2018 Volume 41 Issue 7 Pages 1071-1077
2018 Volume 41 Issue 7 Pages 1071-1077
Potential biologically active derivatives of the curcumin were prepared by the cyclocondensation reaction cyclohexanone 2, imino pyrimidine 3, pyrmidinones 4, thiopyrimidine 6 and pyranone 5, 7 when treated with acetylacetone, guanidine, ureaethylcyanoacetate, thiourea and ethylacetoacetate, respectively. The structures of compounds (2–7) were elucidated by means of microanalysis as well as spectral measurements such as IR, 1H-NMR, MS. The anti-diabetic potential of curcumin derivatives were evaluated by assessing amylase inhibition assay, also inhibition of histamine release activity of curcumin derivatives were assessed by U937 human monocytes. The results for amylase inhibition activity revels that the curcumin inhibits α-amylase in a concentration dependent manner. Compounds 4 and 5 exhibited significant inhibitory activity against amylase enzyme and was comparable with that of acrabose. Also, compounds 5, 6 and 7 exhibited significant inhibitory activity against histamine. Our results concluded that curcumin pyrmidinones and pyranone derivatives have highly effects as anti-diabetic and anti-histamine activities.
Curcumin, which imparts the yellow color to curry, is a natural product of the spice turmeric, Curcuma longa L. (Zingiberaceae). Curcumin exhibits a variety of pharmacologic activities, including anti-inflammatory, anticancer, antioxidant, wound-healing, antimicrobial effects,1) antiallergic activity2,3) and inhibits degranulation of the RBL-2H3 tumor mast cell line in culture.4,5) Also, it prevents biliary disorders, anorexia, coughs, diabetes, hepatic disorders, rheumatism, sinusitis, cancer and Alzheimer’s disease.6,7)
The ethyl acetate extract of Curcuma longa L. and curcumin were found to decrease histamine release from mast cells by blocking intracellular signalling events in mast cells. The anti-allergy activities of curcumin and curcumin-related compounds in relation to their antioxidant activities. Most of these compounds were shown to inhibit histamine release from RBL-2H3 cells induced by concanavalin A or a calcium ionophore.8)
Flavonoid-rich fractions of plants have been reported to be effective as antihyperglycemic and antihyperlipidemic agents in animal models of diabetes.9) Plants derived polyphenols such as quercetagetin, fisetin and quercetin, which belong to the flavonoid family have been shown to be effective inhibitors of mammalian alpha-amylase with IC50s in the order of micromolar.10) Since curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) could be viewed as a flavonoid of polyphenolic compounds, in this investigation we researched the inhibitory effect of curcumin on α-amylase and the effect of curcumin orally on blood glucose levels in streptozotocin (STZ) induced diabetic and normal rats.11)
Pyrimidines exhibit a wide broad as biologically active agents they showed antioxidant,12) antifungal,13) antibacterial,14) antihypertensive,15) antitumor,16) activity. Also pyranone which is six membered oxygenated unsaturated ester heterocyclic compounds that shares chemical and physical properties reminiscent of alkene and aromatic compounds showed a wide range of biological activity as antileukemic,17) antitumor,18) anti-fungal19) inhibiting, human immunodeficiency virus (HIV) protease20) qualities also it was found that In the last several decades, cyclohexenone derivatives have received considerable attention due to their wide range of applications. Cyclohexenone derivatives exhibit antitumor,21) anti-bacterial,22) antimicrobial23) agents this promoted the author to convert curcumin into this newly heterocyclic derivatives to enhance its biological activity.
All melting points are uncorrected. IR spectra (KBr) were recorded with a PerkinElmer, Inc. Spectrum RXIFT-IR system. 1H-NMR were measured with a Varian Gemini 200 MHz instrument using tetramethylsilane (TMS) as internal standard and mass spectra were measured with a Shimadzu GC-MS-QP 100 EX mass spectrometer.
Synthesis of 6-Acetyl-3,5-bis(4-hydroxy-3-methoxystyryl)cyclohexa-2,4-dienone (2)A mixture of 1 (0.01 mol) and acetylacetone (0.01 mol) in ethanol (50 mL) was refluxed for 6 h and left to cool. The separated solid was filtered off, washed with ethanol, dried and recrystallized from acetic acid. Yield 85% and melting point (mp) 198°C.
Analysis of 2 C26H24O6 (432) (%) Calcd for C, 72.21; H, 5.59. Found: C, 72.18; H, 5.62.
Synthesis of 4,4′-(1E,1′E)-2,2′-(2-Imino-1,2-dihydropyrimidine-4,6-diyl)bis(ethene-2,1-diyl)bis(2-methoxyphenol) (3)A mixture of 1 (0.01 mol) and guanidine. HCl (0.01 mol) in ethanol (50 mL) was refluxed for 6 h and left to cool. The separated solid was filtered off, washed with ethanol, dried and recrystallized from acetic acid. Yield 85% and mp 204°C.
Analysis of 3 C22H21N3O4 (391) (%) Calcd for C, 67.51; H, 5.41; N, 10.74. Found: C, 67.55; H, 5.40; N, 10.71.
Synthesis of 4,6-Bis((E)-4-hydroxy-3-methoxystyryl)pyrimidin-2(1H)-one (4)A mixture of 1 (0.01 mol) and urea (0.01 mol) in ethanol (50 mL) was refluxed for 6 h and left to cool. The separated solid was filtered off, washed with ethanol, dried and recrystallized from ethanol. Yield 78% and mp 217°C.
Analysis of 4 C22H20N2O5 (392) (%) Calcd for C, 67.34; H, 5.14; N, 7.14. Found: C, 67.40; H, 5.10; N, 7.12.
Synthesis of 3-Acetyl-4,6-bis((E)-4-hydroxy-3-methoxystyryl)-2H-pyran-2-one (5)A mixture of 1 (0.01 mol) and ethylacetoacetate (0.01 mol) in ethanol (50 mL) was refluxed for 6 h and left to cool. The separated solid was filtered off, washed with H2O, dried and recrystallized from ethanol. Yield 68% and mp 253°C.
Analysis of 5 C25H22NO7 (434) (%) Calcd for C, 69.12; H, 5.10. Found C, 69.00; H, 5.23.
Synthesis of 4,6-Bis((E)-4-hydroxy-3-methoxystyryl)pyrimidine-2(1H)-thione (6)A mixture of 1 (0.01 mol) and thiourea (0.01 mol) in N,N-dimethylformamide (DMF) (50 mL) was refluxed for 6 h flitter while hot. The separated solid, washed with ethanol, dried and recrystallized from ethanol. Yield 74% and mp 228°C.
Analysis of 6 C22H20N2O4S (408) (%) Calcd for C, 64.69; H, 4.94; N, 6.86; S, 7.85. Found: C, 64.74; H, 5.00; N, 6.82; S, 7.83.
Synthesis of 5-(1-(4-Bromophenyl)ethylidene)-4,6-bis((E)-4-hydroxy-3-methoxystyryl)-2-oxo-5,6-dihydro-2H-pyran-3-carbonitrile (7)A mixture of 1 (0.01 mol), (0.01 mol) of p-bromoacetophenone, and ethylcyanoacetate (0.01 mol) in sodium ethoxide (0.5 g in 20 mL) was refluxed for 5 h. The solid product that separated after cooling and pouring into ice was filtered off, washed well with water, dried and recrystallized from EtOH. Yield 77% and mp 250°C.
Analysis of 7 C32H26BrNO6 (599) (%) Calcd for C, 64.01; H, 4.36; Br, 13.31; N, 2.33. Found: C, 63.98; H, 4.38; Br, 13.29; N, 2.36.
Biological AssessmentAll chemicals were received from Sigma-Aldrich (St. Louis, MO, U.S.A.).
Determination of α-Amylase Inhibitory ActivityWe used the assay of colorimetric microplate to determined the activity of α-amylase inhibitory by utilizing a well-established protocol.24) The reaction of enzyme consisted of 4 mg α-amylase of porcine pancreatic (type VI-B, ≥10 units/mg solid), 1.25 mM p-nitrophenyl-α-D-maltopentaoside (PNPG-5) and 1 mg of samples in the 96-well plate. The microplate reader was using to monitor the reaction of enzyme at 405 nm. The following equation was using to calculated the percentage of α-amylase inhibition:
Equation
 |
The IC50 value was defined as the concentration of alpha-amylase inhibitor to inhibit 50% of its activity under the assay conditions.
In Vitro Anti-inflammatory AssaysHistamine Release AssayU937 human monocytes were used to study the effect of samples on histamine release. Approximately 50000 U937 cells were plated in a 96-well cell culture plate (Corning Life Sciences, Lowell, MA, U.S.A.) and treated with various concentration (1000–7.81) of samples, in the presence or absence of 20 nM phorbol myristate acetate (PMA) (Sigma-Aldrich) for 1 h. The cell culture supernatants collected from either untreated control or treated cultures were clarified at 10000 g for 5 min at 4°C and assessed for released histamine by a commercially available EIA kit (SPI-Bio, France).25)
The new derivatives were prepared following the reaction sequences depicted in Chart 1.
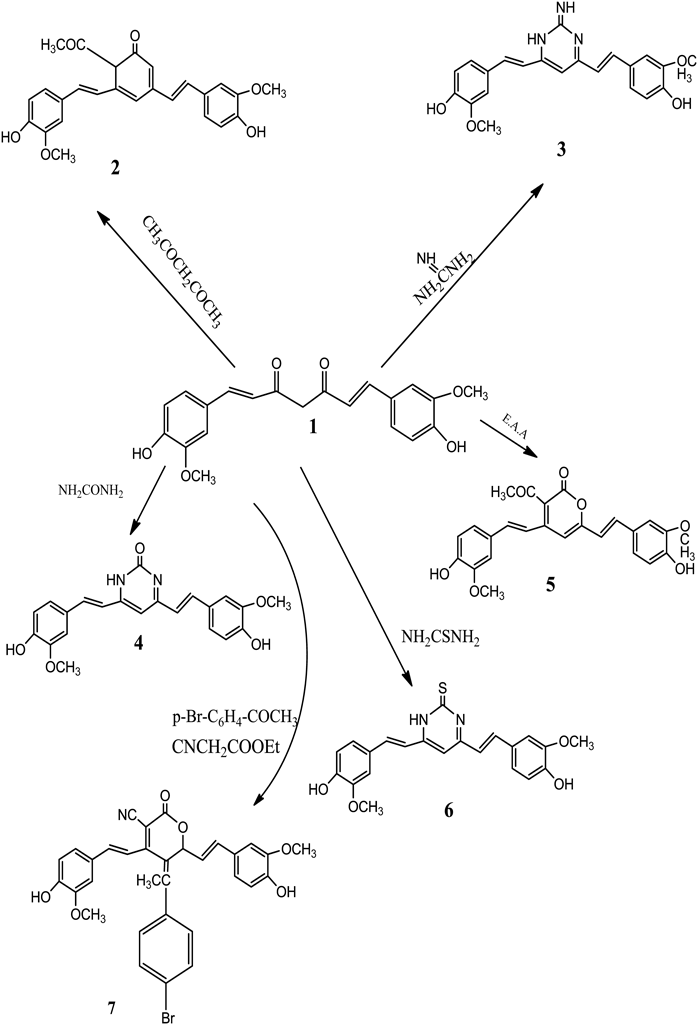
Treatment of curcumin with acetylacetone in ethanol afforded the cyclohexa-2,4-dienone derivative (2). Its IR (KBr, cm−1): 3300 (O–H), 1722, 1692 (C=O); 1629 (C=C), 1150 (C–O). 1H-NMR (DMSO-d6, δ ppm): 9.22 (s, 2H-OH), 7.78–7.25 (m, 6H, Ar-H), 6.44 (s, 4H, CH=CH), 3.04 (s, 6H, OCH3), 2.90 (s, 3H, CO–CH3) MS: molecular ion peak at m/z 432 (8.15%); molecular fragments at m/z 77 (100%) for C6H5.
The condensation of curcumin with guanidine. HCl in ethanol formed-imino-dropyrimidine (3) which showed IR (KBr, cm−1): 3250 (O–H) absence of (C=O); 1614 (C=N), 1200 (C–O). 1H-NMR (DMSO-d6, δ ppm): 9.02 (s, 2H-OH), 7.78–6.85 (m, 6H, Ar-H), 6.92 (s, 4H, CH=CH), 4.06 (s, 1H, C=NH), 4.96 (s, 1H, NH), 3.54 (s, 6H, OCH3); MS: molecular ion peak at m/z 391 (15.3%); molecular fragments at m/z 77 (100%) for C6H5·+.
While condensation with urea and thiourea gave pyrimidin-2(1H)-one (4), pyrimidine-2(1H)-thione (6) which spectral data gave the following data for (4). IR (KBr, cm−1): 3400 (OH–NH), 1735 (C=O), 1608 (C=N), 1200 (C–O). 1H-NMR (DMSO-d6, δ ppm): 7.90–7.20 (m, 6H, Ar-H), 5.01 (s, 2H-OH), 4.11 (s, 1H-NH), 2.89 (s, 1H, CH-CN), 6.03 (s, 4H, CH=CH), 3.41 (s, 6H, OCH3); MS: molecular ion peak at m/z 392 (8.3%); molecular fragments at m/z 77 (100%) for C6H5·+.
And for (6) IR (KBr, cm−1): 3420 (OH–NH) absence of (C=O), 1608 (C=N), 1405 (C=S), 1150 (C–O). 1H-NMR (DMSO-d6, δ ppm): 9.18 (s, 2H-OH), 7.97–6.89 (m, 6H, Ar-H), 5.84 (s, 4H, CH=CH), 4.42 (s, 1H-NH), 3.56 (s, 1H, CH-CN), 3.24 (s, 6H, OCH3); MS: molecular ion peak at m/z 408 (9.05%); molecular fragments at m/z 77 (100%) for C6H5·+.
While the condensation of curcumin using active methylene group afforded the pyran-2-one (5) and pyran-3-carbonitrile (7) when treated with ethylacetoacetate and ethylcyanoacetate, respectively.
The spectral data of (5) showed. IR (KBr, cm−1): 3356 (O–H), 1720 (C=O), 1772 (C=O lactam), 1620 (C=C), 1185 (C–O). 1H-NMR (DMSO-d6, δ ppm): 9.12 (s, 2H-OH), 7.97–7.01 (m, 6H, Ar-H), 5.75 (s, 4H, CH=CH), 3.54 (s, 6H, OCH3), 2.27 (s, 3H, COCH3), 2.02 (s, 2H, CH2); MS: molecular ion peak at m/z 436 (24.5%); molecular fragments at m/z 77 (100%) for C6H5·+.
While for (7). IR (KBr, cm−1): 3420 (OH), 2150 (C≡N), 1755 (C=O), 1611 (C=N), 1210 (C–O). 1H-NMR (DMSO-d6, δ ppm): 9.07 (s, 2H-OH), 7.86–7.01 (m, 10H, Ar-H), 5.79 (s, 4H, CH=CH), 3.52 (s, 6H, OCH3), 2.78 (s, 1H, CH-CN); MS: molecular ion peak at m/z 599 (11.02%); molecular fragments at m/z 77 (100%) for C6H5·+.
α-Amylase Inhibitory Activity by Curcumin DerivativesThe α-amylase inhibiting activity of curcumin derivatives (2–7) at different concentrations (7.81–1000 µM) was studied, and the results are depicted in the Table 1 and Fig. 1. Compounds 4 and 5 exhibited significant inhibitory activity against amylase enzyme and was comparable with that of acarbose and original curcumin. Compound 5 showed 18.34±1.3, 25.41±1.5, 35.21±0.58, 44.85±1.3, 51.78±1.5, 59.32±0.72, 62.50±58 and 71.63±1.2% inhibition at 7.81, 15.63, 31.25, 62.5, 125, 250, 500 and 1000 µM/L concentrations, respectively, IC50=95.5. Compound 4 was found to possess pronounced inhibitory activity with 31.11±1.2, 36.25±1.5, 42.16±0.63, 57.46±0.58, 61.78±1.2, 65.24±1.5, 72.14±0.63 and 79.32±0.72% inhibition at 7.81, 15.63, 31.25, 62.5, 125, 250, 500 and 1000 µM/L concentrations, IC50=47.26. Acarbose and original curcumin showed 86.32±0.63 and 76.37±0.71% inhibition at 1000 µM/L concentration, IC50=34.71 and 49.13, respectively. Compound 4 (IC50=47.26 mM) and Compound 5 (IC50=95.5 mM) are the most potent compared to other compounds. The inhibition of this compound may be due to pyrimidin and pyran rings.12–19)
| Sample conc. (µM) | 7.81 | 15.63 | 31.25 | 62.5 | 125 | 250 | 500 | 1000 | IC50 |
|---|---|---|---|---|---|---|---|---|---|
| Control (acarbose) | 37.81±1.20 | 40.75±1.50 | 48.84±1.20 | 59.31±1.50 | 60.17±0.63 | 69.37±1.20 | 80.14±0.58 | 86.32±0.63 | 34.71 |
| Original curcumin | 29.98±1.31 | 33.75±1.55 | 41.76±0.63 | 56.16±0.58 | 60.7±1.27 | 62.84±1.53 | 70.34±0.63 | 76.37±0.71 | 49.13 |
| 2 | 0 | 0 | 0 | 14.68±1.50 | 24.32±1.20 | 33.25±0.63 | 51.46±0.58 | 56.38±1.20 | 351.6 |
| 3 | 0 | 11.46±1.20 | 16.85±0.72 | 21.38±1.30 | 37.41±0.72 | 58.32±1.50 | 61.34±1.80 | 67.21±1.40 | 200.2 |
| 4 | 31.11±1.20 | 36.25±1.50 | 42.16±0.63 | 57.46±0.58 | 61.78±1.20 | 65.24±1.50 | 72.14±0.63 | 79.32±0.72 | 47.26 |
| 5 | 18.34±1.30 | 25.41±1.50 | 35.21±0.58 | 44.85±1.30 | 51.78±1.50 | 59.32±0.72 | 62.50±0.58 | 71.63±1.20 | 95.5 |
| 6 | 0 | 0 | 12.75±1.30 | 18.35±0.58 | 24.32±2.10 | 48.31±0.63 | 53.24±0.58 | 62.15±1.20 | 335.7 |
| 7 | 0 | 0 | 17.35±1.30 | 26.84±0.63 | 31.85±2.10 | 49.37±0.58 | 61.72±1.20 | 73.24±1.60 | 262.75 |
All determinations were carried out in triplicate manner and values are expressed as the mean±S.D. The IC50 value is defined as the concentration of inhibitor to inhibit 50% of its activity under the assayed conditions.
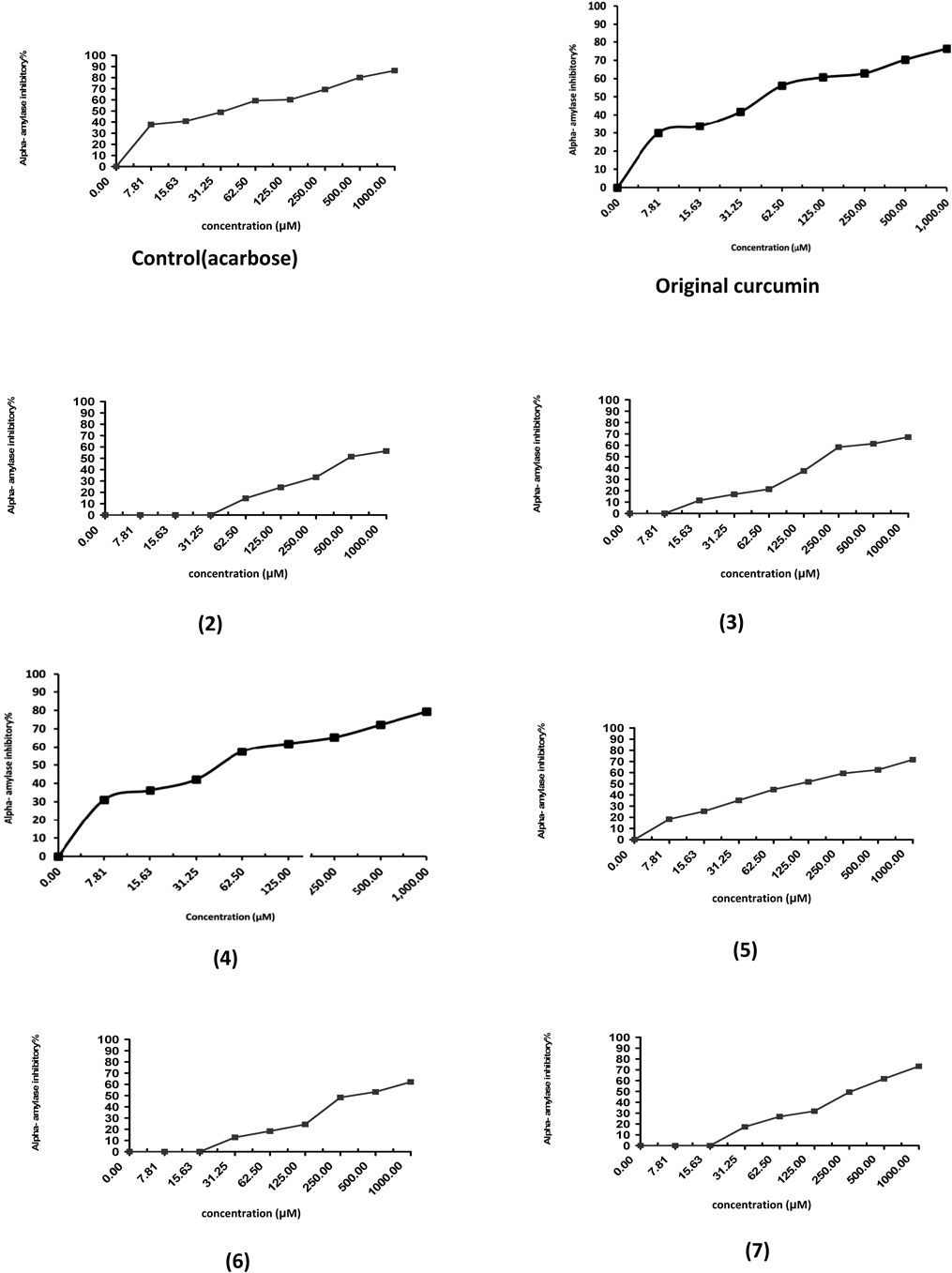
Our results agree with Najafian11) he reported that curcumin inhibited α-amylase with an half maximal inhibitory concentration IC50 value 51.32 µM. Curcumin at different concentrations, i.e., 10, 50, 100 µg showed dose dependent effect where as the standard indomethacin at 50 and 100 µg exhibited much higher activity. Pancreatic and intestinal glucosidases are the key enzymes of dietary carbohydrates digestion and inhibitors of these enzymes may be effective in retarding glucose absorption. Pancreatic α-amylase enzyme inhibition potential of curcumin might be the most promising mechanism for the observed antidiabetic effect.26)
Inhibition of Histamine Release by Curcumin DerivativesThe histamine inhibiting activity of curcumin derivatives (2–7) at different concentrations (7.81–1000 µM) was studied, and the results are depicted in the Table 2 and Fig. 2. Compounds 5, 6 and 7 exhibited significant inhibitory activity against histamine and was comparable with that of original curcumin. Compound 5 showed 29.35±2.5, 36.82±2.1, 41.85±1.6, 58.41±1.3, 62.41±0.58, 68.72±1.2, 74.65±2.1 and 79.31±1.8% inhibition at 7.81, 15.63, 31.25, 62.5, 125, 250, 500 and 1000 µM/L concentrations, respectively, IC50=46.6. Compounds 6 and 7 were found to possess pronounced inhibitory activity with (11.32±0.63 and 23.58±1.5), (28.41±0.72 and 36.28±1.2), (52.34±1.2 and 52.13±1.2), (56.32±0.58 and 54.32±0.63), (61.91±0.72 and 60.17±1.2), (66.81±1.5 and 63.25±0.58), (70.14±2.1 and 76.34±1.2) and (73.25±1.6 and 82.31±1.5)% inhibition at 7.81, 15.63, 31.25, 62.5, 125, 250, 500 and 1000 µM/L concentrations, respectively, IC50=29.7 and 29.15, respectively. Original curcumin showed 73.37±1.51% inhibition at 1000 µM/L concentration, IC50=52.26. Compounds 5 (IC50=46.6 µM), 6 (IC50=29.7 µM) and 7 (IC50=29.15 µM) are the most potent compared to other compounds. The inhibition of this compound may be due to pyrimidin and pyran rings.12–19)
| Sample conc. (µM) | 0 | 7.81 | 15.63 | 31.25 | 62.5 | 125 | 250 | 500 | 1000 | IC50 |
|---|---|---|---|---|---|---|---|---|---|---|
| Original curcumin | 13.11±0.32 | 17.56±1.40 | 26.26±1.31 | 43.17±1.20 | 53.33±0.62 | 60.19±1.35 | 64.29±0.60 | 69.35±1.34 | 73.37±1.51 | 52.26 |
| 2 | 0 | 0 | 0 | 0 | 13.45±1.2 | 21.35±1.5 | 34.13±2.2 | 49.28±1.5 | 58.32±0.58 | 539.8 |
| 3 | 0 | 9.25 | 17.36 | 24.32 | 33.85±1.6 | 46.34±1.5 | 52.41±0.58 | 55.62±1.2 | 61.85±0.63 | 200.37 |
| 4 | 0 | 6.38±1.5 | 13.25±0.85 | 19.35±1.2 | 31.25±0.72 | 46.31±0.72 | 53.18±0.58 | 59.32±1.2 | 71.34±1.5 | 192.13 |
| 5 | 0 | 29.35±2.5 | 36.82±2.1 | 41.85±1.6 | 58.41±1.3 | 62.41±0.58 | 68.72±1.2 | 74.65±2.1 | 79.31±1.8 | 46.6 |
| 6 | 9.35±1.2 | 11.32±0.63 | 28.41±0.72 | 52.34±1.2 | 56.32±0.58 | 61.91±0.72 | 66.81±1.5 | 70.14±2.1 | 73.25±1.6 | 29.7 |
| 7 | 0 | 23.58±1.5 | 36.28±1.2 | 52.13±1.2 | 54.32±0.63 | 60.17±1.2 | 63.25±0.58 | 76.34±1.2 | 82.31±1.5 | 29.15 |
All determinations were carried out in triplicate manner and values are expressed as the mean±S.D. The IC50 value is defined as the concentration of inhibitor to inhibit 50% of its activity under the assayed conditions.

Curcumin has been reported to have anti-allergic effects and can inhibit the release of histamine from mast cells.27) The histamine release from compound 48/80-treated RPMCs was reduced in a dose-dependent manner by curcumin (58 and 80% inhibition at 25 and 50 µM, respectively). Curcumin inhibits mast cell-mediated anaphylactoidresponses by suppressing histamine release from RPMCs.28)
Histamine levels were found to be raised by 8 fold from the baseline upon induction with PMA. Curcumin when treated at different concentration 10, 250 and 1000 ng mL−1 showed decrease in the percentage rise in histamine levels by 82.70, 67.51 and 55.69% from U937 cells.25) Curcumin also suppressed the immunoglobulin E (IgE)-dependent PSA reaction in a mast cell-dependent in vivo model of systemic allergic reaction29) with a potency equivalent to that of the H1 histamine antagonist.30)
From this study we have synthesized new pyrimidine, pyranone, cyclohexanone derivatives from curcumin and proved its structure by different spectral data. This newly prepared compounds had showed remarkable biological activity as antidiabetic and anti-histamine. The curcumin derivatives (4 and 5) showed a prominent inhibition of α-amylase with IC50=47.26 and 95.5, respectively. On the other hand, curcumin derivatives (5, 6 and 7) exhibited significant inhibitory activity as anti-histamine.
The authors declare no conflict of interest.