2018 Volume 41 Issue 8 Pages 1211-1218
2018 Volume 41 Issue 8 Pages 1211-1218
To explore the role of the abnormal expression of the bile salt export pump (BSEP) and multidrug resistance protein 2 (MRP2) in isoniazid (INH)-induced liver injury, we assessed the liver injury induced by INH in rats and HepG2 cells in vitro. The regulatory pathways via Sirtuin 1 (SIRT1) and farnesoid X receptor (FXR) were also determined. Rat liver injury was assessed by histopathological and biochemical analysis and HepG2 cytotoxicity was assessed by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test. The levels of protein were determined by Western blot. The results indicated that INH could induce hepatotoxicity in vivo and in vitro in a dose dependent manner. The liver index and serum biochemical analysis, especially the levels of total bile acids (TBA), total bilirubin (TBIL), and direct bilirubin (DBIL), were significantly increased in rats. The INH hepatotoxicity was severe in the high dose group, and occurred alongside the down-regulation of BSEP and MRP2 in vivo and in vitro, leading to the accumulation of toxic substrates in the hepatocytes. The SIRT1/FXR pathway was identified as being important for the down-regulation of transporters. In summary, our study indicated that the down-regulation of BSEP and MRP2 represents one mechanism of INH-induced liver injury and the down-regulation of SIRT1/FXR may be a key regulator. This will inform the development of novel therapies and enable the prevention of INH-induced liver injury.
Drug-induced liver injury (DILI) is a potentially life threatening adverse reaction and is becoming a common health problem among the general population.1) DILI is responsible for the majority of acute liver failure cases and is now the most common reason for a liver transplant. In the U.S.A., DILI is responsible for nearly 10% of all drug adverse reactions.2)
It has recently been found that DILI may be associated with the alteration of hepatobiliary transporters, which is expressed at the surface domain of hepatocytes. This is especially the case for efflux transporters, which transport bile acids and bilirubin into the biliary tract.3) Bile acid efflux is increased during cholestasis to alleviate the accumulation of bile acids, bilirubin, and toxic substances in the liver. The efflux processes of bile acids and bilirubin are mainly mediated by bile salt export pump (BSEP) and multidrug resistance protein 2 (MRP2), which are expressed on the canalicular membrane of hepatocytes. BSEP is responsible for transport of monovalent bile acids and MRP2 plays an important role in mediating divalent bile acids.4) In addition to cooperating with the excretion function of bile acids as BESP, MRP2 plays an indispensable role in the biliary excretion of bilirubin glucuronides.5) Defects in BSEP and MRP2 function lead to a blockage of bile flow and cholestasis.
Tuberculosis (TB) is a global health issue, especially in developing and undeveloped countries in regions such as Africa, Asia, and Latin America. Isoniazid (INH) is an anti-TB agent that can be used in combination or alone in the treatment and prevention of TB.6) Despite the beneficial effects of INH, severe adverse effects (especially hepatoxicity) are associated with INH treatment. The incidence of INH-induced liver injury is relatively high, and is the most widely reported reason for liver injury cases in the U.S.A., with drug-induced liver injury (DILI) ranking second.7,8) Among the oral anti-TB drugs, in 15–20% of people INH causes an increase in aspartate and alanine transaminase, and in about 1% of these individuals liver injury is observed.9) However, the exact mechanism of INH-induced liver injury is unknown. Some studies have shown that INH-induced hepatotoxicity is most probably caused by toxic metabolites, but the transporters and nuclear receptors also play a critical role in the elimination of drugs and their metabolites.10) Guo et al. demonstrated that the hepatic transporters are involved in the process of INH/rifampicin (RIF) in mice, and Gonzalez et al. showed that RIF altered transporter expression in human hepatocytes.11,12) However, whether INH alone has an effect on the expression of transporter, especially the regulator, has not been reported.
In this study, the altered expression of transporter Bsep and Mrp2 induced by INH were assessed in rats. Liver injury in rats was induced by the oral administration of INH. Serum and tissue biochemical parameters and a histopathology examination were conducted to assess the hepatotoxicity. To investigate the differences among species, a human hepatoma HepG2 cell line was amplified to explore the alteration of MRP2 and BSEP in vitro. Farnesoid X receptor (FXR) is an important regulatory pathway for the transport and biosynthesis of bile acids via regulating the expression of transporters and CYP 7A1.13,14) It is a bile acid sensitive receptor that negatively regulates and improves the cholestasis situation. Sirtuin 1 (SIRT1), a class III oxidized form of nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase, is involved in lipid, glucose, and bile acid metabolism. Some studies have reported that SIRT1 can modulate FXR-stimulated transcriptional signaling by deacetylation of this nuclear receptor and control the activation of FXR.15) Therefore, we tested the hypothesis that INH suppresses the FXR pathway by reducing the expression of SIRT1, leading to the dysregulation of bile acid transport via the alteration of hepatic efflux transporter MRP2 and BSEP. If true, a potential mechanism and targets associated with INH-induced liver injury would be identified, enabling the prevention or treatment of this adverse reaction in INH application.
Specific pathogen free male Sprague–Dawley (SD) rats (220–250 g) were purchased from the Experimental Animal Centre of Jilin University (permit number SCXK 2013-0001), and the test procedures were approved by the animal experiments committee of the First Hospital of Jilin University. The rats were housed in a controlled environment (temperature 20–22°C, humidity 50–55%, and a 12 h light/12 h dark cycle) and fed with water and a chow diet.
Drugs and ReagentsINH (Lot No. MKBV9475V) was purchased from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.) with a purity ≥99%. Resveratrol was perchased from Meilun Co. (Dalian, China) with a purity ≥99%. Rabbit anti-MRP2 was purchased from Abcam (Shanghai, China), rabbit anti-BSEP was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.), rabbit anti-FXR was purchased from Abcam, rabbit anti-SIRT1 (Biosynthesis Biotechnology, Beijing, China), rabbit anti-CYP 7A1 (Biosynthesis Biotechnology), and rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was purchased from Goodhere Biotechnology (Hangzhou, China). The water used in the experiment was generated by a Millipore water system (Billerica, MA, U.S.A.).
Treatment of AnimalsForty male Wistar rats were randomly divided into four groups and an INH solution was orally administered for seven days at 50 (low dose), 200 (middle dose), and 350 mg/kg·d (high dose). The human dose (mg/kg) was near to 6.3-fold of rat dose (mg/kg), and the rat dose(mg/kg) was setted as 6–8 fold human dose (mg/kg) as a middle dose in common. Thus, the middle dose 200 mg/kg was nearly the highest dose in clinical in this study. And in order to induce the acute liver injury, the high dose in this study was near two-fold of the highest dose in clinical. Water was administered as a vehicle control. The number of the days for INH administation in this study was 7 d, which was comfirmed according to our pre-test. Twenty-four hours after the seventh administration, the rats were anesthetized with chloral hydrate, blood was collected from the abdominal aortic puncture, and then the rats were sacrificed. Serum was obtained for biochemical analysis after centrifugation at 3000 rpm and stored at −20°C. Liver tissue specimens were fixed in 10% formaldehyde for hematoxylin and eosin (H&E) staining and immunohistochemical analysis. The remaining liver tissues were frozen with liquid nitrogen and stored at −80°C for further study.
Ethical ApprovalAll procedures performed in studies involving animals were in accordance with the ethical standards of the animal experiments committee of the First Hospital of Jilin University.
Biochemical Analysis of SerumAspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bile acids (TBA), total bilirubin (TBIL), and direct bilirubin (DBIL) were determined using an automatic biochemistry analyzer (Hitachi, 7600–210, Tokyo, Japan).
Rat Liver Index AnalysisRat body weight was measured before they were sacrificed. After collection from rats, livers were wiped with filter paper and weighed. The liver index represented the liver/body weight ratio, which was calculated as (liver weight/body weight)×100%.
Histopathology ExaminationThe samples of liver tissue were fixed in 10% formaldehyde for 10 d and then embedded in paraffin. Liver sections (5 µm thickness) were stained with H&E and examined using a BX51 light microscope (Olympus, Tokyo, Japan). A researcher assessed the degree of liver damage with a blinded evaluation method for each section at 400× magnification.
Immunohistochemistry AnalysisThe 5 µm thick sections were deparaffinized and rehydrated and an immunohistochemistry analysis was performed by the 3,3′-diaminobenzidine (DAB) method. Primary antibody MRP2 (1 : 500) and BSEP (1 : 500) were incubated overnight at 4°C and then the labeled streptavidin-peroxidase (SP) technique was applied. The immunoreaction products were observed with a BX51 light microscope (Olympus) and blind evaluated at 200× magnification.
Cell Culture and Viability AssayHepG2 cells were used in this study because they express hepatic transporters and nuclear receptors, which are pre-requisites for bile acid transportation and hepatotoxicity associated with the administrated INH. HepG2 cell lines were obtained from the Shanghai Cell Bank of the Chinese Academy of Science (Shanghai, China). Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin G/streptomycin in a humidified incubator at 37°C and 5% CO2. Cells were used at passages 3–10, and 70 to 80% of confluent cells were subcultured in standard cell culture plates at an appropriate seeding density. For 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, cells were seeded in 96-well cell culture plates at a density of 5×103 cells/well. For Western blot analysis, 1×105 cells/well were seeded in six-well cell culture dishes and allowed to adhere for 24 h. Five different concentrations of INH (0, 10, 20, 50, and 100 mM) were selected for this study and untreated cells served as a control group. After the cells were harvested, cell viability was assessed by an MTT assay following the manufacturer’s instructions. The MTT experiments were performed at least in triplicate and repeated at least three times.
Western Blot AnalysisEach well of HepG2 cells seeded in six-well with 1×105 cells/well and 20 mg Liver tissue was homogenized in 200 µL radioimmunoprecipitation (RIPA, Beyotime Biotechnology, Shanghai, China) buffer (including 1% phenylmethylsulfonyl fluoride (PMSF)), and centrifuged at a speed of 12000 rpm for 10 min at 4°C. After protein concentrations were determined using a BCA protein assay kit (Beyotime Biotechnology). Samples were loaded onto a precast 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to a polyvinylidene fluoride (PVDF) membrane. Membranes were blocked with 5% non-fat milk in Tris-buffered saline with 0.05% Tween-20 (TBST) for 1 h at room temperature. The membranes were incubated overnight at 4°C, with rabbit anti-MRP2 (1 : 2000), rabbit anti-BSEP (1 : 2000), rabbit anti-FXR (1 : 2000), rabbit anti-CYP 7A1 (1 : 1000), and rabbit anti-GAPDH (1 : 2000). The membranes were rinsed three times for 10 min with TBST and incubated with secondary antibodies for 2 h at room temperature. Immunoreactive bands were visualized using an ECA kit (GE Healthcare, Amersham, U.K.) and the signals were normalized to GAPDH as an internal standard. The density of the immunoreactive bands was analyzed with Image J software (Bethesda, MD, U.S.A.).
Assessment of SIRT1 Agonist on INH-Treated HepG2 CellsResveratrol (RES), a selective SIRT1 agonist, was used to demonstrate the role of SIRT1-FXR pathway. As the MTT assay and Western blot methods, the HepG2 cells were devided into 3 groups: Control, INH (100 mM), INH (100 mM)+RES (50 µM). The effects of cell viability and expression of SIRT1, FXR, MRP2 and BSEP was assessed with MTT and Western blot.
Statistical AnalysisAll the data were presented as a mean±standard deviation (S.D.) and analyzed by SPSS 18.0 software. The difference between groups was analyzed using a one-way ANOVA followed by a Tukey test when equal variances were assumed. Results were considered to be statistically significant when p<0.05.
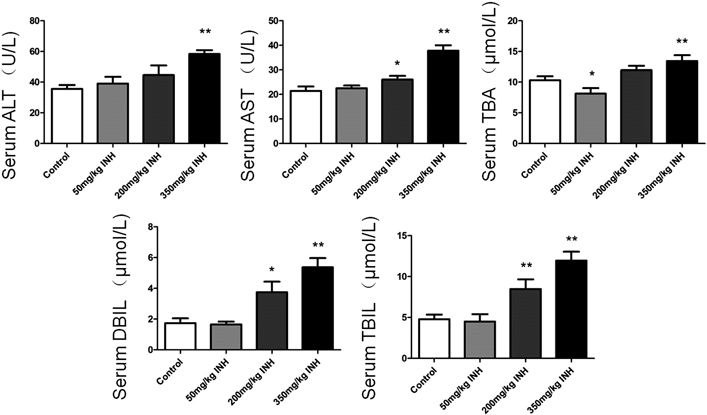
The levels of ALT, AST, TBA, DBIL, and TBIL in serum indicated that hepatic functions were quantified by standard clinical methods (Fig. 1). The levels of ALT, AST, TBA, DBIL, and TBIL were all significantly increased in the high dose group compared with the control group (p<0.01). In the middle dose group, the parameters were similar to the control group and only the levels of ALT, DBIL, and TBIL increased significantly (p<0.05). For the low dose group, none of the serum biochemical assessments were significantly different from the control group (p>0.05), although the level of TBA was significantly lower than in the control group (p<0.05).
The results in Table 1 show that the liver index increased as the dose of INH increased. The body weight decreased gradually as the dose of INH increased, and compared to the control group, the liver index was much higher in the high dose group (p<0.01) and middle dose group (p<0.05). The higher liver index indicated a hepatotoxicity induced by INH.
| Parameter | Control | Isoniazid | ||
|---|---|---|---|---|
| 50 mg/kg | 200 mg/kg | 350 mg/kg | ||
| Body weight (g) | 267.67±8.78 | 265.33±3.93 | 241.33±15.69 | 205±10.08 |
| Liver weight (g) | 9.33±0.39 | 9.45±0.58 | 9.27±0.73 | 10.07±0.83 |
| Liver index (%) | 3.49±0.06 | 3.56±0.23 | 3.85±0.35* | 4.92±0.44** |
* p<0.05 versus the control group; ** p<0.01 versus the control group.
Following the light microscopy assessment at 400× magnification (Fig. 2), histopathological observations revealed serious liver injury in the high dose group. The section from the high dose group indicated degenerative and necrotic changes of the hepatocytes, hepatic steatosis, and mild dilatation of hepatic sinusoids. In the middle dose group, only hepatic steatosis was observed, while there were normal hepatocytes in the low dose group. The section from the control group had normal and eumorphic hepatocytes.

Immunohistochemistry analysis revealed the expression of Mrp2 and Bsep transporters in rat hepatocytes. Under the microscope, the positive expression of Mrp2 and Bsep was showed as brown. In our study, the Mrp2 and Bsep transporter had a lower expression in the high- and middle-dose groups than in the control group which showed a less scale and weak degree of brown color. The expression in the low dose group was similar to that in the control group (Fig. 3).

The Western blot assays indicated that the expression of Mrp2 and Bsep in the high dose administrated INH group was significantly decreased by 0.31-fold (p<0.01) and 0.33-fold (p<0.01), respectively, compared to the control group, and the SIRT1/FXR signal pathway was also significantly decreased by 0.52-fold (p<0.05) and 0.33-fold (p<0.01), respectively. In the middle dose group, the expressions of Mrp2, Bsep, SIRT1, and FXR were significantly decreased by 0.63-fold (p<0.01), 0.58-fold (p<0.05), 0.68-fold (p<0.05), and 0.71-fold (p<0.05), respectively. However, in the low dose group, the expressions of transporters and signal pathways were similar to those of the control group. The trends of Mrp2 and Bsep expression assessed by the Western blot analysis were revealed in Fig. 4.

The relative expression of protein was caculated as band intensity of target protein normalized to GAPDH (* p<0.05 versus the control group; ** p<0.01 versus the control group).
The viability of INH treated HepG2 cells was significantly prohibited by INH at concentrations of 50 and 100 mM compared with the control group (p<0.01). It was found that HepG2 cells treated with 50 and 100 mM INH experienced cytotoxicity (Fig. 5). The expression of MRP2 and BSEP were significantly decreased after treatment with 100 mM INH (p<0.01), and the SIRT1/FXR signal was also down-regulated (p<0.01). After treatment with INH at a concentration of 50 mM, MRP2, SIRT1, and FXR were down-regulated, but there was no significant difference in BSEP expression with the control group. After treatment with INH at concentrations of 10 and 20 mM, the expressions of MRP2, BSEP, SIRT1, and FXR were similar to those of the control group (Fig. 6).

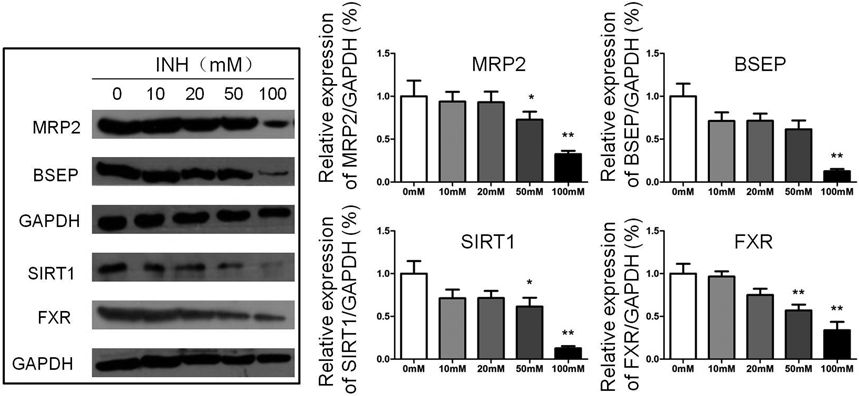
The relative expression of protein was caculated as band intensity of target protein normalized to GAPDH (* p<0.05 versus the control group; ** p<0.01 versus the control group).
In order to assess the possible influence for biosynthesis of bile acids by INH, the expression of CYP 7A1, which is the rate-limiting enzyme in the formation of bile acids was determined by Western blot. The protein expression of CYP 7A1 was similar in each group both in rat liver and HepG2 cells (Fig. 7). It suggested that INH did not affect the bile acids synthesis.

The data in Fig. 8 showed that decreased viability of 100 mM INH-treated HepG2 cells was significantly reversed by combined with 50 µM RES (p<0.01). INH (100 mM)+RES (50 µM) group significantly increased expression of SIRT1 compared to INH (100 mM) group (p<0.01). Furthermore, increased SIRT1 by treated-RES also obviously up-regulated the expression of FXR in HepG2 cells (p<0.05). Ultimately, as the down-stream of FXR, the expression of hepatic transporter MRP2 and BSEP were both significantly increased by SIRT agonist (p<0.01).

The relative expression of protein was caculated as band intensity of target protein normalized to GAPDH (* p<0.05 versus the control group; ** p<0.01 versus the control group; # p<0.05 versus the INH group; ## p<0.01 versus the control group).
In this study, we demonstrated that the down-regulated expression of hepatic efflux transporters MRP2 and BSEP were associated with INH-induced liver injury in rats and HepG2 cells. Moreover, the changes in transporter expression could be regulated by the SIRT1/FXR pathway. Down-regulation of SIRT1 and FXR could cause the dysregulation of bile acid homeostasis and damage to hepatocytes (Fig. 9). The role of the SIRT1/FXR signal pathway in INH-induced liver injury and the hepatotoxicity of INH to HepG2 cells has not been reported previously.

After their synthesis or conjugation, a majority of bile acids are effluxed into bile by BSEP. The small amount of bile acids that have been modified by the sulfate or glucuronide are effluxed by MRP2, while most of direct or undirect bilirubin are secred into bile via MRP2. In our study, oral administration of INH decreases expression of BSEP and MRP2 via down-regulation of SIRT1/FXR in rats and HepG2 cells. This dysregulation of protein expression is associated with the severity of liver injury due to the decreased transport of bile acids into the bile, resulting in an accumulation of toxic substrates in liver.
Our study showed that INH-induced liver injury was accompanied by the down-regulation of BSEP and MRP2 in the liver. As bile acid transporters, BSEP and MRP2 mediate the efflux of individual bile constituents from the liver into the biliary tract, and play a pivotal role in pharmacokinetics, pharmacology, and toxicology.16) The occurrence of liver injury and the degree of severity was in accordance with the down-regulated levels of Bsep and Mrp2 in rats. This might be associated with excretion blockage and the accumulation of substrates in liver, especially bile acids, bilirubin, and toxic metobolites of INH. The liver/body weight ratio and levels of TBA, TBIL, and DBIL in serum were significantly increased in the high dose group, which were associated with pathological changes in the liver. This indicated that the cholestasis situation occurred after the administration of high doses of INH. It has been confirmed that some diseases are caused by the dysregulated function and expression of BSEP and MRP2, leading to bile acid and bilirubin secretion disorders. Progressive familial intrahepatic cholestasis type 1 or 2 (PFIC 1/2) and Dubin–Johnson syndrome are such diseases, and are due to a deficiency of BSEP and MRP2, respectively.17,18) In our study, we also verified the alteration of the expression of BSEP and MRP2 after treatment with INH in HepG2 cells. The down-regulation of BSEP and MRP2 was observed after treatment with 100 mM INH, which was associated with a decrease in cell viability. The tests using the HepG2 cell line from humans and rats liver in vivo had the same trends between hepatotoxicity and the expression of transporters. Interestingly, CYP 7A1, the first and rate-limiting enzyme, was not changed after treated INH in rats and HepG2 cells, which indicated that the process of bile acids biosynthesis was not affected by INH. This demonstrated that the dysregulation of bile acids might be only induced by dysfuntion of transporting. Therefore, up-regulation of BSEP and MRP2 was considered a therapy target, which could transport excessive amounts of bile acids or toxicants into the biliary tract and weaken the cholestasis in the liver.
We demonstrated that the SIRT1/FXR pathway might play an important role in regulating the transport and biosynthesis of bile acids during the process of INH-induced liver injury. FXR is one of the most critical upstream nuclear receptors for maintaining hepatic bile acid homeostasis.19) As a bile acid sensor, FXR negatively regulates the biosynthesis of bile acids and positively regulates hepatic efflux transporters such as BSEP and MRP2, as well as playing an important role in reducing liver injury upon hepatic bile acid overload.20–22) While the role of FXR in cholestasis has been known for a long time, the importance of SIRT1 for hepatic lipid and glucose metabolism has only recently been realized, and it can also regulate the FXR pathway by directly deacetylating it. We found that SIRT1 was down-regulated following oral administration of INH at 350 mg/kg for seven days in rats or following 50 and 100 mM INH treatments in HepG2 cells in vitro. We speculated that SIRT1 might be associated in INH-induced liver injury and was the upstream regulator of FXR and transporter BSEP and MRP2. Similarly, latest reports suggested that the expression of FXR is decreased not only in a SIRT1 deficient liver, but also in SIRT1 deficient hepatocytes, also indicating that SIRT1 directly regulates the expression of FXR.15,23–26) Based on the effects of down-regulated SIRT1, an agonist of SIRT1 might be a potential protective agent in the treatment of INH induced liver injury. There have been few studies of agonists of SIRT1, and to the best of our knowledge resveratrol and SRT1720 are the only known compounds that can activate SIRT1 expression and then reverse the liver injury.27,28) In our study, resveratrol could protect against INH-induced HepG2 apoptosis via increasing the expression of SIRT1 and FXR. And the hepatic efflux transporters MRP2 and BSEP, as the down-stream of FXR, were also increased with RES administration than INH-treated cells. It indicated that INH-induced hepatotoxicity might be partly due to block the efflux of bile acids and toxic compounds via SIRT1/FXR pathway inhibition. Therefore, SIRT1 activation may be an important process with regard to the prevention of INH-induced liver injury and further research should be conducted to confirm this.
In conclusion, our data supports the hypothesis that INH can induce liver injury via the down-regulation of hepatic efflux transporters BSEP and MRP2, and the SIRT1/FXR pathway may be the primary regulator with regard to the expression of transporters and the biosynthesis of bile acids in the liver.
This work was supported by the 8th Youth Foundation of the First Hospital of Jilin University under Grant number JDYY82017026; the National Natural Science Foundation of China under Grant number 81503168.
The authors declare no conflict of interest.