2018 Volume 41 Issue 8 Pages 1257-1268
2018 Volume 41 Issue 8 Pages 1257-1268
Chrysanthemum zawadskii var. latilobum (CZ) has been used as a traditional medicine in Asian countries for the treatment of inflammatory diseases. Recently, CZ extract was shown to inhibit differentiation of osteoclasts and provide protection against rheumatoid arthritis. The aim of this study was to investigate the molecular mechanisms of BST106, the ethanol extract of CZ, for cartilage protection in monosodium iodoacetate (MIA)-induced osteoarthritis (OA), particularly focusing on apoptosis and autophagy. BST106 (50, 100, and 200 mg/kg) was orally administered once daily to MIA-induced OA rats. Swelling, limping, roentgenography, and histomorphological changes were assessed 28 d after MIA injection. Biochemical parameters for matrix metalloproteinase (MMP), apoptosis, and autophagy were also assessed. BST106 ameliorated the severity of swelling and limping after MIA injection. Roentgenographic and histomorphological examinations revealed that BST106 reduced MIA-induced cartilage damage. BST106 decreased MIA-induced increases in MMP-2 and MMP-13 mRNA levels. Increased levels of serum cartilage oligomeric matrix protein and glycosaminoglycan release were attenuated by BST106. Furthermore, BST106 suppressed the protein expression of proapoptotic molecules and increased the protein expression of autophagosome- and autolysosome-related molecules. These findings indicate that BST106 protects against OA-induced cartilage damage by inhibition of the apoptotic pathway and restoration of impaired autophagic flux.
Osteoarthritis (OA) is a joint pathology characterized by progressive loss of cartilage that affects millions of people worldwide. Previous studies demonstrated that cartilage destruction in OA results from inflammation-induced matrix metalloproteinase (MMP) activation.1) Furthermore, compelling studies reported that chondrocyte cell death such as apoptosis and necrosis participates in OA development, which appears to be complex.2) Despite extensive studies on cartilage loss and degradation in OA, the exact pathologic mechanisms are not fully understood. The main symptoms of OA include pain, inflammation, stiffness, and loss of mobility in joints. To date, nonsteroidal anti-inflammatory drugs have been a conventional treatment for pain and inflammation in OA. However, these medications also have various gastrointestinal (GI) adverse effects such as peptic ulcer and GI tract bleeding, therefore significant efforts are being made to investigate new strategies for the treatment of OA with more precise mechanisms and better safety.
Apoptosis is a process of programmed cell death that is involved in development, homeostasis, and aging in multicellular organisms. Apoptosis plays a key role in chondrocyte loss and has been considered one of the major factors in the pathogenesis of OA. In osteoarthritic human cartilage, apoptosis is closely linked with the severity of cartilage destruction and loss of matrix.3) In a human chondrocyte cell line, inhibition of caspase-3 activity prevented chondrocyte apoptosis and maintained cellular function by the retention of type II collagen promoter activity.4) Moreover, cilostazol, a selective phosphodiesterase type III inhibitor, reduced nitric oxide-induced apoptosis, which protected rat articular chondrocytes in a monoiodoacetate (MIA)-induced rat OA model.5)
Autophagy, self-digestion process, is a dynamic process by which damaged or dysfunctional cytosolic materials including organelles and proteins are sequestered into double-membraned autophagosomes and then delivered to the lysosome for degradation. Autophagy is regarded as an adaptive response to limit cellular injury in various human diseases, including infections, inflammatory and neurodegenerative diseases, and cancer.6) Recent studies have suggested that autophagy has a protective effect against chondrocyte degeneration. Activation of autophagy by rapamycin reduced inflammation and protected against chondrocyte loss and extracellular matrix damage in mice.7) Furthermore, there is an interrelationship between autophagy and apoptosis in several inflammatory diseases such as inflammatory bowel diseases, sepsis, and arthritis. Autophagy attenuated advanced glycation end product-induced apoptosis and MMP activation in rat chondrocytes.8) Silencing of autophagy-related gene (Atg) 5 caused increases in MMP-13 gene expression and protein levels of cleaved poly (ADP-ribose) polymerase (PARP) and cleaved caspase-9 in human chondrocytes.9) Knee joints of aging mice exhibited decreased autophagy together with a reduction in cartilage cellularity and increase in apoptosis.10)
Chrysanthemum zawadskii var. latilobum (CZ) is a perennial flowering plant belonging to the genus Chrysanthemum in the family Asteraceae. It is widely distributed in East Asia and has been traditionally used as a tea in Korea and China. CZ has been also used as an herbal medicine to alleviate vertigo, hypertensive symptoms, and several inflammatory diseases such as colitis and stomatitis.11) Extracts from CZ flower showed many pharmacological activities, including anti-allergic, anti-inflammatory, and anti-cancer activities.12–14) In lipopolysaccharide (LPS)-treated macrophages, CZ leaf extract inhibited inflammation by decreasing levels of inflammatory mediators and inducing heme oxygenase-1 (HO-1).15) Recently, Gu et al. suggested the therapeutic potential of CZ extract in inflammatory bone diseases by showing its inhibitory effect on differentiation and formation of osteoclasts from bone marrow cells.16) Moreover, an extract of CZ protected mice against rheumatoid arthritis through suppression of nuclear factor-kappaB (NF-κB)-mediated inflammation.17)
Therefore, in this study, we aimed to investigate the molecular mechanisms of BST106, the ethanol extract of CZ, for cartilage protection in MIA-induced OA, in particular focusing on apoptosis and autophagy.
CZ was collected from the aroma of dreams (Gu-Jeol-Cho Farming Association Corporation, Jeongeup-si, Korea) and identified by professor Youngbae Suh (Natural Products Research Institute, Seoul National University, Seoul, Korea).
Preparation of BST106The ethanol extract of CZ (BST106) was prepared according to the previously published paper.17) BST106 was obtained from the GreenCross Wellbeing Corporation (GCWB, Seongnam-si, Korea). BST106 was extracted from the stems and leaves of CZ using ethanol at 50°C, concentrated using a rotary evaporator at 50°C, and dried using a vacuum dry oven at 50°C. The yield was 18.7% of the extract. BST106 was prepared by dissolving in dimethyl sulfoxide (DMSO) for the in vitro assays, and as a suspension in 0.5% carboxymethyl cellulose (CMC) for the in vivo study.
AnimalsMale Sprague–Dawley rats (200–220 g) and male New Zealand white rabbits (1.5–2.0 kg) were provided by Orient Bio Inc. (Seong-nam, Korea). Animals were housed in a room with controlled temperature and humidity (25±1°C and 55±5%, respectively) and a 12 h light–dark cycle. All animal procedures were approved by the Sungkyunkwan University Animal Care Committee (SKKUIACUC-2016-05-0001-1) and were performed in accordance with National Institutes of Health Guidelines of the Care and Use of Laboratory Animals (NIH, Department of Health and Human Services Publication No. 85-23, revised 1985).
Cartilage Glycosaminoglycan AssayIn accordance with method by Sandy et al.,18) cartilage pieces were placed in cell culture dishes and treated with BST106 at 0.5, 1, 5, 10, 20, and 50 µg/mL. One hour after BST106 treatment, 5 ng/mL of recombinant human interleukin (rhIL)-1α (R&D Systems, Minneapolis, MN, U.S.A.) was added. After incubation with rhIL-1α for 72 h, the release of glycosaminoglycan (GAG) was assessed from culture medium using the Blyscan Sulfated GAG Assay kit (Biocolor Ltd., County Antrim, U.K.).
Induction of OA and Experimental DesignRats were intraarticularly injected with 2 mg MIA (Sigma-Aldrich, St. Louis, MO, U.S.A.) in a total volume of 50 µL saline after light anesthetization with diethyl ether. BST106 (50, 100, and 200 mg/kg) or 0.5% CMC (vehicle) was administered orally once daily for 28 d after MIA injection. Celecoxib (Celebrex, Pfizer Inc, U.S.A.) was used as a positive control and administered orally at 60 mg/kg once daily for 28 d after MIA injection. The dosage of BST106 treatment was determined based on a previously study.17) Animals were randomly separated into seven groups: (a) vehicle-treated control (control), (b) BST106 100 mg/kg-treated control (BST106), (c) vehicle-treated MIA (MIA), (d) BST106 50 mg/kg-treated MIA (MIA+BST106 50), (e) BST106 100 mg/kg-treated MIA (MIA+BST106 100), (f) BST106 200 mg/kg-treated MIA (MIA+BST106 200), and (g) celecoxib 60 mg/kg-treated MIA (MIA+celecoxib, positive control). At 28 d after MIA injection the rats were killed under ketamine (55 mg/kg) and xylazine (7 mg/kg) anesthesia. Blood was collected from the inferior vena cava and knee cartilage was isolated; both were stored at −75°C for later analysis.
Gross Observation and RoentgenographyAfter MIA injection, rats were weighed and carefully checked for knee joint swelling and gait disturbances every 2 d. Swelling and limping were classified as no change, mild, moderate, and severe.19) For the roentgenography, rats were imaged by X-ray and divided into four grades based on the Kellgren–Lawrence scoring system.20) In this classification, knee with minimal osteophytes is given a score of 1, knee with small but definite osteophytes and unimpaired joint space is given a score of 2, knee with moderately impaired joint space and osteophytes is given a score of 3, and knee with substantially impaired joint space, severe osteophytes, and sclerosis of subchondral bone is given a score of 4.
Serum Cartilage Oligomeric Matrix Protein (COMP) LevelCommercial COMP (MyBioSource, San Diego, CA, U.S.A.) enzyme linked immunosorbent assay (ELISA) kits were used.
Histological AnalysisFor histological analysis, formalin-fixed knee joint samples were decalcified. Each knee joint was embedded in paraffin, serially sectioned (3–4 µm) and stained with hematoxylin and eosin (H&E) and safranin O. Histopathological changes in the knee joint were assessed by Mankin scoring systems.21) Briefly, mankin score (maximum=12) is totals of each index including cartilage surface damages (scored 0–3), chondrocyte numbers (scored 0–3), clone formations (scored 0–3) and matrix intensity of safranin O (scored 0–3). The higher Mankin score, the higher level of OA (semiquantitative scores; maximum=12). The histological images were evaluated by a pathologist with a light microscope (Model Eclipse 80i, Nikon, Tokyo, Japan) in a blinded manner.
Terminal Deoxynucleotidyl Transferase (TdT)-Mediated Deoxyuridine Triphosphate (dUTP)-Biotin Nick End Labeling (TUNEL) AssayApoptotic cells were detected in situ by TUNEL staining with a commercially available kit (Apoptosis Detection kit, TaKaRa Bio Inc., Shiga, Japan). The percentage of TUNEL-positive cells was measured by an automated digital image analyzer (iSolution FL ver. 9.1, IMT i-Solution Inc. Vancouver, BC, Canada).
Immunohistochemistry (IHC)Apoptosis-related molecules, cleaved caspase-8, cleaved caspase-3, and cleaved PARP were detected using purified specific antibody with an avidin–biotin complex (ABC) and peroxidase substrate kit (Vector Labs, Burlingame, CA, U.S.A.). At least five histological fields in each prepared knee joint histological section were evaluated to calculate the mean histomorphometric value.
Total RNA Extraction and RT-PCRArticular cartilage samples were pulverized in liquid nitrogen using a pestle and mortar. Total RNA was extracted using RNA isoPlus (TaKaRa Bio Inc.). The cDNA was synthesized through reverse transcription (EcoDry™ cDNA Synthesis Premix, TaKaRa Bio Inc.) and amplified by real-time PCR using a thermocycler (Lightcycler® Nano, Roche Applied Science, Indianapolis, IN, U.S.A.) and a SYBR Green detection system (Roche Applied Science). The primer sequences are presented in Table 1. mRNA expression levels were normalized to those of glyceraldehyde-3-phosphate dehydrogenase, and are expressed relative to the average of all delta cycle threshold (Ct) values in each sample using the Ct method. All experiments were conducted in duplicate to ensure amplification integrity.
| Gene | Primer sequences (5′→3′) | Product length (bp) |
|---|---|---|
| MMP-2 | Sense: GACCTTGACCAGAACACCATCG | 178 |
| Antisense: CCCGAGCAAAGGCATCATCC | ||
| MMP-13 | Sense: CTTACAGAATTGTGAACTACACC | 170 |
| Antisense: GAAGTCACCATGTTCTTTAGTTCC | ||
| p62 | Sense: AAGTTCCAGAGGCACAG | 992 |
| Antisense: AGCAGTTATCCGACTCCA | ||
| GAPDH | Sense: GGCATTGCTCTCAATGACAAC | 223 |
| Antisense: TGTGAGGGAGATGCTCAGTG |
Articular cartilage samples were pulverized in liquid nitrogen using a pestle and mortar and homogenized in NE-PER (Pierce Biotechnology, Rockford, IL, U.S.A.) for cytosolic fractions. The homogenate was centrifuged at 600×g for 10 min and the supernatant was centrifuged for 5 min at 15000×g. The 15000×g supernatant was centrifuged at 100000×g for 30 min. The resulting supernatant was used as the particulate-free cytosolic fraction. The protein content of the cytosol fractions was determined using the Bradford method.
Western BlottingArticular cartilage samples were pulverized in liquid nitrogen using a pestle and mortar and homogenized in PRO-PREP™ solution (iNtRON Biotechnology Inc., Seongnam, Korea) for total fractions. Samples from lysate were run on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto polyvinylidene difluoride membranes, and detected using an ECL detection system (iNtRON Biotechnology Inc.). Western blotting bands was analyzed using TotalLab TL120 software (Nonlinear Dynamics Ltd., Newcastle, U.K.). The following primary antibodies were used: tumor necrosis factor receptor-1 (TNFR1), Atg3, Atg12–Atg5 complex (Cell Signaling Technology, Beverly, MA, U.S.A.); sequestosome-1/p62 (p62) (Abcam, Cambridge, MA, U.S.A.); cleaved caspase-8, cleaved caspase-9, cytochrome C, Bax, cathepsin B, Rab7 (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.); LC3-II and lysosome-associated membrane protein (LAMP)-2 (Novus Biologicals, Littleton, CO, U.S.A.); and β-actin (Sigma-Aldrich). Protein densities were standardized to those of β-actin.
StatisticsAll results are presented as means±standard error of the mean (S.E.M.) The overall significance of the experimental results was examined by one-way ANOVA. Differences between groups were considered statistically significant at p<0.05 with the appropriate Bonferroni corrections made for multiple comparisons.
At 28 d after MIA injection, swelling and limping were attenuated by treatment with BST106 at 50 and 100 mg/kg (Table 2). To assess morphological changes of knee articular bone, rats were examined with roentgenography. In the MIA-treated group, knees showed osteophyte formation and impaired joint space. These changes were attenuated by BST106 100 and 200 mg/kg treatment (Fig. 1). Based on these results, BST106 100 mg/kg was selected as the optimal effective dose for evaluating the protective mechanisms of BST106 in MIA-induced rats.
| Observatory changes (%) | Score | Control | MIA | ||||
|---|---|---|---|---|---|---|---|
| Vehicle | BST106 50 mg/kg | BST106 100 mg/kg | BST106 200 mg/kg | Celecoxib 60 mg/kg | |||
| Swelling | |||||||
| No change | 0 | 20/20 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 |
| Mild | 1 | 0/20 | 8/20 | 16/20 | 16/20 | 12/20 | 18/20 |
| Moderate | 2 | 0/20 | 12/20 | 4/20 | 4/20 | 8/20 | 2/20 |
| Severe | 3 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 |
| Average score | 0.0±0.0 | 1.6±0.1** | 1.2±0.1**,+ | 1.2±0.1**,+ | 1.4±0.1** | 1.1±0.1**,+ | |
| Limping | |||||||
| No change | 0 | 20/20 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 |
| Mild | 1 | 0/20 | 4/20 | 14/20 | 12/20 | 12/20 | 14/20 |
| Moderate | 2 | 0/20 | 16/20 | 6/20 | 8/20 | 8/20 | 6/20 |
| Severe | 3 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 |
| Average score | 0.0±0.0 | 1.8±0.1** | 1.3±0.1**,++ | 1.4±0.1**,+ | 1.4±0.1**,+ | 1.3±0.1**,++ | |
The results represent the means±S.E.M. from twenty animals per group. Swelling and limping scores were classified as no change=0, mild=1, moderate=2, and severe=3 based on severity. The symbols denote significant difference ** p<0.01 compared with the control group; + p<0.05 compared with MIA group; ++ p<0.01 compared with the MIA group.
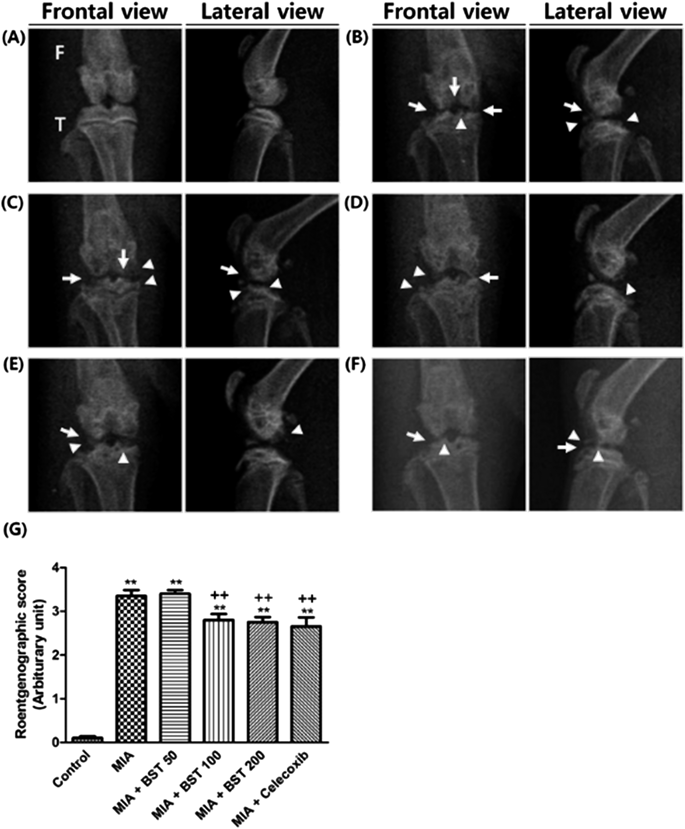
Roentgenography of knee joint in the control group (A), vehicle-treated MIA group (B), BST106 50 mg/kg-treated MIA group (C), BST106 100 mg/kg-treated MIA group (D), BST106 200 mg/kg-treated MIA group (E), celecoxib 60 mg/kg-treated MIA group (F). Morphological changes of knee articular bones were evaluated with roentgenographic score (G). F, femur; T, tibia. Arrow, impaired joint space; Arrowhead, osteophyte. The results represent the means±S.E.M. from twenty animals per group. The symbols denote significant difference ** p<0.01 compared with the control group; ++ p<0.01 compared with the MIA group.
For detailed histopathological analysis, the femur and tibia articular cartilage in the knee was evaluated with H&E and safranin O staining (Figs. 2A, B). In the MIA-treated group, rats showed a marked increase in surface cartilage damage and clone/osteophyte formation and decreases in chondrocyte cellularity and safranin O staining intensities on the both femur and tibia articular cartilage, with a consequent increase in the Mankin score (Fig. 2C). These changes were attenuated by BST106 100 mg/kg treatment.
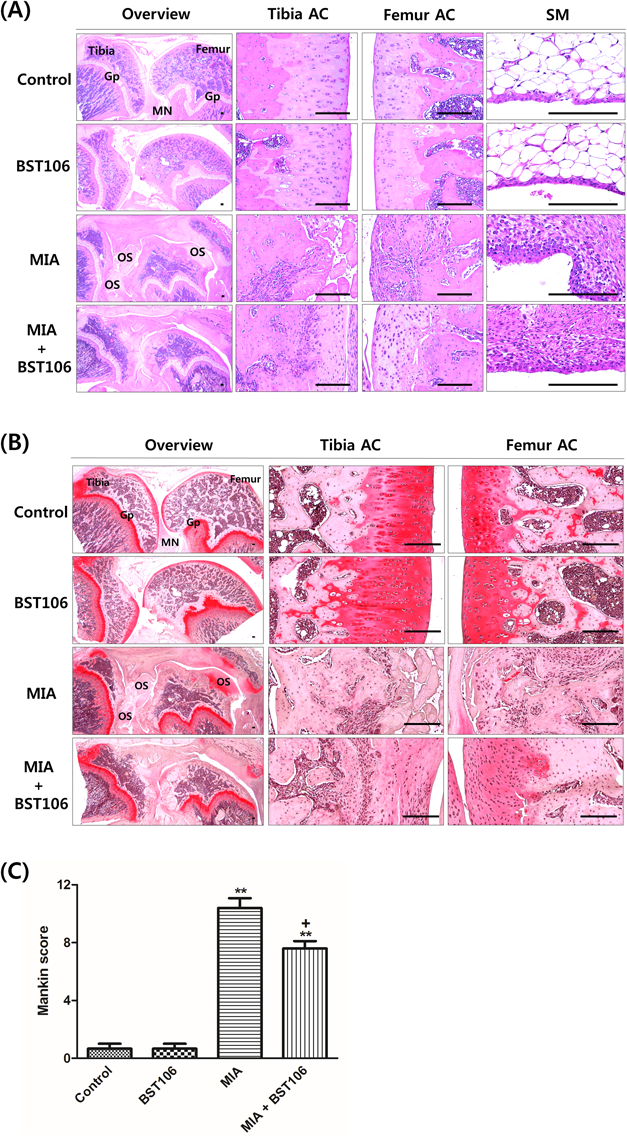
Representative images of H&E staining (A) and safranin O staining (B). (C) Mankin score (maximum=12) is totals of each index including cartilage surface damages, chondrocyte numbers, clone formations and matrix intensity of safranin O. AC, articular cartilage; SM, synovial membrane; Gp, growth plate; MN, meniscus; OS, osteophyte. Scale bars=160 µm. The results represent the means±S.E.M. from five animals per group. The symbols denote significant difference ** p<0.01 compared with the control group; + p<0.05 compared with MIA group. (Color figure can be accessed in the online version.)
COMP is a member of the thrombospondin family of extracellular matrix glycoproteins that is released into knee joint fluid and serum after joint cartilage injury.22) The serum level of COMP significantly increased after MIA injection, and this increase was attenuated by BST106 (Fig. 3A). In addition, GAG release was measured in explant cultures of rabbit knee articular cartilage. In the control group, the level of GAG from culture medium remained constant at 0.24±0.05 µg/mg cartilage. After 72 h of rhIL-1α treatment, the level of GAG in the culture medium significantly increased compared to that of the control group. Treatment with BST106 0.5, 1, 5, and 10 µg/mL inhibited the elevation in GAG release (Fig. 3B).
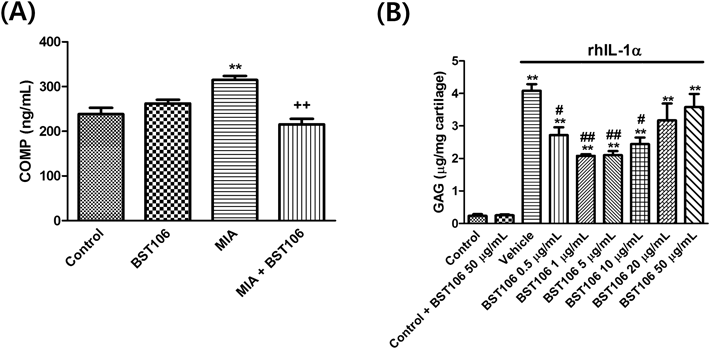
(A) Serum COMP level in MIA-induced OA rats. (B) Effect of BST106 (0.5, 1, 5, 10, 20, and 50 µg/mL) on GAG release in rabbit articular cartilage explant cultures at 72 h. The results represent the means±S.E.M. from seven animals per group for serum COMP level and three articular cartilage explants cultures per group for GAG release, respectively. The symbols denote significant difference ** p<0.01 compared with the control group; ++ p<0.01 compared with MIA group; # p<0.05 compared with the rhIL-1α group; ## p<0.01 compared with rhIL-1α group.
After MIA injection, the levels of MMP-2 and MMP-13 mRNA expression significantly increased compared with those of the control group. These increases were attenuated by BST106 treatment (Figs. 4A, B).
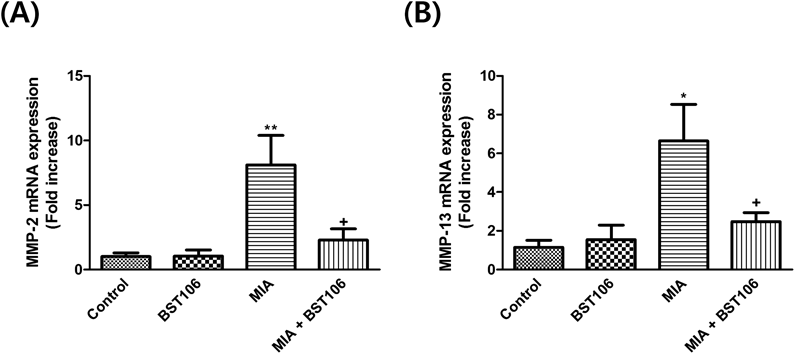
(A) MMP-2 and (B) MMP-13 mRNA expressions in knee joint of MIA-induced OA rats. The results represent the means±S.E.M. from seven animals per group. The symbols denote significant difference * p<0.05 compared with the control group; ** p<0.01 compared with the control group; + p<0.05 compared with the MIA group.
In the MIA-treated group, levels of cleaved caspase-3, cleaved caspase-8, and cleaved PARP immunoreactive cell percentages significantly increased in both femur and tibia articular cartilage compared with those of the control group. The percentage of TUNEL-positive cells in both femur and tibia articular cartilage significantly increased after MIA injection compared with that of the control group. These changes were attenuated by BST106 treatment (Figs. 5A–D and Table 3). Furthermore, the levels of TNFR1, Bax, cytosolic cytochrome C and cleaved caspase-8, cleaved caspase-9 protein expression significantly increased after MIA injection compared with those of the control group. BST106 attenuated the increases in the levels of TNFR1, Bax, cleaved caspase-8 and cleaved caspase-9 protein expression (Figs. 5E–I).
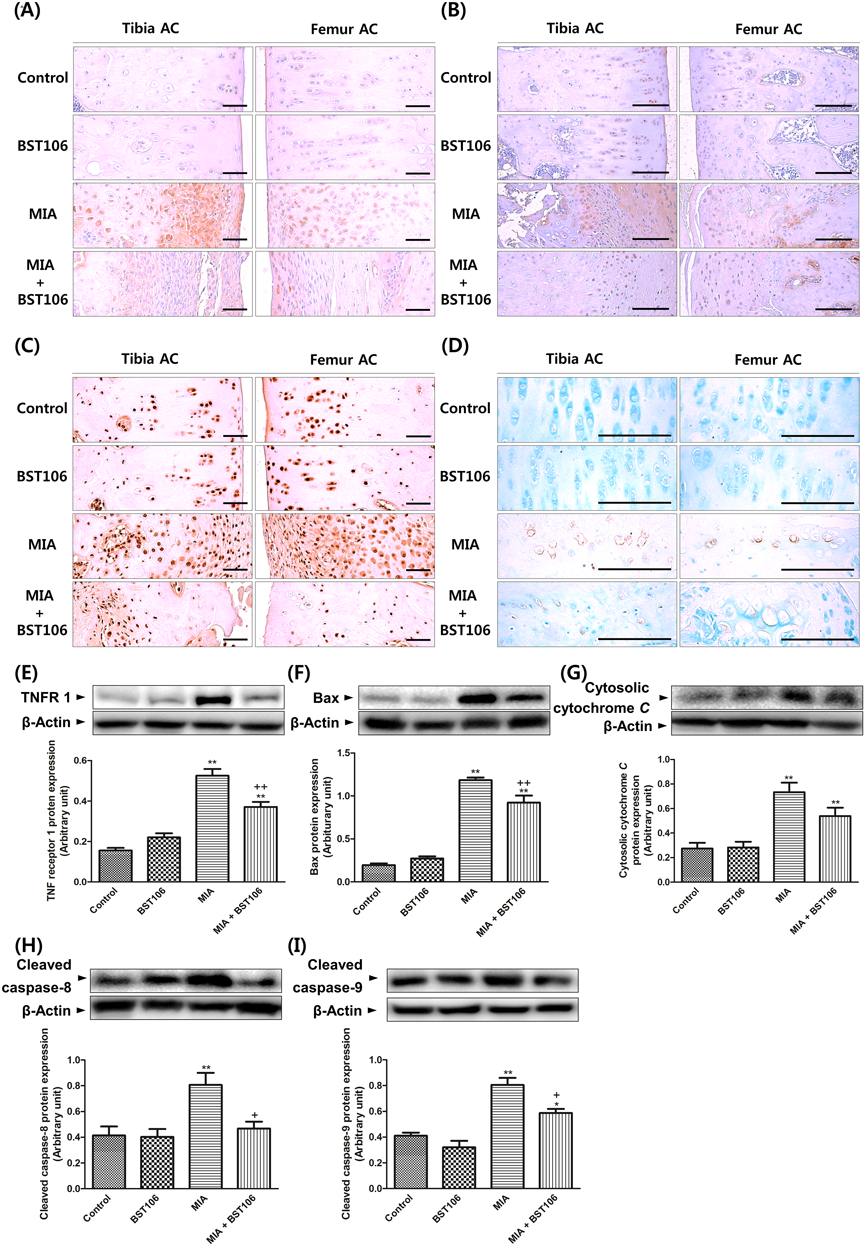
Immunohistochemistry with cleaved caspase-3 (A), cleaved caspase-8 (B), cleaved PARP (C), and TUNEL (D) in knee joint of MIA-induced OA rats. Effects of BST106 on the protein expression levels of TNFR1 (E), Bax (F), cytosolic cytochrome C (G), cleaved caspase-8 (H), and cleaved caspase-9 (I) in knee joint of MIA-induced OA rats. The results represent the means±S.E.M. from eight animals per group. The symbols denote significant difference * p<0.05 compared with the control group; ** p<0.01 compared with the control group; + p<0.05 compared with the MIA group; ++ p<0.01 compared with MIA group. Scale bars=120 µm. (Color figure can be accessed in the online version.)
| Index (Unit) | TUNEL+ cells (%/AC) | Cleaved caspase-8+ cells (%/AC) | Cleaved caspase-3+ cells (%/AC) | Cleaved PARP+ cells (%/AC) | ||||
|---|---|---|---|---|---|---|---|---|
| Groups | Femur | Tibia | Femur | Tibia | Femur | Tibia | Femur | Tibia |
| Control | ||||||||
| Vehicle | 2.9±0.9 | 4.9±1.1 | 4.6±0.8 | 5.5±0.7 | 35.5±1.8 | 40.1±2.1 | 35.3±2.3 | 33.9±4.3 |
| BST106 | 3.1±0.6 | 4.9±1.2 | 4.3±0.6 | 4.6±1.0 | 33.6±3.6 | 42.4±1.8 | 32.9±1.5 | 39.2±2.5 |
| MIA | ||||||||
| Vehicle | 63.4±2.7** | 59.7±4.9** | 64.1±7.7** | 53.3±1.9** | 78.9±4.4** | 76.7±5.1** | 77.4±1.3** | 78.3±4.0** |
| BST106 | 20.5±2.4**,++ | 22.9±3.5++ | 30.2±2.1*,++ | 29.8±3.7**,++ | 53.2±3.3*,++ | 54.7±1.7++ | 56.4±4.1**,++ | 52.8±4.5++ |
The results represent the means±S.E.M. from five animals per group. The symbols denote significant difference ** p<0.01 compared with the control group; ++ p<0.01 compared with MIA group.
In MIA-treated group, the protein expression levels of LC3-II, an autophagosome marker, and p62, an autophagy substrate, significantly increased compared with those of the control group. BST106 attenuated the increase in the level of p62 protein expression (Figs. 6A, B). Furthermore, the level of p62 mRNA expression also significantly increased compared with that of the control group in the MIA-treated group, and this increase was attenuated by BST106. (Fig. 6C). There were no significant differences in the protein expression levels of beclin-1 and Atg5-12 complex, phagophore-related proteins, among any of the experimental groups (Figs. 6D, E). In the MIA-treated group, the level of Atg3 protein expression significantly decreased compared with that of the control group and this decrease was attenuated by BST106 (Fig. 6F). In the MIA-treated group, the levels of LAMP-2 and Rab7, lysosomal membrane proteins, and the lysosomal protease cathepsin B protein expression significantly decreased compared with those of the control group. BST106 attenuated the decreased levels of Rab7 and cathepsin B protein expression (Figs. 6G–I).
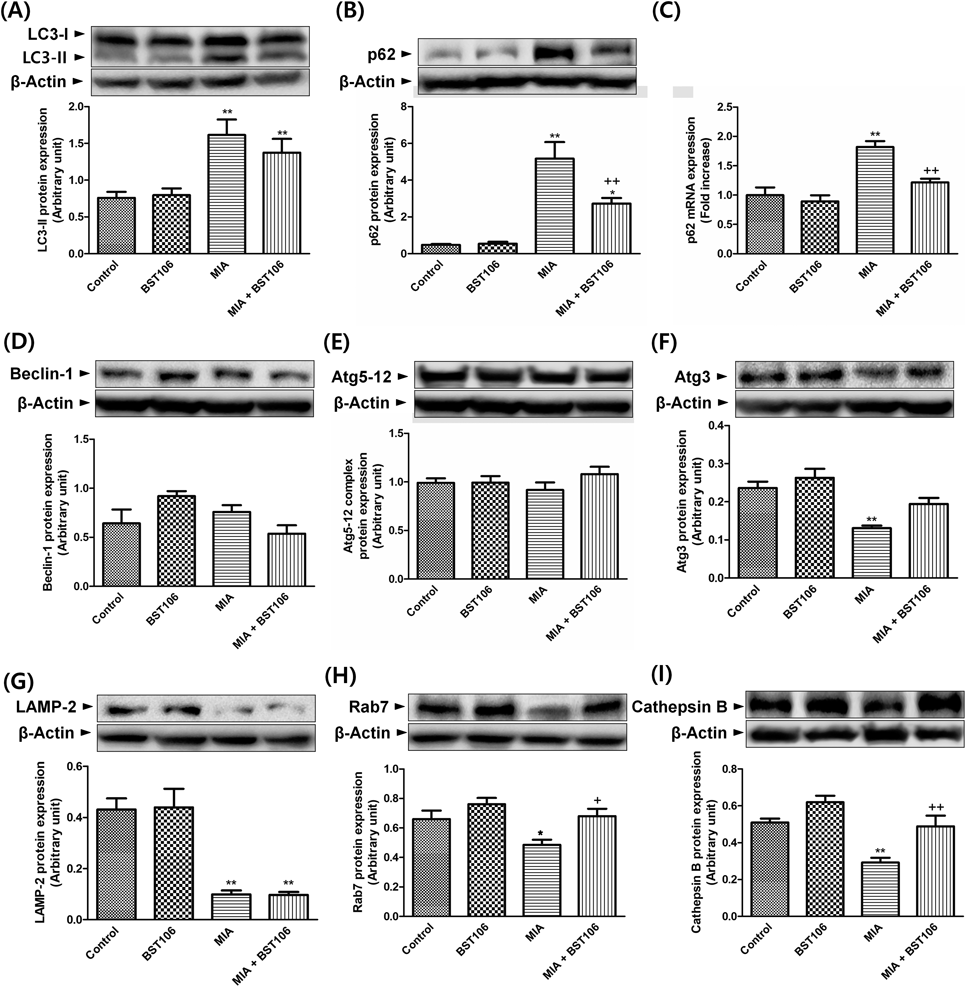
Protein expression levels of LC3-II (A), p62 (B), mRNA expression level of p62 (C), protein expression levels of beclin-1 (D), Atg5–12 complex (E), Atg3 (F), LAMP-2 (G), Rab7 (H), and cathepsin B (I) in knee joint of MIA-induced OA rats. The results represent the means±S.E.M. from eight animals per group. The symbols denote significant difference * p<0.05 compared with the control group; ** p<0.01 compared with the control group; + p<0.05 compared with the MIA group; ++ p<0.01 compared with MIA group.
The pathogenesis of OA appears to be extremely complex because the diverse interactions among inflammation, cell death, and metabolism signaling occurs in chondrocytes. The current aims of OA treatment are to alleviate pain and to improve the function of the joint. Non-steroidal anti-inflammatory drugs and various analgesics are served as a first-line treatment of OA.23) Since these agents do not treat the underlying disease process and their chronic use causes adverse effects, there is an urgent need for the development of more effective and safer medicines.24) CZ has known as a traditional herbal medicine for various inflammatory diseases and its anti-inflammatory activity has been studied. CZ extract increased the induction of HO-1 and inhibited IL-1β, IL-6, and cyclooxygenase-2 mRNA levels in LPS-stimulated RAW264.7 cells.12,25) Recently, Kim et al. reported that extract from CZ ameliorated arthritis symptoms and bone erosion in collagen-induced rheumatoid arthritis.17) Here, we demonstrated the protective effect of BST106 against OA-related cartilage damage.
Typical pathological changes of OA are degradation and focal loss of articular cartilage, osteophyte formation, and subsequent joint space narrowing. Intra-articular injection with MIA, an inhibitor of glycolysis, is a clinically relevant animal model of OA that closely mimics human OA pathology and pain.26) MIA induces loss of articular cartilage with progression of subchondral bone remodeling by 28 d.27) In the present study, we investigated the effect of BST106 on global OA symptoms including pain-related behaviors and morphological changes in MIA-induced OA. In the MIA-treated group, swelling and limping were first observed 7 d after MIA injection. These symptoms reduced and reemerged at 14 d. They worsen at 21 d (data not shown) and were most severe at 28 d. Administration of BST106 50 and 100 mg/kg for 28 d attenuated the degree of swelling and limping. Roentgenographic examinations revealed that BST106 100 and 200 mg/kg ameliorated MIA-induced degenerative changes, such as irregularity or osteophytes on the surface of the cartilage in femur and tibia. BST106 50 and 100 mg/kg showed different pharmacological response. BST106 is plant extract composed of various active components, which may represent the pharmacological effect through harmonizing with each other, like orchestra, depending on their dose and distribution ratio. Thus, the reason for this contradiction in results depending on dose of BST106 remains question. Further studies are needed to conclude an association between dose and biological optimality. Meanwhile, BST106 suppressed the MIA-induced increase in the serum level of COMP, a biomarker for monitoring progression of cartilage destruction. Moreover, histological analysis with H&E and safranin O staining showed that MIA increased cartilage surface damage, osteophyte formation, and inflammatory cell infiltration, and decreased chondrocyte cellularity and matrix intensity. BST106 was found to remarkably attenuate these changes, demonstrating significant protection of cartilage against OA. These results suggest that BST106 may potentially have clinical applications in the treatment of OA.
An imbalance between anabolism and catabolism of extracellular matrix (ECM) components is the central feature in cartilage destruction.1) The massive loss of ECM results from cleavage of type II collagen and aggrecan, a predominant proteoglycan, and the consequent release of GAG which is covalently linked to core proteins to form proteoglycans.28) MMPs, a family of proteolytic enzymes, play pivotal role in the degradation of ECM and mediate cartilage destruction and joint erosion.29) Compared with other MMPs, MMP-13 and MMP-2 play important roles in OA. MMP-13 predominantly degrades type II collagen. MMP-2 as a gelatinase is required for completion of collagen degradation after cleavage of collagen molecules by collagenases. These enzymes are also able to degrade aggrecan molecules.30,31) Elevated mRNA levels of MMP-13 and MMP-2 resulted in increased proteolysis of the aggrecan and collagen in human OA articular cartilage treated with IL-1α.32) Administration of specific MMP-13 inhibitors significantly reduced the severity of the pathology in both human and animal models of OA.33,34) Resveratrol, a natural antioxidant, reduced MIA-induced pain by inhibiting MMP-13 mRNA expression and inflammatory cytokines in rats.35) Our previous study showed that GCSB-5, a herbal formulation, protected against articular cartilage destruction by suppressing MMP-2 activity and cyclooxygenase-2 expression in a MIA-induced OA rat model.28) In our present study, BST106 decreased the levels of MMP-2 and MMP-13 mRNA as well as the release of GAG from knee articular cartilage explants. These results indicate that BST106 inhibits cartilage destruction through downregulation of MMP-13 and MMP-2 at the transcriptional level.
Chondrocyte death and matrix depletion may form a vicious cycle, with each one aggravating the other. Earlier reports revealed a positive correlation between the severity of cartilage damage and chondrocyte apoptosis.36) Apoptosis schematically occurs through two main pathways, the death receptor-mediated extrinsic pathway and the mitochondria-mediated intrinsic pathway. The two pathways converge at the point of activation of an executioner caspase, caspase-3, which consequently cleaves PARP, a key component for DNA damage repair.37) In both in vitro and in vivo rat OA models TNF-related apoptosis-inducing ligand (TRAIL) induced chondrocyte apoptosis.38) In human OA cartilage, TNFR1 was localized in chondrocytes at the sites of focal loss of cartilage proteoglycans.39) The loss of mitochondrial membrane potential and reactive oxygen species production induced caspase-3 activation in MIA-treated primary rat chondrocytes.40) Na et al.41) reported that ginsenoside Rb1 protected rat chondrocytes against H2O2-induced apoptosis through inhibition of the mitochondrial permeability transition and the increased levels of Bax and caspase-3. Malvidin, an anthocyanidin with anti-inflammatory activity, reduced OA-induced pain and inflammation by inhibiting chondrocyte apoptosis and NF-κB signaling.42) In our study, BST106 inhibited MIA-induced activation of TNFR1, Bax, caspase-8, caspase-9, and caspase-3 and cleavage of PARP. These biochemical parameters of apoptotic cell death paralleled the morphological changes observed with TUNEL staining. These results suggest that BST106 suppresses both the extrinsic and intrinsic apoptotic pathways in OA.
Autophagy has recently been recognized as an important regulator of normal chondrocyte homeostasis. Autophagy inhibits chondrocyte apoptosis and blocks the degeneration of articular cartilage and OA development.9) Decreased autophagy was accompanied by increased apoptotic cell death in a surgically induced-OA mouse model.43) In the MIA-induced OA rat, mRNA and protein levels of LC3-II were downregulated, which positively correlated with the degree of OA lesions.44) Furthermore, local intra-articular injection of an autophagy inducer such as rapamycin delayed articular cartilage degradation in a murine model of OA.45) Measuring both LC3-II and autophagy substrate p62/SQSTM1 has been used to monitor autophagic flux changes because LC3-II and p62 are removed by autophagic degradation with ubiquitinated protein aggregates.46) Impaired autophagic flux was led to aberrant accumulation of LC3-II and p62.47) Recently, Cetrullo et al. demonstrated that autophagic flux was impaired by excessive oxidative stress in human chondrocytes.48) In t-butyl hydroperoxide-treated-chondrocytes, trehalose attenuated apoptosis through restoration of autophagic flux via increased LC3-II and decreased p62 protein expression.49) p62 acts as a cargo receptor for autophagic degradation and is a target gene for transcription factor, nuclear factor-E2-related factor 2 (Nrf2) creating a positive feedback loop by inducing antioxidant response element-driven gene transcription. Then, phosphorylation of p62 increases binding affinity of p62 to Keap1, resulting in the escape of Nrf2 from the Keap1 interaction. Keap1 is degraded together with the autophagic cargo.50) In our study, MIA significantly increased the levels of LC3-II and p62 protein expression and BST106 attenuated p62 protein expression. Furthermore, the level of p62 mRNA expression significantly increased compared with that of the control group in the MIA-treated group, and this increase was attenuated by BST106. Collectively, our result suggests that the elevation of p62 protein expression by MIA is mediated by the transcriptional upregulation as well as autophagic impairment.
Autophagy machinery is important for execution of the complete autophagy process. Dynamic process of autophagy includes membrane isolation, phagophore elongation, autophagosome formation, and fusion between autophagosome and lysosome for degradation.51) After membrane isolation by beclin-1 complex formation, autophagosome formation requires two conjugation processes such as Atg12–Atg5 conjugation and LC3 lipidation. Moreover, Atg3 and Atg7 are crucial molecules for the LC3-I to LC3-II conversion, known as LC3 lipidation. LC3-I in the cytoplasm is activated by Atg7 and transferred to Atg3, forming the intermediate LC3-II. Finally, mature autophagosome is fused with the lysosome, which is important process for degradation of its contents.52) The small GTPase Rab7 and LAMP are required for membrane trafficking and the autophagosome-endosome-lysosome (autolysosome) fusion process.53) These autolysosomes acquire the lysosomal proteases cathepsins and acid phosphatases and finally autophagic substrate is degraded.54) Aging-related mouse OA suppressed autophagy through reduced beclin-1 and LC3-II protein expression, which was accompanied by increased chondrocyte cell death.43) In OA patients, beclin-1 overexpressing chondrocytes improved autophagy, reduced apoptosis, and inhibited MMP-3 and MMP-13 expression.55) Cartilage-specific deletion of Atg7 in mice enhanced chondrocyte death and decreased chondrocyte proliferation.56) The extract of Butea monosperma, a plant-derived nutraceutical, enhanced the autophagy flux and increased formation of autolysosomes in human OA chondrocytes.57) In our study, MIA did not affect beclin-1 or Atg5–12 but significantly decreased Atg3 protein expression. BST106 attenuated decreased level of Atg3 protein expression. Interestingly, MIA significantly decreased levels of LAMP-2, Rab7, and cathepsin B protein expression and BST106 attenuated the decreases in Rab7 and cathepsin B protein expression. These results suggest that BST106 activates autophagic clearance through increasing LC3 lipidation and autophagosome–lysosome fusion in OA.
In summary, BST106 showed the cartilage and chondroprotective effect through inhibition of GAG release from knee articular cartilage explants and decreased serum level of COMP in MIA-induced OA rats. The effect of preventing cartilage destruction was achieved by the suppression of MMP-2 and MMP-13 mRNA levels. Moreover, BST106 inhibited the extrinsic and intrinsic apoptotic pathway and restored impairment of autophagic flux through increasing LC3 lipidation and autophagosome–lysosome fusion. Taken together, we propose that BST106 may be a new therapeutic candidate for OA treatment.
This research was supported by the National Research Foundation of Korea (NRF) Grant funded by the Ministry of Education, Science, and Technology in Korea (MEST; 2012R1A5A2A28671860). Jeong-Min Hong received ‘Global Ph.D. Fellowship Program’ support (NRF-2016H1A2A1909480) from NRF funded by the MEST in Korea.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.