China (NSFC No. 81202545) and Beijing Natural Science Foundation (No. 7172140).
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Feldman SR, Evans C, Russell MW. Systemic treatment for moderate to severe psoriasis: estimates of failure rates and direct medical costs in a north-eastern U.S. managed care plan. J. Dermatolog. Treat., 16, 37–42 (2005).
- 2) Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J. Am. Acad. Dermatol., 41, 401–407 (1999).
- 3) Schon MP, Boehncke WH. Psoriasis. N. Engl. J. Med., 352, 1899–1912 (2005).
- 4) Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature, 445, 866–873 (2007).
- 5) Christophers E. Psoriasis–epidemiology and clinical spectrum. Clin. Exp. Dermatol., 26, 314–320 (2001).
- 6) Sadahira Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc. Natl. Acad. Sci. U.S.A., 89, 9686–9690 (1992).
- 7) Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu. Rev. Biochem., 78, 743–768 (2009).
- 8) Manggau M, Kim DS, Ruwisch L, Vogler R, Korting HC, Schafer-Korting M, Kleuser B. 1α,25-Dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J. Invest. Dermatol., 117, 1241–1249 (2001).
- 9) Vogler R, Sauer B, Kim DS, Schafer-Korting M, Kleuser B. Sphingosine-1-phosphate and its potentially paradoxical effects on critical parameters of cutaneous wound healing. J. Invest. Dermatol., 120, 693–700 (2003).
- 10) Schuppel M, Kurschner U, Kleuser U, Schafer-Korting M, Kleuser B. Sphingosine 1-phosphate restrains insulin-mediated keratinocyte proliferation via inhibition of Akt through the S1P2 receptor subtype. J. Invest. Dermatol., 128, 1747–1756 (2008).
- 11) Schaper K, Dickhaut J, Japtok L, Kietzmann M, Mischke R, Kleuser B, Baumer W. Sphingosine-1-phosphate exhibits anti-proliferative and anti-inflammatory effects in mouse models of psoriasis. J. Dermatol. Sci., 71, 29–36 (2013).
- 12) Sharma S, Mathur AG, Pradhan S, Singh DB, Gupta S. Fingolimod (FTY720): First approved oral therapy for multiple sclerosis. J Pharmacol Pharmacother, 2, 49–51 (2011).
- 13) Jin J, Hu J, Zhou W, Wang X, Xiao Q, Xue N, Yin D, Chen X. Development of a selective S1P1 receptor agonist, Syl930, as a potential therapeutic agent for autoimmune encephalitis. Biochem. Pharmacol., 90, 50–61 (2014).
- 14) Tian Y, Jin J, Wang X, Han W, Li G, Zhou W, Xiao Q, Qi J, Chen X, Yin D. Design, synthesis and docking-based 3D-QSAR study of novel 2-substituted 2-aminopropane-1,3-diols as potent and selective agonists of sphingosine-1-phosphate 1 (S1P1) receptor. Med. Chem. Commun., 4, 1267–1274 (2013).
- 15) Bosman B, Matthiesen T, Hess V, Friderichs E. A quantitative method for measuring antipsoriatic activity of drugs by the mouse tail test. Skin Pharmacol., 5, 41–48 (1992).
- 16) Wolf R, Shechter H, Brenner S. Induction of psoriasiform changes in guinea pig skin by propranolol. Int. J. Dermatol., 33, 811–814 (1994).
- 17) Weinstein GD, Jeffes E, McCullough JL. Cytotoxic and immunologic effects of methotrexate in psoriasis. J. Invest. Dermatol., 95, 49S–52S (1990).
- 18) Jeffes EW 3rd, McCullough JL, Pittelkow MR, McCormick A, Almanzor J, Liu G, Dang M, Voss K, Voss J, Schlotzhauer A, Weinstein GD. Methotrexate therapy of psoriasis: differential sensitivity of proliferating lymphoid and epithelial cells to the cytotoxic and growth-inhibitory effects of methotrexate. J. Invest. Dermatol., 104, 183–188 (1995).
- 19) Amarji B, Garg NK, Singh B, Katare OP. Microemulsions mediated effective delivery of methotrexate hydrogel: more than a tour de force in psoriasis therapeutics. J. Drug Target., 24, 147–160 (2016).
- 20) Galluzzo M, D’Adamio S, Bianchi L, Talamonti M. Brodalumab for the treatment of psoriasis. Expert Rev. Clin. Immunol., 12, 1255–1271 (2016).
- 21) Wasilewska A, Winiarska M, Olszewska M, Rudnicka L. Interleukin-17 inhibitors. A new era in treatment of psoriasis and other skin diseases. Postepy Dermatol Alergol, 33, 247–252 (2016).
- 22) Gonzalez-Cabrera PJ, Brown S, Studer SM, Rosen H. S1P signaling: new therapies and opportunities. F1000Prime Rep., 9, 109 (2014).
- 23) Vaclavkova A, Chimenti S, Arenberger P, Hollo P, Sator PG, Burcklen M, Stefani M, D’Ambrosio D. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet, 384, 2036–2045 (2014).