2018 Volume 41 Issue 8 Pages 1194-1202
2018 Volume 41 Issue 8 Pages 1194-1202
Temozolomide (TMZ) is currently the first-line drug used for clinical postoperative or non-surgical chemotherapy for glioma, but acquired and intrinsic resistance to TMZ limits its application. The anti-proliferative effect of formononetin on human glioma cells had been confirmed. To improve therapeutic effects of TMZ, we studied the effect of formononetin in combination with TMZ on C6 glioma cells. The anti-proliferative effect of C6 cells was tested by 3-4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT) assay. The synergy was evaluated by Chou–Talalay method. Morphological changes were observed by hematoxylin–eosin (HE) staining. The effect of formononetin in combination with TMZ on apoptosis of C6 cells was investigated by flow cytometry. The effect of formononetin in combination with TMZ on migration of cells was investigated by wound healing assay and transwell assay. The expression of proteins related to apoptosis and migration were detected by Western blot. These results showed that formononetin or TMZ alone could inhibit the growth of C6 cells in dose-dependent manner and formononetin in combination with TMZ had synergy effect on C6 cells. Further changes in cell morphology could be observed in drug combination by HE staining. Drug combination enhanced the expression of Bax, Cleaved Caspase-3, Cleaved Caspase-9, decreased the expression of Bcl-2, and promoted tumor cells apoptosis. In addition, the expression of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) were down-regulated via drug combination which resulted into inhibiting migration of C6 cell. In conclusion, formononetin in combination with TMZ can play a synergistic role in anti-C6 cells, the mechanisms of synergy depended on multiple pathways.
Glioblastoma is an invasive tumor of the central nervous system, characterized by high recurrence rate and higher mortality, which accounts for 60–70% of primary brain tumors.1) Traditional surgical treatment of glioma is difficult to achieve completely removal of the tumor, making the tumor itself easy to relapse. In addition, owning to the existence of drug resistance, the efficacy of chemotherapy is limited in clinical application.2,3) How to significantly improve the outcomes of chemotherapy is a urgent clinical issue.
In the clinical settings, a single antitumor drug has been given to the patient for a long time, the drug resistance might be induced by the mechanisms underlying the changes in expression levels of some important proteins involved, gene mutation, and deletion of stem cell target.4) Multiple targets and reduction of the emergence of drug resistance would achieve the purpose of reducing toxicity and improving efficacy.5) Anti-tumor drug synergistic research focused on the regulation of apoptosis signaling pathway, reversal of multi-drug resistance, inhibition of tumor migration and invasion and other directions. More attention has been paid to the research on the synergistic effects of antitumor drugs.6–8)
Temozolomide (TMZ) (Fig. 1B) is the second generation of oral ketamine and demonstrate efficacy for patients with Glioblastoma. It can easily permeated into the lesion sites through the blood–brain barrier.9,10) TMZ attacks the tumor cell DNA, causing DNA alkylation damage. However, multidrug resistance remains primarily obstacle to the successful treatment of human Glioblastoma.11) Formononetin is the active ingredient that isolated from Astragalus membranaceus (Fig. 1A), and belongs to isoflavonoid. The study of formononetin in anti-tumor has been proved to have certain inhibitory effect on various tumors, such as prostate cancer, cervical cancer and breast cancer.12–14) Formononetin has also been verified that after human non-small cell lung cancer cells were treated with formononetin, apoptosis-related proteins (Bcl-2, Bax and Cleaved Caspase-3) changed and the apoptosis rate was increased.15) Evidence also indicated that formononetin inhibited breast cancer cell migration via decreasing the expression of matrix metalloproteinases (MMPs).16) TMZ inhibited the migration of the glioma cells in vitro,17) apoptosis signal pathways in C6 cells were significantly affected by the synergistic alteration of lidamycin and temozolomide via regulating expression of Bax, procaspase-3.18) Those research displayed that a synergistic effect could be induced by combining formononetin with TMZ via regulating the apoptosis and migration for C6 cells.

In this study, we devoted to investigate the synergistic effect by treating with TMZ and formononetin. Furthermore, the synergies mechanism was explicited .
C6 rat glioma cells were obtained from China Pharmaceutical University. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (KeyGEN, Nanjing, China) supplemented with 10% fetal bovine serum (FBS) at 37°C with a 5% CO2 humidified atmosphere. Formononetin (purity≥99%) was purchased from Jiangsu Yongjian Pharmaceutical Co., Ltd. (Taizhou, Jiangsu, China) (Fig. 1A). TMZ was presented by Jiangsu Heng Rui Medicine Co., Ltd. (Lianyungang, Jiangsu, China) (Fig. 1B). Dimethyl sulfoxide (DMSO) was purchased from KeyGEN BioTECH (Nanjing, Jiangsu, China).
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium Bromide (MTT) AssayC6 cells were taken in logarithmic growth phase, which were seeded onto 96-well culture plates at a density of 6000 cells/well, then cultured for 24 h. When cells adhered, the original medium was replaced with a complete medium containing the drug, DMSO was used as the vehicle control (DMSO<1‰). And cultured for 48 h, 50 µL MTT (KeyGEN, Nanjing, China) solution was added into each well. The cells were incubated for 4 h at 37°C, then 150 µL DMSO was added and shaken evenly in each well, measurements were performed with the microplate reader (TECAN, Switzerland) at 565 nm. The C6 cells viability=(the optical density (OD) values of treated groups/the OD values of control group)×100%. The experiment was repeated at least in triplicate.
Combination Study with TMZC6 cells (6×103) were treated with formononetin (20–320 µM) and TMZ (125–2000 µM) alone or in combination at 1 : 6.25 (formononetin : TMZ) fixed molar ratio. After 48 h, the inhibitory effect of C6 cells was evaluated by MTT assay, and combination index (CI) was calculated by Chou–Talalay method,19,20) with Calcusyn software, dose reduction index (DRI) represents the fold reduction of drug as a result of synergistic combination compared to the concentration of drug treatment alone needed to reach the same effect, and CI=(D)1/(DX)1+(D)2/(DX)2. DRI1=(DX)1/(D)1 and DRI2=(DX)2/(D)2. (D)1 and (D)2 were the doses of the drugs in combination to elicit the same effect. Additionally, (DX)1 and (DX)2 were the doses of each drug alone to exert x% effect. CI>1 indicated antagonism, CI=1 additive effects, and CI<1 synergy.
Hematoxylin–Eosin (HE) StainingThe C6 cells were selected at their logarithmic growth period, which were digested by trypsin and the density of cell suspension was adjusted at a density of 2×105/well in a 6-well plate placed with sterile coverslips. After the cells were adhered, four groups were set: control group without drug, formononetin group (80 µM), TMZ group (500 µM) and drug combination group (TMZ+formononetin). After C6 cells were cultured for 48 h, coverslips with cells were took out. Then C6 cells were placed in 4% paraformaldehyde and fixed for 10 min for HE staining. The morphological changes were observed under inverted microscope (Nikon, Japan).
Cell Migration by Wound Healing Assay and Transwell AssayWound Healing AssayThe logarithmic growth phase cells were seeded at a density of 1.0×106/mL in 6-well plates and cultured in DMEM containing 2% FBS. An artificial scratching was made using 10 µL pipette tips when the cells grew to 80% or more of the plate bottom. C6 Cells were washed gently with sterile phosphate buffered saline (PBS) to remove suspended cells, they were exposed to formononetin (80 µM) and TMZ (500 µM) alone or in combination for 12 h. At 0 and 12 h, cells migration was observed under an inverted phase contrast microscope and pictures were taken.
Transwell AssayC6 cells (3×105) were resuspended in 200 µL of serum-free DMEM medium containing formononetin (80 µM) and TMZ (500 µM) alone or in combination. And inoculated in the upper chamber of 8.0-µm-pore polycarbonate membrane insert (Corning Inc., U.S.A.). Cells were cultured for 12 h. Then the upper chamber was taken out, the growth medium was discarded in the upper chamber, and washed twice with the calcium-free PBS, then the cells were fixed with methanol for 30 min. Let upper chamber dry. Stained with 0.1% crystal violet (Yifeixue Bio Tech, Jiangsu, China) for 20 min, gently wiped the upper chamber of non-migratory cells with cotton swab, washed with PBS in triplicate. Finally, the number of migrated cells was counted under microscope (Nikon, Japan) in five random fields, and the membranes tested in triplicate.
Cell Apoptosis by Flow CytometryThe apoptosis of glioma C6 cells by TMZ combined with formononetin was detected by flow cytometry (BD, U.S.A.). Selecting C6 cells in the logarithmic growth period, which were digested with trypsin and the density of the cell suspension was adjusted at a density of 2×105/well in a 6-well plate. Then, cultured with formononetin (80 µM) and TMZ (500 µM) alone or in combination for 48 h. Keep in incubator for 48 h. Each set of three wells. After the culture is completed, cells were washed in triplicate with cold PBS. C6 cells were digested with trypsin without ethylenediaminetetraacetic acid (EDTA). After centrifugation at 1000 r/min for 5 min, the cells were washed twice with PBS, then C6 cells were centrifuged with 300 µL of PBS, 500 µL Binding Buffer was added, 5 µL Annexin V-fluorescein isothiocyanate (FITC), 5 µL propidium iodide (PI) (KenGEN, Nanjing, China) sequentially. After mixing to avoid light reaction for 10 min at room temperature, and detection by flow cytometry. At least 2×104 C6 cells were counted in each measurement. C6 cells were stained with Annexin V-FITC and propidium iodide (PI). Necrotic and apoptotic C6 cells were quantified by flow cytometry. Four subpopulations were defined aslower left, Annexin V/PI-double negative, i.e., normal live cells; lower right, Annexin V-positive but PI-negative, i.e., early apoptotic cells; upper right, Annexin V/PI-double positive, i.e., late apoptotic cells; upper left, Annexin V-negative but PI-positive, i.e., necrotic cells.
Western Blot AnalysisC6 cells (1×106) were exposed to formononetin (80 µM), TMZ (500 µM), or combination of both for 48 h, respectively. The C6 cells were washed and resuspended with PBS, followed by cold centrifugation twice. The cell lysates were added and then the supernatant was obtained for further experiments. Equal amounts of protein (20 µL) were electrophoresed by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and electrotransferred onto polyvinylidene fluoride (PVDF) membrane. After being stained with Ponceau (Aladdin Shanghai, China), the membranes were blocked with 5% non-fat milk (BrightDairy, Shanghai, China) for 2 h at room temperature then incubated with monoclonal anti-Bax (1 : 1080, No. PAB343Ra01), monoclonal anti-Bcl-2 (1 : 333, No. PAB343Ra01), monoclonal anti-Cleaved monoclonal Caspase-3 (1 : 1080, No. PAA626Ra01), monoclonal anti-Cleaved Caspase-9 (1 : 740, No. PAA627Ra01), monoclonal anti-MMP-2 (1 : 500, No. PAA100Ra01), monoclonal anti-MMP-9 (1 : 100, No. PAA553Ra01) or monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies (1 : 5000, No. CAB932Hu01) (Yunkelong, Wuhan, China) at 4°C for overnight. The blots were washed three times with Tris Buffered saline Tween (TBST) and incubated with peroxidase-conjugated secondary antibody (1 : 5000, No. SAA544Rb19, Yunkelong, Wuhan, China). After washing the membrane three times in TBST, the PVDF membrane was immersed in the enhanced chemiluminescence substrate solution for about 1 min in a dark room, then wrapped in preservation film and placed in a cassette for development and exposure.
Statistical AnalysisEach experiment above was repeated at least three times. Statistical analysis and mapping were performed by SPSS and GraphPad Prism. All data was assumed as the mean±standard deviation (S.D.) and analyzed for statistical significance by one-way ANOVA followed by Tukey’s multiple comparison test. * p<0.05 and ** p<0.01 were considered as signifcant and very signifcant, based on at least n=3 experimental repeats.
First, anti-proliferative effects of formononetin on C6 cells was demonstrated by MTT assay. It is exhibited that the growth-inhibition rate of formononetin (20–320 µM) were 6.5–42.7% and 10.1–50.7% respectively after effected for 24 and 48 h on C6 cells (Figs. 2A, C). The results suggested that formononetin can induce anti-proliferative effects of glioma C6 cells. It is exhibited that the growth-inhibition rate of TMZ (125–2000 µM) were 3.7–38.5% and 13.8–61.3% respectively after effected for 24 and 48 h on C6 cells(Figs. 2B, D). Formononetin or TMZ alone can inhibit the growth of C6 cells in a dose-dependent manner. Then 1 : 6.25 (formononetin : TMZ) ratio for combination in C6 cells were selected. Compared with formononetin or TMZ alone in the 48 h inhibition rate of C6 cells, formononetin in combination with TMZ significantly increased C6 cells growth-inhibition (Fig. 3A). According to the MTT data, Chou–Talalay method was used to calculate the combination index (CI) (Fig. 3B). The combination index was at the lowest (0.513, CI<1) in combination of formononetin (80 µM) and TMZ (500 µM) (Table 1). Suggesting that the synergistic effect is the strongest. According to DRI parameters, for 50% and 75% of cells growth-inhibition, formononetin reduced 2.491- and 3.360-fold of TMZ in C6 cells respectively (Table 2). These results showed the efficacy of formononetin to improve TMZ-mediated cytotoxicity by reducing its concentration. Therefore, formononetin in combination with TMZ have a synergistic effect on the proliferation inhibition of glioma cells in a specific concentration range.
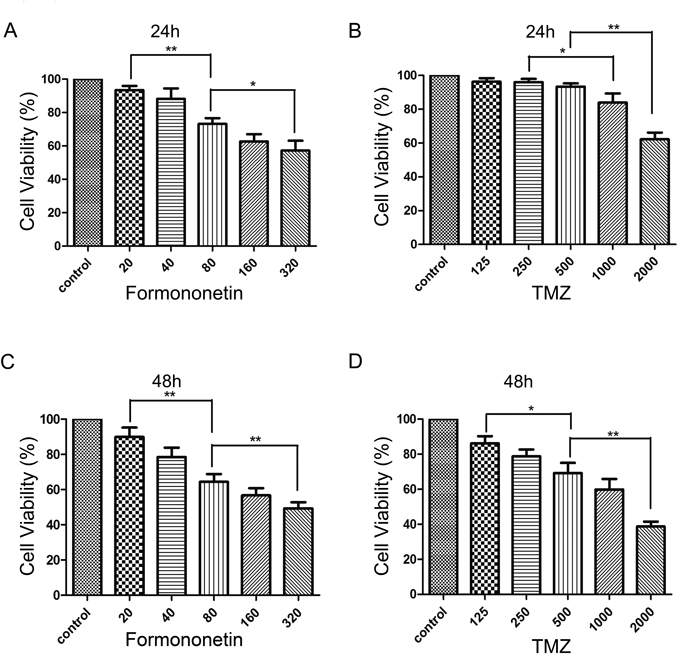
C6 cells (6000/well) were seeded onto 96-well microplates and treated with formononetin (0–320 µM) for 24 and 48 h. (A, C) C6 Cells were also treated with TMZ (0–2000 µM) for 24 and 48 h (B, D). Cell viability were evaluated by MTT assay. Data represented as the mean±standard deviation (S.D.) of three independent experiments. * p<0.05 or ** p<0.01.

(A) C6 cells were exposed to either formononetin (20–320 µM) or TMZ (125–2000 µM) alone or in combination at 1 : 6.25 (formononetin : TMZ) fixed molar ratio and cultured for 48 h. Each value is obtained as the mean±S.D. of three independent experiments. * p<0.05. (B) According to Chou–Talalay method, the combination index (CI) was calculated by Calcusyn software. CI>1,=1 and <1 indicate antagonism, additivity and synergism respectively.
| Formononetin (µM) | TMZ (µM) | Molar ratio (formononetin : TMZ) | CI | 95% Confidence interval | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| 20 | 125 | 1 : 6.25 | 1.767 | 1.334 | 2.200 |
| 40 | 250 | 0.876 | 0.552 | 1.419 | |
| 80 | 500 | 0.513 | 0.367 | 0.662 | |
| 160 | 1000 | 0.674 | 0.463 | 0.885 | |
| 320 | 2000 | 0.545 | 0.329 | 0.761 | |
CI represents a quantitative measure of the extent of drug interaction. The CI>1 indicates antagonism; CI<1 indicates synergy and CI=1 indicates additive effects. The CI values<1 indicated that interaction between formononetin (40–320 µM) and TMZ (250–2000 µM) was synergistic on C6 cells.
| Drug : Compound | Molar ratio | DRI (50% fa) | DRI (75% fa) |
|---|---|---|---|
| Formononerin : TMZ | 1 : 6.25 | 2.491 | 3.360 |
The DRI represents the fold reduction of TMZ as a result of synergistic combination compared to the concentration of drug treatment alone needed to reach the same effect. Concentrations of TMZ were reduced 2.491- and 3.360-fold in C6 to achieve 50 and 75% fa (fraction affected level), when C6 cells were exposed to combination treatment, after 48 h.
Cells in control group remained unchanged after staining and maintained the original growth shape suggested by HE staining, and nucleus integrity after cultured for 48 h. Cells treated with formononetin and TMZ for 48 h showed less live cells, reduced cell sizes and more chromatin condensed compared with control group and single drug group (Fig. 4).

Cells were treated with formononetin (80 µM) and TMZ (500 µM) alone or in combination for 48 h. The experiment was conducted in triplicate.
To find out whether combination of TMZ and Formononetin can induce cell apoptosis, C6 cells were treated with two drugs in combination or alone respectively and apoptosis was detected using Annexin V/PI Dual staining by flow cytometry (Fig. 5A). The results indicated that compared with TMZ (500 µM, p<0.01) and formononetin (80 µM, p<0.01) used alone, the apoptosis rate in drug combination group (formononetin 80 µM+TMZ 500 µM) was significantly increased, which indicated that formononetin in combination with TMZ could elicited apoptosis of C6 cells significantly (Fig. 5B). In order to explore the underlying molecular mechanisms of the effects of formononetin in combination with TMZ involved in glioma cell apoptosis, the protein levels of Bax, Bcl-2, Caspase-3 and Caspase-9 on drug combination group and single drug groups in C6 cells were measured by Western blot. Drug combination group significantly enhanced the expression of Bax and decreased the expression of Bcl-2, compared with control group and single drug groups (Figs. 5C, E, F). Expression levels of Cleaved Caspase-3 and Cleaved Caspase-9 in drug combination group were significantly higher than other groups (Figs. 5D, G, H). These data showed that formononetin improved the effects of TMZ on C6 cells apoptosis via promoting Bcl-2, inhibiting Bax, and elevating the activation of Cleaved Caspase-3 and Cleaved Caspase-9.

(A) The apoptosis rate after 48 h treated with formononetin (80 µM) and TMZ (500 µM) alone or in combination. The apoptosis induced by the two drugs in combination compared to drug alone was evaluated by flow cytometry. (B) The apoptosis rate is represented by Bar graphs. (C, D) Levels of expression of Bcl-2, Bax, Caspase-3, Caspase-9 were analyzed by Western blot on C6 cells after 48 h treatment with formononrtin (80 µM) and TMZ (500 µM) alone or in combination. GAPDH was used as a loading control. (E, F, G, H) Bar graphs showed the Relative optical density of the tested proteins by Image J. Data are presented as the mean±S.D. of three independent experiments. * p<0.05 or ** p<0.01.
Whether drug combination can have effects on inhibition of migration on glioma cells was further explored. Wound-healing assay and transwell assay were used to evaluate the effects of combination treatment with formononetin and TMZ on C6 cells migration. Wound-healing assay suggested that treatment with formononetin or TMZ alone suppressed C6 cells migration, while, combined treatment increased the suppression effects (Fig. 6A). Transwell assay indicated that the number of migrated cells in the drug combination group was significantly less than in single drug groups (Fig. 6B). Quantitative analysis of cell migration in wound healing and transwell assay was executed (Figs. 6C, D). In addition, to determine the mechanism of the combination effects on C6 cells migration, levels of key protein markers, including MMP-2 and MMP-9 were appraised (Figs. 6E, F, G). These results indicated that formononetin enhanced the TMZ-mediated inhibition of glioma cell migration.
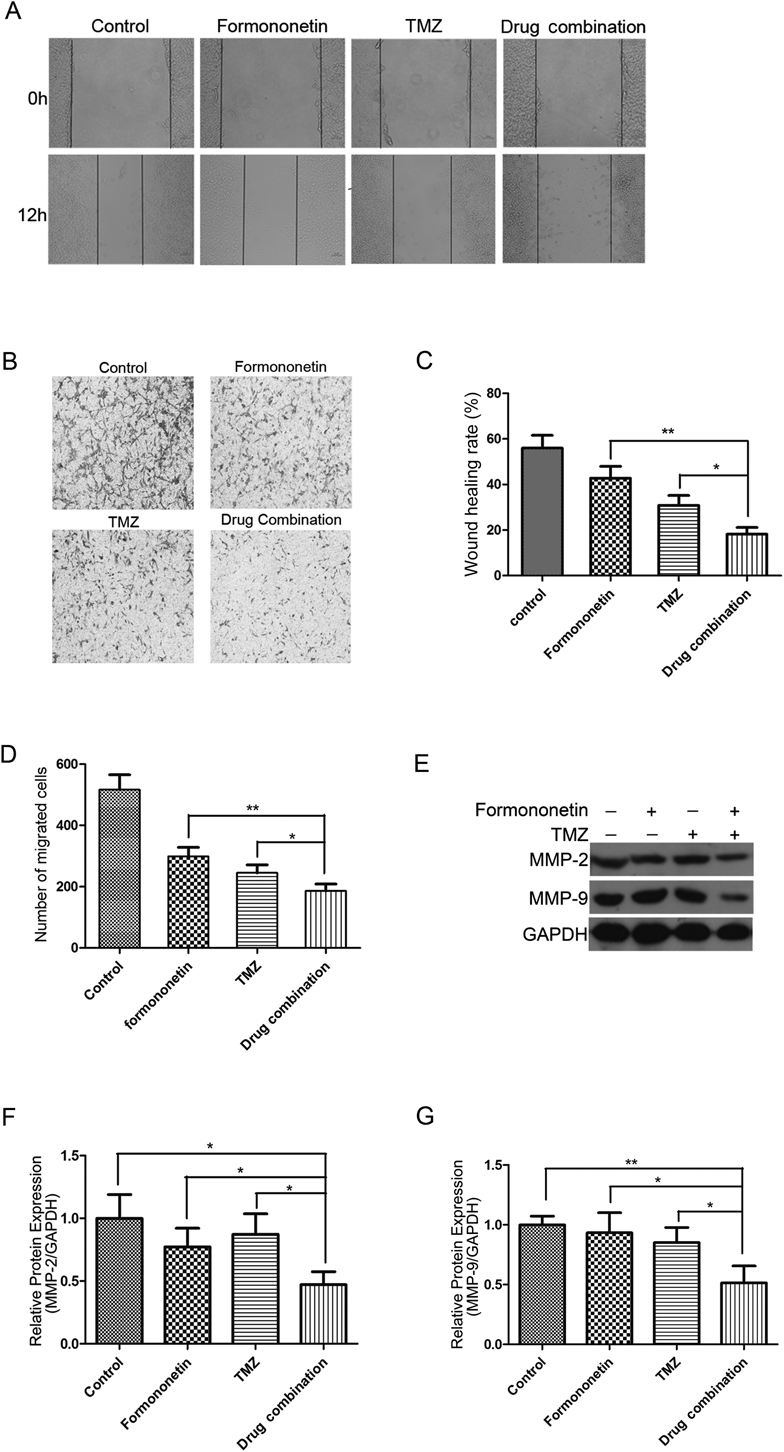
(A, B) Representative images of wound healing assay and transwell assay after 48 h treatment with formononetin (80 µM) and TMZ (500 µM) alone or in combination. (C, D) The wound healing rate and the number of migration cells are represented by Bar graphs respectively, which were calculated from the three independent experiments with five fields counted per experiment. (E) Figures were the expression levels of MMP-2, MMP-9 and GAPDH after 48 h treatment with formononetin (80 µM) and TMZ (500 µM) alone or in combination. (F, G) Bar graphs demonstrated the Relative optical density of the tested proteins by Image J. Data are presented as the mean ±S.D. of three independent experiments. * p<0.05 or ** p<0.01.
There are series of limitations in the current clinical use of chemotherapy drugs in the treatment of glioma. For example, TMZ, as a first-line of anti-glioma chemotherapy drug, multidrug resistance (MDR) is found in the clinical application, resulting in a lower therapeutic effect.21) The main purpose of our experiment is to explore the effect of TMZ combined with formononetin on improving chemotherapy. In vitro, we explored the effects on C6 cells at different time points with different concentrations of the formononetin and TMZ. Our work confirmed that formononetin and TMZ both have inhibitory effects on glioma C6 cells in a dose-dependent manner. It is significant to emphasize that our study indicated that the toxic effect of formononetin on C6 cells was superior to TMZ on C6 cells. Based on the Chou-Talalay method, the strongest synergistic effects of the two drugs was calculated when the concentration of TMZ was 500 µM and the concentration of formononetin was 80 µM. It showed that for glioma C6 cells, in a certain concentration range, formononetin can indeed synergistically enhance the therapeutic effects of chemotherapy for TMZ.
Apoptosis is a process of programmed cell death, and is characterized by typical molecular and cell characteristics such as externalization of phosphatidylserine, cell shrinkage, and condensation of chromatin.22,23) Regulation of cell apoptosis can be observed in all types of cancers. Inducing tumor cell apoptosis can be used as an important method of cancer treatment.24) A number of studies have confirmed that formononetin can achieve the therapeutic effects of tumor by inducing critical pro-apoptotic proteins expression and cell apoptosis.25,26) On the molecular level, the anti-apoptotic gene Bcl-2 and the apoptotic gene Bax formed the dimer to achieve the equilibrium state and regulated the apoptosis of the cell. It was reported that formononetin could induce the apoptosis of human osteosarcoma cell line U2OS by up-regulating the expression of Bax and down-regulating the expression of Bcl-2 in vitro.27) Here, for C6 glioma cells, we also detected that compared with the single drug groups, drug combination group can significantly increase the expression of Bax, down-regulation of Bcl-2 expression. It means that combined drugs can be more effective in promoting the apoptosis of tumor cells through regulation of Bcl-2 and Bax. Mitochondrial apoptotic pathway is one of the main pathways of apoptosis, Bcl-2 and Bax are key regulatory factors in mitochondria-mediated apoptotic pathways. The apoptotic initiation factor, Caspase-9, which located upstream of the cascade amplification effects is activated to activate the downstream apoptotic factor, Caspase-3. Activation of Caspase-3 also marks that apoptosis is involved in irreversible phase and then initiate the cell apoptosis. The expression of Caspase-3 and Caspase-9 in drug combination group was increased, which indicated that the regulation of caspase was one of the mechanisms of this combination therapy.
Tumor migration ability is an important biological characteristics of malignant tumors and inhibition of glioma cell migration is critical to the treatment of malignant gliomas. In fact, there are some literature has confirmed that formononetin can inhibit the growth of human breast cancer and other tumors. Some monomer components such as calycosin has been shown to inhibit the migration of U87 and U251 glioma cells by modulating mesenchymal properties (MMPs).28) However, the study of the migration ability of formononetin to glioma is not sufficient. Matrix metalloproteinases (MMPs) are important modulators of tumor metastasis because of their extracellular matrix (ECM) degradation capacity.29) Studies have shown that MMP-9 is involved in inducing epithelial interstitial transformation (epithelial–mesenchymal transition, EMT), degradation of vascular basement membrane and neovascularization, which is closely related to tumor invasion. In normal cells, MMP-9 shows low expression, but high expression in glioma cells.30,31) TMZ has showed effects on reducing the level of MMP-9.29) Our work also showed that drug combination group can inhibit the migration of glioma cells by down-regulating the expression of MMP-2 and MMP-9 compared with single drug groups. These evidence suggested that it is feasible to inhibit the migration of glioma by the regulation of MMPs.
Cancer cellts gain resistance because the multi-genic abnormalities present in tumor cells allow them to evade the action of these drugs when patients are treated with therapies that target single pathways. Thus, the combination of drugs on the control of multiple targets can show better therapeutic result and lower chance to produce drug resistance.32) Our work also confirmed that synergistic effects of formononetin and TMZ are not regulated by a single mechanism. The mechanism involves glioma cell apoptosis, migration, as well as the regulation of external transporter protein and other multiple pathways to complete the synergistic effects. This article evaluated the Synergistic effects of two drugs in combination in vitro, and lack of pharmacodynamic data in vivo. In the future, further validation of synergy of two drugs in combination in vivo is needed.
Our research showed that the combination of formononetin with TMZ could curb malignant biological behaviors of glioma cell in a synergistic way. Formononetin would be expected to be a potential drug in postoperative adjuvant therapy to improve the sensibility of TMZ for glioma and reduce side effects.
I would like to appreciate Dr. Zhang Xin of China Pharmaceutical University for kindly providing C6 rat glioma cells and all the colleagues who have devoted to the work.
The authors declare no conflict of interest.