2018 Volume 41 Issue 8 Pages 1295-1298
2018 Volume 41 Issue 8 Pages 1295-1298
In our recent study, we reported that kurarinone, one of the most abundant flavonoids found in the dry root of Sophora flavescens (Kushen), is a potent activator of the large-conductance Ca2+-activated K+ (BKCa) channel. Herein, we isolated and characterized other flavonoid components from Kushen. Among the 13 compounds tested, six flavonoids were found to activate the BKCa channel, three of which, 7,4′-dihydroxy-5-methoxy-8-(γ,γ-dimethylallyl)-flavanone, kuraridin, and kuraridinol, are new activators of the BKCa channel.
The large-conductance Ca2+-activated K+ channel known as the BKCa channel or Slo1 requires membrane depolarization and/or increased intracellular Ca2+ for its activation.1,2) Channel opening creates a rapid efflux of K+ ions across the cell membrane. The BKCa channel is related to relaxation of urinary bladder smooth muscle (UBSM) and represents a therapeutic target for overactive bladder (OAB).3,4) In our previous study, kurarinone was established as a novel activator of BKCa channel with therapeutic potential for treating OAB.5) This plant flavonoid contains a flavanone backbone and is abundant in the root of Sophora flavescens AITON (Kushen, Leguminosae).6,7)
S. flavescens AIT. is a medicinal herb widely distributed in northeast Asia.8) Kushen contains diverse flavonoids including kurarinone, kushenol C, and leachianone G, and these individual compounds display anti-inflammatory, anti-microbial, and cytotoxic activities.9,10) In the present study, we tested other flavonoids in Kushen for their potentiating effects on BKCa channel function. Of thirteen different flavonoids and alkaloids purified from Kushen, six compounds including kurarinone significantly increased channel activity. The BKCa channel was activated differentially by the six flavonoids, which variously caused a shift in the conductance–voltage (G-V) relationship to more negative voltages.
Purification and verification of 13 individual compounds were reported previously.11,12) A voucher specimen has been deposited at the Department of Food Science and Nutrition, Pukyong National University. Individual compounds were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, U.S.A.) to generate stock solutions.
Cell-Based Fluorescence Assay and Data AnalysisIn the cell-based assay, hyperactive mutant BKCa channels (G803D/N806K) were stably overexpressed in AD-293 cells derived from human embryonic kidney 293 cells, as previously described.5,13) The FluxOR potassium channel assay (Invitrogen, U.S.A.) was used to measure the activity of the BKCa channel following the manufacturer’s instructions. Fluorescence signals were measured using FlexStation 3 (Molecular Devices, U.S.A.) and SoftMax Pro software was used for data transformation. Fluorescence signals were recorded every 2 s for 2 min. Measured fluorescence values were normalized against the basal level of each trace to give normalized values in relative fluorescence units (RFU). Activation by individual compounds was compared in terms of the rate of increase in the initial fluorescence estimated by linear fitting of the first three timepoints after addition of the stimulus solution. Data analysis was performed using the OriginPro 9.1 program (OriginLab Corp., U.S.A.).
Electrophysiological Recording and Data AnalysisElectrophysiological recording was performed using the α-subunit of the rat BKCa channel (Slo1) expressed in Xenopus laevis oocytes. The Gigaohm-seal patch-clamp method was used for current recordings in an outside-out configuration as previously described.5,14) Currents were digitized at a rate of 10 or 20 points/ms. The resting potential was −100 mV, and BKCa channels were activated by voltage pulses from −80 to 200 mV in 10 mV increments. For recording, KMHG [120 mM potassium gluconate, 10 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), 4 mM KCl, and 5 mM ethylene glycol bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) (pH 7.2)] was used as both an extra- and intra-cellular (pipette) solution to suppress endogenous Ca2+-activated chloride currents in Xenopus oocytes. To provide the precise free concentration of intracellular Ca2+ ([Ca2+]i), the appropriate amount of total Ca2+ to be added to the intracellular solution was calculated using MaxChelator software (http://maxchelator.stanford.edu/).15) Data acquisition and analysis were performed using Clampex 8.0 and Origin 9.1. Data are presented as means±standard error of the mean (S.E.M.) with n replicates, and statistical differences were determined using the two-sample t-test in Origin 9.1. A p-value of <0.05 was considered statistically significant.
In the present study, we tested flavonoids purified from Kushen for their effects on BKCa channel activity using the aforementioned cell-based fluorescence assay (Fig. 1A). Among the 13 flavonoids tested, four compounds were described in our previous study.7) At a final concentration of 10 µM, five compounds (7,4′-dihydroxy-5-methoxy-8-(γ,γ-dimethylallyl)-flavanone, kuraridin, kuraridinol, kushenol C, and leachianone G) were found to significantly activate the channel. Kuraridinol, a prenylated chalcone, exhibited the highest potency for channel activation among the Kushen flavonoids (Figs. 1A, 1B). The chemical structures of these compounds are shown in Fig. 1C and supplementary Fig. 1.
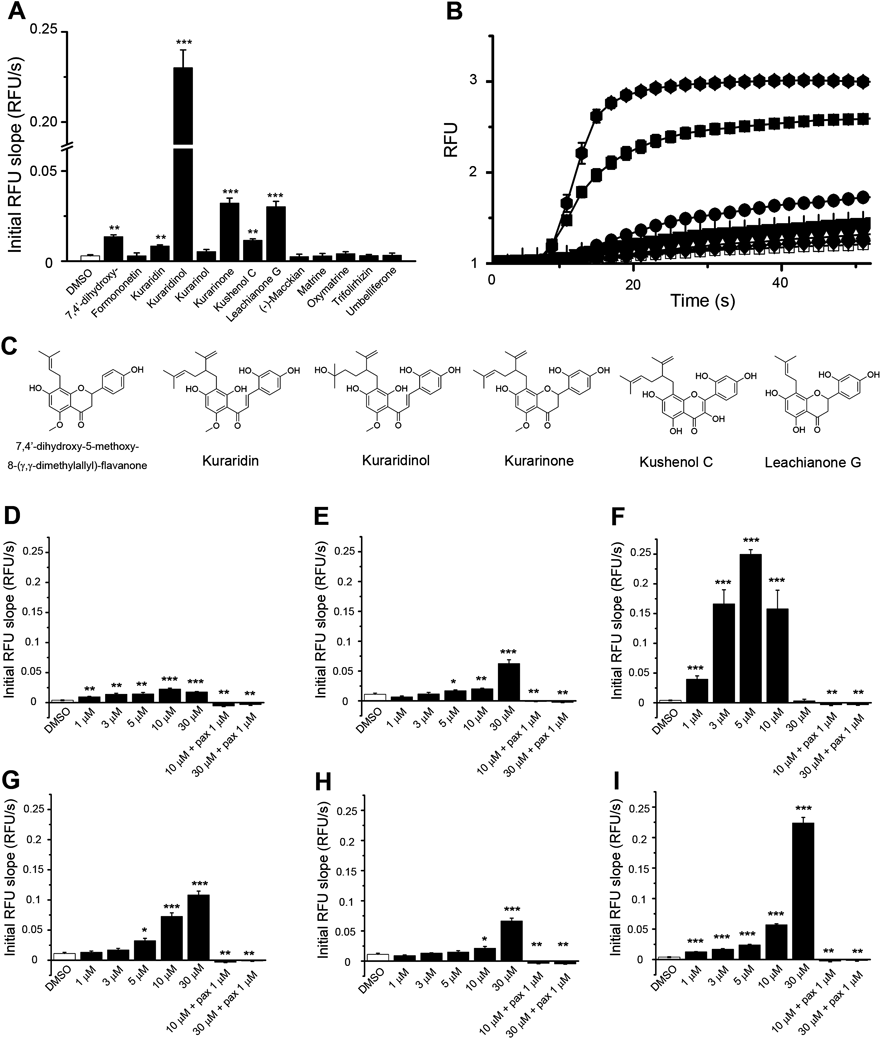
A. Initial increase in RFU following addition of each of 13 flavonoids (n=4). B. Time-dependent RFU increase by the each of the six compounds exhibiting the strongest effect on BKCa channel activity. Symbols are as follows: 7,4′-dihydroxy-5-methoxy-8-(γ,γ-dimethylallyl)-flavanone (▼), kuraridin (◆), kuraridinol (hexagon), kurarinone (■), kushenol C (▲) and leachianone G (●) C. Chemical structures of the flavonoids. D–I. Initial RFU increases were plotted for 7,4′-dihydroxy-5-methoxy-8-(γ,γ-dimethylallyl)-flavanone (D), kuraridin (E), kuraridinol (F), kurarinone (G), kushenol C (H), and leachianone G (I). (n=4; * p<0.05, ** p<0.01, *** p<0.001).
The potentiating effects of six compounds including kurarinone were further investigated at different concentrations (1−30 µM). All compounds except kuraridinol progressively increased the fluorescence signal in a dose-dependent manner (Figs. 1D–1I). The fluorescence signal evoked by each compound was completely blocked by co-treatment with 1 µM paxilline, a known BKCa channel inhibitor, confirming that the Tl+ fluorescence induced by the compounds was due to the activation of BKCa channels.16)
Effects of Kushen Flavonoids on Macroscopic Currents of the BKCa ChannelWe further validated the effects of the six Kushen flavonoids electrophysiologically. Macroscopic BKCa channel currents were evoked by voltage pulses from −80 to 200 mV in the absence and presence of extracellular flavonoids. Both outward and inward tail currents were increased by all six flavonoids at a concentration of 10 µM (Fig. 2A). However, the degree of potentiation varied among the different flavonoids. The effects of each flavonoid were further quantified by plotting the G-V relationship (Figs. 2B, 2C). All compounds shifted the G-V curve toward more negative voltage to varying degrees. In the presence of 10 µM compound, the shift in V1/2 was −27 mV for 7,4′-dihydroxy-5-methoxy-8-(γ,γ-dimethylallyl)-flavanone, −38 mV for kuraridin, −102 mV for kuraridinol, −54 mV for kurarinone, −12 mV for kushenol C, and −66 mV for leachianone G. All six flavonoids also increased the maximum conductance (Gmax) by 1.12-fold for 7,4′-dihydroxy-5-methoxy-8-(γ,γ-dimethylallyl)-flavanone, 1.16-fold for kuraridin, 1.33-fold for kuraridinol, 1.28-fold for kurarinone, 1.28-fold for kushenol C, and 1.38-fold for leachianone G (Fig. 2E). Thus, the results indicate that all six Kushen flavonoids can potentiate BKCa channel activation with varying degrees of potency.
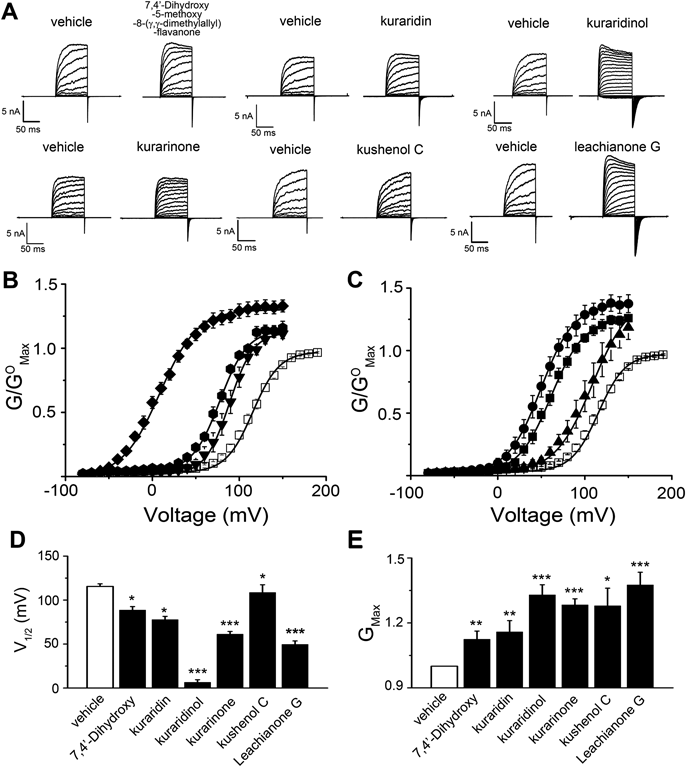
A. Representative BKCa channel current traces in the presence of different Kushen flavonoids. [Ca2+]i was 3 µM and the pulse duration was 100 ms for each step. B,C. Effects of the flavonoid on the G-V relationship. Conductance was recorded at 0.8 s after termination of the stimulus pulse. Symbols are as follows: vehicle (□, n=25), 7,4′-dihydroxy-5-methoxy-8-(γ,γ-dimethylallyl)-flavanone (▼, n=6), kuraridin (hexagon, n=5), kuraridinol (◆, n=4), kurarinone (■, n=5), kushenol C (▲, n=5), and leachianone G (●, n=5). The Boltzmann equation (G/GMax={(GMax−GMin)/(1+exp[(V1/2−V) / k])}+GMin) was used for data fitting. D. Effects of flavonoids on V1/2 values. E. Effects of flavonoids on GMax values (* p<0.05, ** p<0.01, *** p<0.001).
Kushen contains various natural compounds including flavonoids and alkaloids.17) Since flavonoids are the most abundant class of chemicals in Kushen, and since kurarinone, a major flavonoid in Kushen, can potently active the BKCa channel, we investigated the effects of other Kushen flavonoids on channel activity.5,18)
In addition to flavonoids with a flavanone backbone reported previously, two other flavonoids with a chalcone backbone, kuraridinol and kuraridin, were found to potently activate the BKCa channel.5) Among these flavanones and chalcone pairs, we found that the chalcone displayed higher activity. In fact, no potentiation was observed for kurarinol in the present study or in our previous report.5) Thus, opening of the C ring between O1 and C2 may allow freedom of rotation and the adoption of a conformation better suited to channel binding.
It is worth noting that the effect of kuraridinol on BKCa channel is biphasic. While kuraridinol potently activates the channel below 5 µM, higher concentrations of the compound inhibit the channel (Fig. 1F). This biphasic effect could be explained by the presence of more than two or more binding sites for the compound with opposite effects. Assuming two distinct binding sites, an ‘activation site’ of high affinity and an ‘inhibition site’ of lower affinity, we can envisage kuraridinol activating the channel by occupying the activation binding site at low concentrations and inhibiting the channel by binding to the inhibition site at higher concentrations. It remains to be further examined if such binding sites exist and how occupancy of these sites can evoke opposite functional consequences in terms of channel activity.
In summary, we showed that the chalcones kuraridinol and kuraridin activate the channel from the extracellular side. Since activation of the BKCa channel by kurarinone can induce the relaxation of UBSM and relieve the symptoms of OAB,5) the flavonoid components described in this study could represent new BKCa channel-targeting agents for the treatment of diseases such as OAB syndrome.
This work was supported by Grants from the National Leading Research Laboratories supported by the National Research Foundation of Korea [2011-0028665] and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Agri-Bioindustry Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) [2017-352] to CSP.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.