Abstract
Cardiorenal syndrome (CRS) is a frequently encountered clinical condition when the dysfunction of either the heart or kidneys amplifies the failure progression of the other organ. CRS remains a major global health problem. Qiliqiangxin (QLQX) is a traditional Chinese herbs medication, which can improve cardiac function, urine volume, and subjective symptoms in patients with chronic heart failure. In the present study, we aim to investigate the role of QLQX in the treatment of CRS type I and the possible mechanism through establishment of a rat model of myocardial infarction. Rats in CRS-Q group were orally treated with QLQX daily for 2 weeks or 4 weeks, while in sham group and CRS-C group were treated with saline at the same time. Enzyme-linked immunosorbent assay (ELISA) analysis showed that QLQX significantly reduced the levels of angiotensin II (AngII), brain natriuretic peptides (BNP), creatinine (CRE), cystatin C (CysC), tumor necrosis factor (TNF)-α, interleukin (IL)-6, microalbuminuria (MAU), and neutrophil gelatinase-associated lipocalin (NGAL) in plasma induced by myocardial infarction. Western blot analysis showed that QLQX significantly reduced the expressions of AngII, non-phagocytic cell oxidase (NOX)2, and B-cell lymphoma (Bcl)2 associated X protein (Bax), and increased the expressions of Bcl2 and Angiotensin II Type 1 receptor (ATR) in the kidney as compared with the CRS-C group. Fluorescence microscopy showed that the content of reactive oxygen species (ROS) was significantly reduced in the kidney as compared with the CRS-C group. We also examined the apoptosis level in kidney by using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining, and the result showed that QLQX significantly reduced the apoptosis level in kidney induced by myocardial infarction. Taken together, we suggest that QLQX may be a potentially effective drug for the treatment of CRS by regulating inflammatory/oxidative stress signaling.
Cardiorenal syndrome (CRS) is defined as an interaction of cardiac disease with renal dysfunction that leads to diuretic resistance and renal function worsening, mainly with heart failure exacerbation.1) CRS has be considered as a complex molecular interplay of neurohumoral pathway activation including the sympathetic nervous system, the renin angiotensin aldosterone axis, the endothelin system and the arginine vasopressin system which were induced by vascular inflammation, oxidative stress, accelerated atherosclerosis, cardiac hypertrophy and both myocardial and intrarenal fibrosis with progression of CRS treatment.2)
The contribution of inflammatory reactions and oxidative injury in the pathogenesis of heart failure, renal failure, and CRS has attracted some attention in the past few years.3) Augmented levels of plasma oxidative stress have been documented in patients with CRS as well as in mouse models of experimental heart failure.4–6) Angiotensin II (AngII) has been shown to be involved in various inflammatory and oxidative reactions. Ruiz-Ortega et al. revealed that infusion of AngII increased tumour necrosis factor (TNF) production in the kidney, increased renal synthesis of interleukin (IL)-6 and monocyte chemoattractant protein-1 (MCP-1) and elevated tissue levels of activated nuclear factor κB (NFκB).7) It has been shown that exogenous AngII augments renal mitochondrial oxidative stress and induces albuminuria in rats with heart failure.8) In addition, AngII activates several subunits of the membrane-bound multicomponent nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and also increases reactive oxygen species (ROS) formation in the mitochondria.9)
Qiliqiangxin (QLQX) is a traditional Chinese herbs medication, which is composed of eleven distinct herbs including astragali radix, ginseng radix et rhizoma, aconite lateralis radix preparata, Salvia miltiorrhiza radix et rhizoma, semen descurainiae lepidii, alismatis rhizoma, cinnamomi ramulus, Polygonati odorati rhizoma, carthami flos, periploca cortex, and citri reticulatae pericarpium.10–12) QLQX has effect on balancing the pro-inflammatory and anti-inflammatory cytokines during myocardial infarction.13) Previous studies indicated that the major active ingredients of QLQX, such as Ginseng, Radix Astragali and Salvia miltiorrhiza may significantly inhibit the enzymatic activity of xanthine (XA)/xanthine oxidase (XO)14,15) and decrease the production of ROS.16,17) It has also been reported that QLQX can inhibit AngII-induced transdifferentiation of rat cardiac fibroblasts through suppressing IL-6.18)
In the present study, we hypothesize that QLQX can protect against renal injury in rat with CRS through regulating the inflammatory/oxidative stress signaling. We investigated the role of QLQX in the treatment of CRS and examined the effect of QLQX on the level of AngII and inflammatory/oxidative stress signaling.
MATERIALS AND METHODS
Crude MaterialsQLQX was provided by Shijiazhuang Yiling Pharmaceutic (Hebei, China). QLQX consists of Radix Astragali, Aconite Root, Ginseng, Salvia miltiorrhiza, Semen Lepidii Apetali, Cortex Periplocae Sepii Radicis, Rhizoma Alismatis, Carthamus tinctorius, Polygonatum odorati, Seasoned Orange Peel and Rumulus Cinnamomi. The herbal drugs were authenticated and standardized on marker compounds according to the Chinese Pharmacopoeia 2010. The drug powder was dissolved with normal saline at the concentration of 2.67 g/mL.
AnimalsAdult male Sprague–Dawley rats (n=80, 180–250 g) were obtained from the Animal Center of Wuhan University. The animals were kept in a temperature-controlled environment (20±1°C) on a 12 h light–dark cycle with abundant food and water. For experiments, rats were intragastric treatment with QLQX (4 g/kg/d) or saline for 2 or 4 weeks. Sham operated rats were also given QLQX (4 g/kg/d) or saline for 2 or 4 weeks. The Animal Ethics and Use Committee of Wuhan University approved all experimental procedure. All animals were maintained according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Myocardial Infarction Rat Model and Experimental GroupsThe myocardial infarction model was established via ligation of the left anterior descending coronary artery as previously described.19) Briefly, rats were anaesthetized with an intraperitoneal (i.p.) injection of 5% sodium pentobarbital. The coronary artery was ligated approximately 2.0 mm from its origin. Electrocardiography was used to demonstrate ST elevation and thereby confirm the success of surgery. The sham operation was performed in a similar manner, except for ligation of the coronary artery. Forty surviving rats that underwent ligation were randomly divided into four groups (n=10): CRS control 1 group (CRS-C1, treating with saline for 2 weeks after coronary occlusion), CRS control 2 group (CRS-C1, treating with saline for 4 weeks after coronary occlusion), CRS-QLQX 1 group (CRS-Q1, treating with QLQX (4 g/kg) for 2 weeks after coronary occlusion), CRS-QLQX 2 group (CRS-Q2, treating with QLQX (4 g/kg) for 4 weeks after coronary occlusion). Twenty sham-operated rats were randomly divided into two groups (n=10): sham 1 group (Sham1, treating with saline for 2 weeks after sham operation), sham 2 group (Sham2, treating with saline for 4 weeks after sham operation). All rats were fed ad libitum. The timing and dosage of QLQX administration was based on a previous study on a model of myocardial infarction.20,21)
Enzyme-Linked Immunosorbent Assay (ELISA) AnalysisThe levels of AngII, brain natriuretic peptides (BNP), creatinine (CRE), cystatin C (CysC), TNF-α, IL-6, microalbuminuria (MAU) and neutrophil gelatinase-associated lipocalin (NGAL) in plasma were quantified using Quantikine ELISA kit (Elabscience, Wuhan, China). The assay was performed according to the manufacturer’s instructions. Plates were read in an ELISA microplate reader DR-200Bs (Wuxi Hiwell Diatek Instruments Co., Ltd., Wuxi, China) at 450 nm. The values thus obtained were plotted into the standard plot prepared by using serial dilutions of the standard provided the kit.
Western Blot AnalysisThe protein extract (20 µg) prepared from tissue sample was separated on 12% (for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), AngII, non-phagocytic cell oxidase (NOX)2, B-cell lymphoma (Bcl)2 associated X protein (Bax), Bcl2, and angiotensin II Type 1 receptor (ATR) sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, MA, U.S.A.). The membrane was blocked with 10% milk in Tris-buffer saline solution (pH 7.6) containing 0.1% Tween-20 (TBS/T), incubated with the primary antibodies overnight at 4°C, and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 2 h at room temperature. The primary antibodies used were polyclonal anti-AngII antibody (Abcam, Cambridge, U.K.), polyclonal anti-NOX2 antibody (Abcam), polyclonal anti-Bcl2 antibody (Abcam), polyclonal anti-Bax antibody (Abcam), and monoclonal anti-GAPDH (Sigma-Adlrich, St. Louis, MO, U.S.A.). The immunoreactive proteins were visualized using ECL plus detection reagents (Millipore). ImageJ software was used for the densitometry analysis.
Measurements of Renal Reactive Oxygen Species (ROS)Renal ROS was quantified by a fluorescence microscope using the fluorescent probe Dihydroethidium (DHE) (NanJing KeyGen Biotech Co., Ltd., Nanjing, China). Briefly, Sections of kidney tissue (10 µm thick) were cut at cryostat (Leica CM 1850) and then incubated with fresh DHE (5 mM/30 min) at 37°C. Then washed twice with PBS and the DHE fluorescence was measured by the fluorescence microscope.
Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate Nick-End Labeling (TUNEL) StainingFluorescent TUNEL staining was performed according to the manufacturer’s protocol and a previous study.22) TUNEL staining was used to detect apoptotic cells in the kidney in CRS rats. Fluorescence microscopy images were observed by the Olympus IX51 inverted microscope system (Olympus, Japan). TUNEL-positive cells in three different fields per animal in the kidney were counted under Olympus microscopy at ×400 magnification in a blind manner. The number of TUNEL-positive cells per square millimetre predicted the severity of renal injury. Negative controls were performed using a labelling solution without TUNEL reagent.
Statistical AnalysisData were analyzed by using Graphpad 6.0 software and were expressed as means±standard error of the mean (S.E.M.) values. The one-way ANOVA followed by a Post Hoc’ multiple comparison test was used to compare control and treated groups. p value of <0.05 was considered statistically significant difference.
RESULTS
QLQX Attenuates Cardiorenal Injury Induced by Myocardial InfarctionPlasma samples were collected to measure BNP, MAU, CRE, and cysteine (Cys)-C levels using ELISA kit on weeks 2 and 4 post myocardial infarction. BNP levels are used in the evaluation of patients with heart disease,23) MAU is a strong indicator of long-term death in patients with acute myocardial infarction,24) CRE and Cys-C are important markers in the detection of renal function.25) As shown in Fig. 1, the levels of BNP (Fig. 1A), MAU (Fig. 1B), CRE (Fig. 1C), and Cys-C (Fig. 1D) were significantly increased after 2 and 4 weeks of myocardial infarction, while QLQX significantly inhibited the levels induced by myocardial infarction at weeks 2 and 4. Indicating that myocardial infarction induced an acute CRS, and QLQX has a therapeutic effect on the cardiorenal injury induced by myocardial infarction.
QLQX Inhibits Inflammatory Damage Induced by Myocardial InfarctionPlasma samples were collected to measure AngII, IL-6, TNF-α, and NGAL levels using ELISA kit on weeks 2 and 4 post myocardial infarction. AngII plays a key role in the development of cardiorenal syndrome because of its association with increased vascular ROS production in kidney, which contributes to renal injury.26) NGAL is a 25-kDa glycoprotein found in granules of human neutrophils. It is a proposed scavenger of bacterial products at sites of inflammation and a modulator of inflammation.27) As shown in Fig. 2, the levels of AngII (Fig. 2A), IL-6 (Fig. 2B) and TNF-α (Fig. 2C), and NGAL (Fig. 2D) in plasma were significantly increased after 2 and 4 weeks of myocardial infarction, while QLQX significantly inhibited the levels induced by myocardial infarction at weeks 2 and 4. Indicating that QLQX can inhibit inflammatory response induced by myocardial infarction.
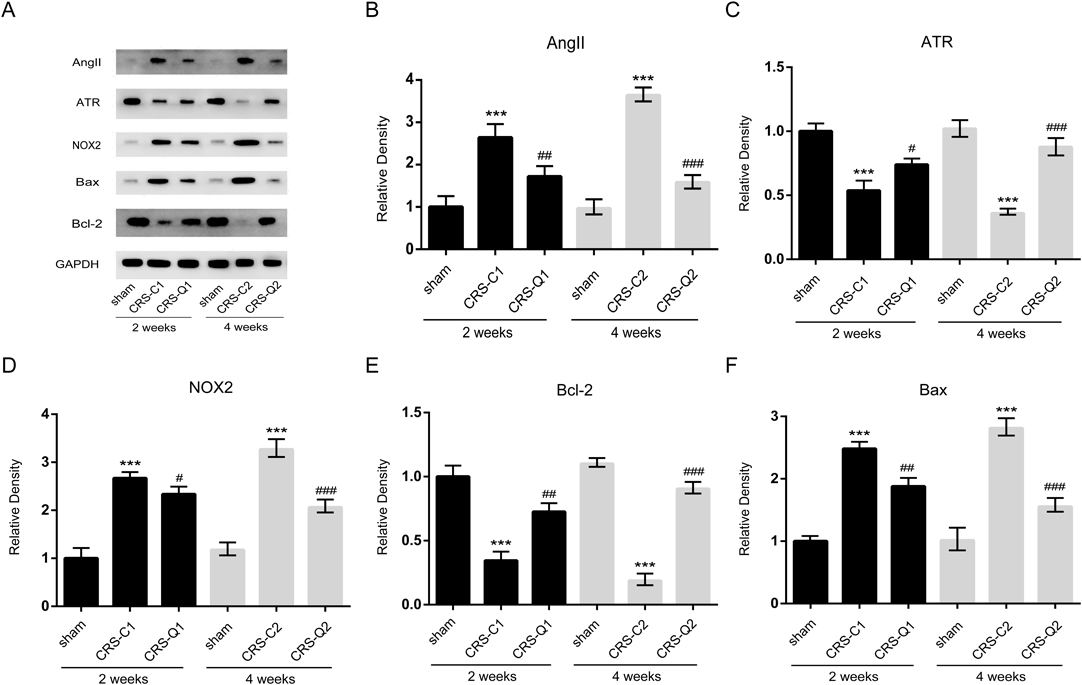
QLQX Inhibits Oxidative Stress Signaling Induced by Myocardial InfarctionKidney samples were collected to measure the expressions of AngII, ATR, NOX2, Bcl2, and Bax using Western blot analysis. AngII acts via ATR to induce NOX activation and subsequently lead to over-production of ROS.28) This elevation of ROS is involved in a wide range of cardiovascular diseases-related changes such as activation of apoptosis and fibrosis in the heart.29) As shown in Fig. 3, the expressions of AngII (Fig. 3B), NOX2 (Fig. 3D), and Bax (Fig. 3F) were significantly increased, and the expressions of ATR (Fig. 3C) and Bcl2 (Fig. 3E) were significantly decreased after 2 and 4 weeks of myocardial infarction, while QLQX significantly inhibited the expression changes induced by myocardial infarction at weeks 2 and 4. Indicating that QLQX can inhibit oxidative stress signaling transmission and cell apoptosis in kidney induced by myocardial infarction.
QLQX Reduced the Content of ROS in Kidney Induced by Myocardial InfarctionRenal ROS production was determined by DHE. As shown in Fig. 4, the red fluorescence was significantly increased after 2 and 4 weeks of myocardial infarction, while the red fluorescence was significantly decreased after 2 and 4 weeks of QLQX treatment as compared with the CRS rats that were treated with saline. Indicating that QLQX inhibited the producing of ROS in kidney induced by myocardial infarction.
QLQX Reduced the Apoptotic Rate in Kidney Induced by Myocardial InfarctionThe cell apoptotic rate was determined by TUNEL staining. As shown in Fig. 5, CRS-C group rats showed more TUNEL staining (brown) in kidney compared with sham group rats after 2 and 4 weeks of myocardial infarction, while QLQX reduced the TUNEL staining in CRS rats after treatment for 2 and 4 weeks. Indicating that QLQX inhibited the apoptotic index of renal cell induced by myocardial infarction.
DISCUSSION
Renal dysfunction is a common and progressive complication of chronic heart failure, with a clinical course that typically fluctuates with the patient’s clinical status and treatment. Despite growing recognition of the frequent presentation of combined cardiac and renal dysfunction, or “cardiorenal syndrome,” its underlying pathophysiology is not well understood, and no consensus as to its appropriate management has been achieved.30) Myocardial infarction-induced bidirectional interaction between progressive left ventricle remodeling and kidney dysfunction is known to advance cardiorenal syndrome (CRS).31) BNP, MAU, CRE, and Cys-C are important markers in the detection of cardiac and renal function.25,32,33) Our results showed that myocardial infarction significantly increased the levels of BNP, MAU, CRE, and Cys-C in the kidney, whereas QLQX significantly inhibited the levels induced by myocardial infarction at weeks 2 and 4. Indicating that myocardial infarction induced an acute CRS, and QLQX has a therapeutic effect on the cardiorenal injury induced by myocardial infarction.
Several studies have suggested that inflammation may play an important role in myocardial remodeling of myocardial infarction-induced cardiomyopathy. It has been reported that the AT1 receptor blocker, olmesartan, attenuated cardiac inflammatory reactions and protected myocardial/coronary structure and function of the failing heart, which has shown that rats treated with the angiotensin-converting enzyme (ACE) inhibitor ramipril (1 mg/kg/d), or the AT1 receptor antagonist, olmesartan (1 mg/kg/d) significantly reduced IL-6 upregulation, and macrophage infiltration and IL-1β expression by myocardial infarction.34) Moreover, it has also been reported that the inflammatory cytokines, IL-6 and TNF-α mRNA expression, as well as microvascular endothelial permeability and tubular cell apoptosis, significantly increased in the kidneys of MI rats.35) In our results, we showed that QLQX significantly reduced the inflammatory cytokines, AngII, IL-6, TNF-α, and NGAL levels in the plasma by myocardial infarction, which were consistent with the previous study. Indicating that QLQX could reduce the inflammatory response in rats with CRS.
Oxidative stress has also been reported to play a pivotal role in the pathogenesis of CRS. Oxidative loss of redox homeostasis in reactive ROS and reactive nitrogen species (RNS) results in an immune system activation and in a prion-flammatory and profibrotic milieu via distinct mechanisms which stimulate renal and cardiovascular structural and functional abnormalities.36–38) The pathogenesis of the CRS is complex, including chronic activation of the renin–angiotensin–aldosterone system (RAAS) and the sympathetic nervous system, together with reduced renal perfusion. Chronic activation of the RAAS can impair mitochondrial function, and increase mitochondrial derived oxidative stress which in turn can lead to renal injury and sodium and water retention. It has been shown that exogenous AngII augments renal mitochondrial oxidative stress and induces albuminuria in rats with heart failure. Administration of AngII also augmented renal mitochondrial dysfunction in aged mice.8) Our results showed that QLQX significantly increased the expressions of AngII, NOX2, and Bax, and significantly inhibited the expressions of ATR and Bcl-2 in the kidney by myocardial infarction. We also examined the content of ROS and the apoptosis rate in the kidney of rats with CRS, the results showed that QLQX significantly reduced the ROS content and apoptosis rate in the kidney by myocardial infarction, which were consistent with the Western blot results. Indicating that QLQX could reduce the oxidative stress response in rats with CRS.
Taken together, we demonstrated that 2 and 4 weeks after treatment with QLQX, the renal injury induced by myocardial infarction in rats was significantly attenuated. Our results suggested that QLQX significantly inhibited the inflammatory and oxidative stress damage induced by myocardial infarction, which may be responsible for the attenuation of CRS.
Acknowledgments
This work was supported by the Project of Hubei Provincial Administration of Traditional Chinese Medicine (From year 2016 to 2017) and the Project of Health and Family Planning Commission of Wuhan, China (No. WZ16A02).
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Materials
The online version of this article contains supplementary materials.
REFERENCES
- 1) Guazzi M, Gatto P, Giusti G, Pizzamiglio F, Previtali I, Vignati C, Arena R. Pathophysiology of cardiorenal syndrome in decompensated heart failure: role of lung-right heart–kidney interaction. Int. J. Cardiol., 169, 379–384 (2013).
- 2) Napoli C, Casamassimi A, Crudele V, Infante T, Abbondanza C. Kidney and heart interactions during cardiorenal syndrome: a molecular and clinical pathogenic framework. Future Cardiol., 7, 485–497 (2011).
- 3) Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat. Rev. Nephrol., 9, 99–111 (2013).
- 4) Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure A randomized, controlled trial. Circulation, 107, 992–995 (2003).
- 5) Clermont G, Lecour S, Lahet J, Siohan P, Vergely C, Chevet D, Rifle G, Rochette L. Alteration in plasma antioxidant capacities in chronic renal failure and hemodialysis patients: a possible explanation for the increased cardiovascular risk in these patients. Cardiovasc. Res., 47, 618–623 (2000).
- 6) Konstantinidis G, Head GA, Evans RG, Nguyen-Huu TP, Venardos K, Croft KD, Mori TA, Kaye DM, Rajapakse NW. Endothelial cationic amino acid transporter-1 overexpression can prevent oxidative stress and increases in arterial pressure in response to superoxide dismutase inhibition in mice. Acta Physiol., 210, 845–853 (2014).
- 7) Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int., 62, S12–S22 (2002).
- 8) Giam B, Kaye DM, Rajapakse NW. Role of renal oxidative stress in the pathogenesis of the cardiorenal syndrome. Heart Lung Circ., 25, 874–880 (2016).
- 9) Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J. Am. Soc. Nephrol., 18, 2439–2446 (2007).
- 10) Wang H, Zhang X, Yu P, Zhou Q, Zhang J, Zhang H, Zhu H, Zhang C, Yao W, Che L, Xu J, Bei Y, Li X. Traditional Chinese medication qiliqiangxin protects against cardiac remodeling and dysfunction in spontaneously hypertensive rats. Int. J. Med. Sci., 14, 506–514 (2017).
- 11) Chen F, Wu JL, Fu GS, Mou Y, Hu SJ. Chronic treatment with qiliqiangxin ameliorates aortic endothelial cell dysfunction in diabetic rats. J. Cardiovasc. Pharmacol. Ther., 20, 230–240 (2015).
- 12) Tao L, Shen S, Li X. Future prospects of Qiliqiangxin on heart failure: epigenetic regulation of regeneration. Front. Genet., 4, 221 (2013).
- 13) Xiao H, Song Y, Li Y, Liao YH, Chen J. Qiliqiangxin regulates the balance between tumor necrosis factor-alpha and interleukin-10 and improves cardiac function in rats with myocardial infarction. Cell. Immunol., 260, 51–55 (2009).
- 14) Shen L, Han JZ, Li C, Yue SJ, Liu Y, Qin XQ, Liu HJ, Luo ZQ. Protective effect of ginsenoside Rg1 on glutamate-induced lung injury. Acta Pharmacol. Sin., 28, 392–397 (2007).
- 15) Yu DH, Bao YM, Wei CL, An LJ. Studies of chemical constituents and their antioxidant activities from Astragalus mongholicus Bunge. Biomed. Environ. Sci., 18, 297–301 (2005).
- 16) Liu X, Chen R, Shang Y, Jiao B, Huang C. Superoxide radicals scavenging and xanthine oxidase inhibitory activity of magnesium lithospermate B from Salvia miltiorrhiza. J. Enzyme Inhib. Med. Chem., 24, 663–668 (2009).
- 17) Chen R, Shao H, Lin S, Zhang JJ, Xu KQ. Treatment with Astragalus membranaceus produces antioxidative effects and attenuates intestinal mucosa injury induced by intestinal ischemia–reperfusion in rats. Am. J. Chin. Med., 39, 879–887 (2011).
- 18) Zhou J, Jiang K, Ding X, Fu M, Wang S, Zhu L, He T, Wang J, Sun A, Hu K, Chen L, Zou Y, Ge J. Qiliqiangxin inhibits angiotensin II-induced transdifferentiation of rat cardiac fibroblasts through suppressing interleukin-6. J. Cell. Mol. Med., 19, 1114–1121 (2015).
- 19) Chen T, Cai MX, Li YY, He ZX, Shi XC, Song W, Wang YH, Xi Y, Kang YM, Tian ZJ. Aerobic exercise inhibits sympathetic nerve sprouting and restores β-adrenergic receptor balance in rats with myocardial infarction. PLOS ONE, 9, e97810 (2014).
- 20) Xiao J, Deng SB, She Q, Li J, Kao GY, Wang JS, Ma YU. Traditional Chinese medicine qiliqiangxin inhibits cardiomyocyte apoptosis in rats following myocardial infarction. Exp. Ther. Med., 10, 1817–1823 (2015).
- 21) Xiao H, Song Y, Li Y, Liao YH, Chen J. Qiliqiangxin regulates the balance between tumor necrosis factor-alpha and interleukin-10 and improves cardiac function in rats with myocardial infarction. Cell. Immunol., 260, 51–55 (2009).
- 22) Duris K, Manaenko A, Suzuki H, Rolland WB, Krafft PR, Zhang JH. α7 Nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke, 42, 3530–3536 (2011).
- 23) Hopkins WE, Chen Z, Fukagawa NK, Hall C, Knot HJ, LeWinter MM. Increased atrial and brain natriuretic peptides in adults with cyanotic congenital heart disease: enhanced understanding of the relationship between hypoxia and natriuretic peptide secretion. Circulation, 109, 2872–2877 (2004).
- 24) Berton G, Cordiano R, Palmieri R, Cucchini F, De Toni R, Palatini P. Microalbuminuria during acute myocardial infarction; a strong predictor for 1-year mortality. Eur. Heart J., 22, 1466–1475 (2001).
- 25) Abu-Assi E, Raposeiras-Roubin S, Riveiro-Cruz A, Rodríguez-Girondo M, González-Cambeiro C, Alvarez-Alvarez B, Gestal-Romaní S, Pereira-López E, Cabanas-Grandío P, García-Acuña JM, Virgós-Lamela A, González-Juanatey JR. Creatinine- or cystatin C-based equations to estimate glomerular filtration rate in acute myocardial infarction: a disparity in estimating renal function and in mortality risk prediction. Int. J. Cardiol., 168, 4300–4301 (2013).
- 26) Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic. Biol. Med., 39, 1–25 (2005).
- 27) Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut, 38, 414–420 (1996).
- 28) Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension. Circulation, 112, 2677–2685 (2005).
- 29) Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes, 57, 3297–3306 (2008).
- 30) Shlipak MG, Massie BM. The clinical challenge of cardiorenal syndrome. Circulation, 110, 1514–1517 (2004).
- 31) Halade GV, Kain V, Serhan CN, Ingle KA. Resolvin D1 programs myocardial infarction-induced cardiorenal syndrome in heart failure pathology. FASEB J., 31, 59 (2017).
- 32) Berton G, Cordiano R, Palmieri R, Cucchini F, De Toni R, Palatini P. Microalbuminuria during acute myocardial infarction; a strong predictor for 1-year mortality. Eur. Heart J., 22, 1466–1475 (2001).
- 33) Hopkins WE, Chen Z, Fukagawa NK, Hall C, Knot HJ, LeWinter MM. Increased atrial and brain natriuretic peptides in adults with cyanotic congenital heart disease; enhanced understanding of the relationship between hypoxia and natriuetic peptide secretion. Circulation, 109, 2872–2877 (2004).
- 34) Sandmann S, Li J, Fritzenkotter C, Spormann J, Tiede K, Fischer JW, Unger T. Differential effects of olmesartan and ramipril on inflammatory response after myocardial infarction in rats. Blood Press., 15, 116–128 (2006).
- 35) Cho E, Kim M, Ko YS, Lee HY, Song M, Kim MG, Kim HK, Cho WY, Jo SK. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol. Dial. Transplant., 28, 2766–2778 (2013).
- 36) Virzi GM, Clementi A, de Cal M, Brocca A, Day S, Pastori S, Bolin C, Vescovo G, Ronco C. Oxidative stress: dual pathway induction in cardiorenal syndrome type 1 pathogenesis. Oxid. Med. Cell Longev., 2015, 391790 (2015).
- 37) Khan SR. Stress oxidative: nephrolithiasis and chronic kidney diseases. Minerva Med., 104, 23–30 (2013).
- 38) Li PL, Zhang Y. Cross talk between ceramide and redox signaling: implications for endothelial dysfunction and renal disease. Handb. Exp. Pharmacol., 10, 171–197 (2013).