2019 Volume 42 Issue 10 Pages 1620-1627
2019 Volume 42 Issue 10 Pages 1620-1627
2′-Fucosyllactose (2FL) is the most abundant component of the oligosaccharide content in human milk. It has been reported that 2FL has the ability to protect against infectious disease caused by bacterial pathogens. In this study, we investigated the protective effects of 2FL on particulate matter (PM)10-induced pro-inflammatory cytokines in HaCaT keratinocytes. 2FL reduced PM10-induced excess expression of interleukin (IL)-6, IL-8, IL-1α and IL-1β in HaCaT keratinocytes. In addition, PM10 also increased hypoxia-inducible factor (HIF)-1α protein levels; however, 2FL inhibited the accumulation of HIF-1α protein and the phosphorylation of phosphatidylinositol 3-kinase (PI3K)/Akt stimulated by PM10. Furthermore, 2FL improved PM10-induced the decrease in epidermal thickness and integrity of the cornified layer in the reconstructed human epidermal skin model (RHE). In our results, 2FL inhibited PM10-induced pro-inflammatory mediators by regulating the HIF-1α/PI3K/Akt pathway and protected the skin epidermis against PM10 irritation. Taken together, these results suggest that 2FL can be used as a primary ingredient in cosmeceutical products to alleviate skin irritation and inflammation caused by urban air pollution.
Human skin is an organ that acts as a barrier to defend the internal organs from various external stimuli. However, external environmental stimuli attack human skin and deteriorate the function of the skin, thereby accelerating skin aging. Airborne pollutants have recently emerged as environmental stimuli that have a major health impact on various organs, including skin. Currently, a number of studies have reported that airborne pollutants are related to several skin diseases that involve a progression of skin inflammation, such as atopic dermatitis, acne, and psoriasis.1–6) Exactly, particulate matter (PM)10 and PM2.5 are defined as particles which pass through a size-selective inlet with a 50% efficiency cut-off at 10 and 2.5 µm aerodynamic diameter, respectively.2,3) PM is known to induce oxidative stress via the production of reactive oxygen species (ROS) and to stimulate the secretion of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1α, IL-6 and IL-8.2,7–10) In addition, these particles carry organic chemicals, such as polyaromatic hydrocarbons (PAHs), which are potent ligands for aryl hydrocarbon receptor (AhR) in keratinocytes.1,11) Activated AhR signaling causes inflammatory skin lesions similar to contact dermatitis.12)
A recent study reported that the AhR pathway contributed to hypoxia-inducible factor 1α (HIF-1α) accumulation associated with the phosphatidylinositol 3-kinase (PI3K)/Akt pathway.13) The transcription factor HIF-1, a key regulator in the adaptation to hypoxia, is a heterodimer composed of HIF-1α and aryl hydrocarbon receptor nuclear translocator (ARNT). Under normoxic conditions, HIF-1α is rapidly degraded by prolyl hydroxylases (PHDs) following proline hydroxylation, and its degradation is facilitated by the von Hippel–Lindau tumor suppressor protein (VHL)-mediated ubiquitin–proteasome pathway. On the other hand, HIF-1α, which is stabilized with decreasing PHD activity, induces transcription of its target genes with adaptive functions under hypoxic conditions. Previous studies demonstrated that HIF-1 activation by hypoxia is closely related to oxidative stress and inflammatory responses in various tissues.13–17)
2′-Fucosyllactose (2FL) is a major constituent of human milk oligosaccharides (HMOs), which can be present at ≤5 g/L in milk. It has been reported that a lower 2FL level in mother’s milk was associated with a higher incidence of diarrhea in breast-fed infants. In addition, 2FL possesses protective effects against infectious diseases, which are caused by pathogenic bacteria and their toxins.18–22) In the present study, we demonstrated the effects of PM10 on skin inflammation and HIF-1α accumulation. Furthermore, we investigated the inhibitory effects of 2FL on PM10-induced HIF-1α accumulation via the PI3K/Akt pathway. Our results suggested that 2FL has protective effects against PM10-induced inflammatory responses by inhibition of the PI3K/Akt signaling pathway in skin keratinocytes.
The HaCaT human keratinocyte cell line was purchased from the American Type Culture Collection (Manassas, VA, U.S.A.). The cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 1% Antibiotic Antimycotic Solution (DMEM; HyClone Laboratories, Inc., Logan, UT, U.S.A.) and 10% fetal bovine serum at 37°C in an atmosphere of 5% CO2. The cells were seeded in 6-well plates (5 × 105 cells/well) and washed with 1.5 mL Dulbecco’s phosphate-buffered saline (DPBS), and the culture medium was replaced after 24 h of incubation. The cells were then exposed not only to 50 µg/mL but also several dose concentrations of PM10 (PM10-like, Sigma-Aldrich) and treated with 2FL in serum-free medium. The dust was collected from the road tunnel Wisłostrada in Warsaw, Poland and mainly composed of PAHs including benzo[a]pyrene, dibenz[a,h]anthracene, indeno[1,2,3-cd]pyrene, benzo[b]fluoranthene, benzo[k]fluoranthene and benzo[j]fluoranthene.
Real-Time RT-PCRTotal RNA was isolated from cells using TRIzol reagent according to the manufacturer’s instruction (TaKaRa, Shiga, Japan). cDNA was synthesized from 2 µg of total RNA using Reverse Transcription Premix (Elpis-biotech, Daejeon, Korea) under the following reaction conditions: 45°C for 45 min and 95°C for 5 min. Gene expression signals were quantified with real-time RT-PCR and the data were analyzed using the StepOne PlusTM system software (Applied Biosystems, Foster City, CA, U.S.A.). Real-time RT-PCR amplification reactions were performed using SYBR Green PCR Master Mix with premixed ROX or TaqMan™ Universal Master Mix II with UNG (Applied Biosystems). The following primer pairs (Bioneer, Daejeon, Korea) were used in the reactions performed in an ABI 7300 following the manufacturer’s protocol: β-actin (F: 5′-GAT GAG ATT GGC ATG GCT TT-3′ and R: 5′-CAC CTT CAC CGT TCC AGT TT-3′), IL-6 (F: 5′-TAC CCC CAG GAG AAG ATT CC-3′ and R: 5′-TTT TCT GCC AGT GCC TCT TT-3′), IL-8 (F: 5′-CCA ACA CAG AAA TTA TTG TAA AGC-3′ and R: 5′-TGA ATT CTC AGC CCT CTT CAA-3′), IL-1α (F: 5′-AGA TGC CTG AGA TAC CCA AAA CC-3′ and R: 5′-CCA AGC ACA CCC AGT AGT CT-3′) and IL-1β (F: 5′-GTC ATT CGC TCC CAC ATT CT-3′ and R: 5′-ACT TCT TGC CCC CTT TGA AT-3′). The expression of β-actin was used as an internal control. HIF-1α expression analysis was performed by Custom Taqman® Array Analysis utilizing the corresponding Taqman® Gene Expression Assay (Hs00153153_m1) and 18S ribosomal RNA (rRNA) (Hs99999901_m1) was used as an internal control (Applied Biosystems). The reaction conditions were as follows: initiation at 50°C for 2 min and 95°C for 10 min, followed by cycling conditions of 95°C for 15 s and 60°C for 1 min for 40 cycles.
Immunoblotting AnalysisThe cells were lysed in RIPA lysis buffer (Sigma-Aldrich) containing 150 mM NaCl, 1.0% IGEPAL® CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS) and 50 mM Tris, pH 8.0. The protein concentrations were determined using the Bradford assay and 45 µg of each sample was separated using a 10% SDS-polyacrylamide gel and then transferred to a nitrocellulose membrane (Bio-Rad, CA, U.S.A.). The membrane was blocked with 5% skim milk (in TBS containing 1% Tween-20) and incubated overnight at 4°C with primary antibodies against HIF-1α, p-PI3K, PI3K, p-Akt, Akt, GAPDH and β-actin (Abcam, MA, U.S.A.). After it was washed with TBST 3 times, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody (Bethyl Laboratories, Inc., Montgomery, TX, U.S.A.) for 2 h at room temperature. The signals were detected using an enhanced chemiluminescence (ECL) detection system (Thermo Scientific, MA, U.S.A.) and visualized with a G:Box Chemi System (Syngene, Cambridge, U.K.).
Reconstructed Human Epidermal Skin Model (RHE) Culture and Hematoxylin and Eosin (H&E) StainingThe RHE™ model, which is a reconstructed human epidermis composed of multilayered normal keratinocytes, was purchased from SkinEthic (France). The reconstructed skins were pre-incubated with culture medium overnight in an atmosphere of 5% CO2 at 37°C. The RHE were then applied with PM10 (50 µg/mL) on the surface of stratum corneum and treated with 2FL (10 µg/mL) in culture medium, simultaneously. The RHE models were collected after 24 h of treatment and fixed in 10% formalin. The effects of PM10 and 2FL on RHE were evaluated by the H&E staining assay. Five-micrometer paraffin-embedded RHE tissue sections were deparaffinized and rehydrated with xylene and ethanol. First, the sections were stained with hematoxylin to visualize the nuclei in purple. Eosin staining was then performed to classify the cytosolic area in red. The stained sections were observed under a light microscope.
Statistical AnalysisThe data are expressed as the mean ± standard deviation (S.D.). For multiple comparisons, p value was calculated with ANOVA using GraphPad Prism software. p-Values <0.05 were considered to be statistically significant.
In recent years, epidemiological studies have shown that PM affects the progression of inflammatory skin diseases, such as contact hypersensitivity, atopic dermatitis and acne.2) Previous studies demonstrated that pro-inflammatory cytokines were highly expressed in lesional skin of patients with skin diseases.23) To determine the effects of PM10 in human HaCaT keratinocytes, we evaluated the mRNA expression levels of pro-inflammatory cytokines in the presence or absence of PM10. HaCaT keratinocytes were treated with PM10 concentrations ranging from 10 to 100 µg/mL for 4 h and the mRNA expression levels were measured by RT-quantitative (q)PCR. As shown in Fig. 1, PM10 increased the mRNA expression levels of pro-inflammatory cytokines, such as IL-6, IL-8, IL-1α and IL-1β, in a dose-dependent manner, compared to that in the untreated control.
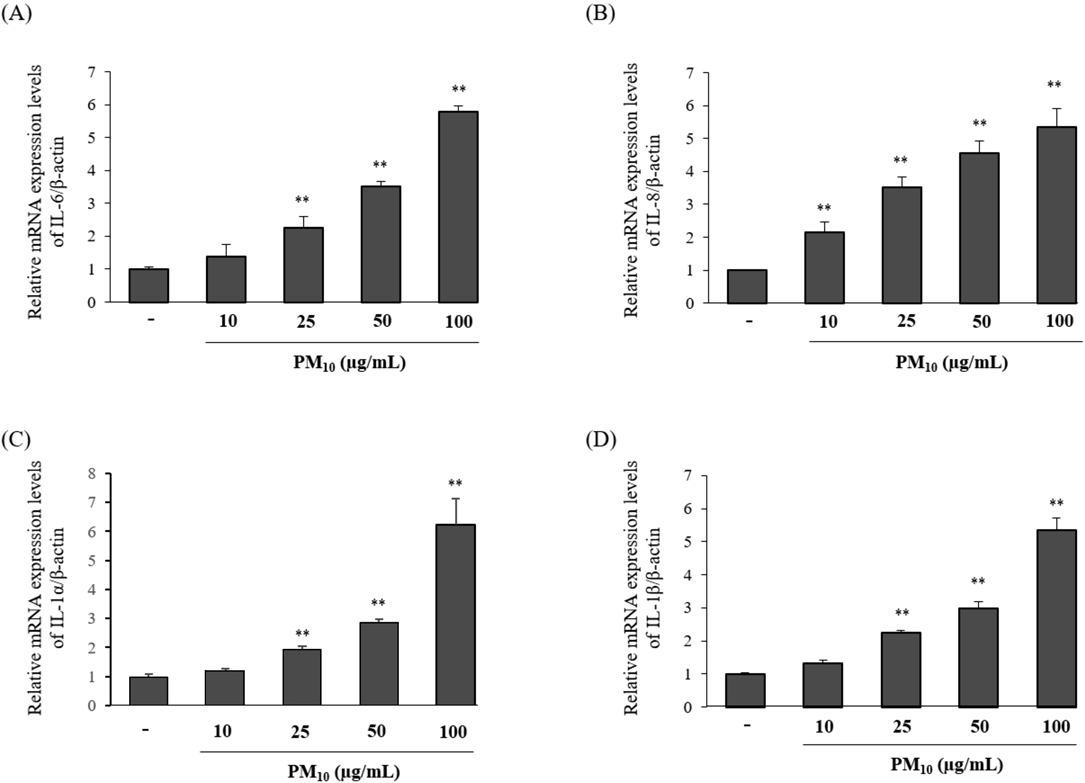
HaCaT keratinocytes were treated with different concentrations (10–100 µg/mL) of PM10 for 4 h. (A) IL-6, (B) IL-8, (C) IL-1α and (D) IL-1β mRNA expression levels were measured by RT-qPCR. Results are expressed as mean ± S.D. (% control) of three independent experiments. ** p < 0.01 compared to the control.
In order to evaluate the inhibitory effects of 2FL on PM10-induced pro-inflammatory cytokines, HaCaT keratinocytes were treated with PM10 (50 µg/mL) with 2FL (10 and 100 µg/mL). RNA was harvested after 4 h of treatment, and the pro-inflammatory gene expression was assessed using RT-qPCR. Since PM10 significantly reduced the HaCaT keratinocyte cell viability at 100 µg/mL in previous experiments (data not shown), here, we treated the HaCaT keratinocytes with 50 µg/mL of PM10. As resulted, PM10 treatment increased the mRNA expression levels of IL-6, IL-8, IL-1α and IL-1β; however, 2FL significantly attenuated (p < 0.01) the increase in the expression of the pro-inflammatory cytokine genes (Fig. 2). These results suggested that 2FL exerted anti-inflammatory effects in PM10-induced HaCaT keratinocytes by suppressing the mRNA expression levels of IL-6, Il-8, IL-1α and IL-1β. Thus, our subsequent studies focused on exploring the molecular mechanism underlying this anti-inflammatory activity of 2FL.

HaCaT keratinocytes were treated with 10 or 100 µg/mL of 2FL and 50 µg/mL of PM10 for 4 h. (A) IL-6, (B) IL-8, (C) IL-1α and (D) IL-1β mRNA expression levels were measured by RT-qPCR. Results are expressed as mean ± S.D. (% control) of three independent experiments. ## p < 0.01 compared to the control.; ** p < 0.01 compared to the PM10-treated control.
To demonstrate the molecular mechanism by which 2FL decreases pro-inflammatory cytokine expression in PM10-treated HaCaT keratinocytes, the accumulation of HIF-1α was examined by immunoblotting and RT-qPCR. It has been reported that HIF-1α accumulation is involved in the regulation of IL-6, IL-8 and IL-1β in endothelial cells.24,25) Here, we examined whether the exposure to PM10 influenced the accumulation of HIF-1α level in HaCaT keratinocytes. As shown in Fig. 3A, PM10 treatment increased the HIF-1α protein levels in a dose-dependent manner, compared to that in the untreated control. Next, to assess the inhibitory effects of 2FL on the protein and mRNA levels of HIF-1α, HaCaT keratinocytes were stimulated with PM10 (50 µg/mL) in the presence or absence of 2FL (1, 10 and 100 µg/mL). As shown in Figs. 3B and C, the treatment of 2FL suppressed the PM10-induced protein and mRNA levels of HIF-1α for 4 h in a dose-dependent manner (10 and 100 µg/mL). In addition, to verify whether the accumulation and inhibition of HIF-1α protein are affected by protein degradation, we utilized cycloheximide (CHX), an inhibitor of protein synthesis, with treatment of PM10 to determine the protein levels of HIF-1α. CHX treatment did not affect the HIF-1α protein levels in PM10-treated keratinocytes. 2FL treatment with CHX showed further slightly reduction, however, there is no significant difference compared to 2FL treatment in the absence of CHX (Fig. 3D). These results imply that PM10 treatment significantly increased HIF-1α mRNA levels, nevertheless PM10 accumulated HIF-1α protein by inhibition of protein degradation and 2FL treatment mainly inhibited PM10-induced HIF-1α increase associated with HIF-1α stabilization.

(A) HaCaT keratinocytes were treated with different concentrations of PM10 (10–100 µg/mL) for 4h. HaCaT keratinocytes were treated with 2FL or LY294002 (10 µM) with PM10 (50 µg/mL) for 4 h and (B) protein level and (C) mRNA level were measured. (D) The cells were pretreated with PM10 (50 µg/mL) and 2FL (100 µg/mL) for 4h, followed by addition of CHX (40 µM) for 1h. The protein levels of HIF-1α were examined by immunoblotting. Equal protein loading was confirmed by staining the membrane with antibodies against β-actin and GAPDH. mRNA expression levels were measured by RT-qPCR. Results are expressed as mean ± S.D. (% control) of three independent experiments. ## p < 0.01 compared to the control.; * p < 0.05 and ** p < 0.01 compared to the PM10-treated control.
It was previously reported that the PI3K/Akt pathway mediates HIF-1α accumulation in keratinocytes.16) In order to determine whether PM10-induced HIF-1α accumulation is due to the PI3K/Akt pathway, we treated HaCaT keratinocytes with PM10 after a pretreatment with LY294002, a strong inhibitor of PI3K. The proteins were harvested, and the protein levels of HIF-1α, phospho-Akt, total Akt, phospho-PI3K, and total PI3K were measured by immunoblotting (Figs. 3B, 4B). PM10 exposure increased the phosphorylation levels of Akt and PI3K (Fig. 4A) and consistently, LY294002 caused a decline in the phosphorylation levels (Fig. 4B).

(A) HaCaT keratinocytes were treated with different concentrations of PM10 (10–100 µg/mL). The protein levels of phospho-Akt and phospho-PI3K were measured by immunoblotting. (B) The cells were pretreated with different concentrations of 2FL or LY294002 (10 µM) for 2 h, followed by treatment with PM10 (50 µg/mL) for 1 h. Equal protein loading was confirmed by staining the membrane with antibodies against β-actin. ## p < 0.01 compared to the control. * p < 0.05 and ** p < 0.01 compared to the PM10-treated control.
We subsequently evaluated the effects of 2FL on the PI3K/Akt pathway that lead to the decrease in HIF-1α accumulation. HaCaT keratinocytes were pretreated with 2FL or LY294002 for 2 h to examine the short-term response of PI3K/Akt phosphorylation and then treated with PM10 for 1 h. As shown in Fig. 4B, 2FL inhibited the increased phosphorylation levels of PI3K and Akt in a dose-dependent manner, compared with the PM10-treated control. These results implied that 2FL decreased the HIF-1α accumulation induced by PM10, via the decrease of PI3K/Akt pathway in HaCaT keratinocytes.
2FL Rescued the PM10-Induced Epidermal Layer Damage in the RHE ModelNext, to verify the effects of 2FL after PM10 treatment, we utilized the RHE model. The RHE was exposed to PM10 and incubated with 10 µg/mL of 2FL for 24 h. Then, the epidermal condition was measured by H&E staining. Morphological alterations caused by PM10 exposure in the epidermis resulted in a thickening of the cornified layer and a thinning of the epidermis in the RHE model, whereas 2FL shown to restore the epidermal thickness and integrity of the cornified layer (Fig. 5). Collectively, our in vitro and RHE model results support the possibility that 2FL may provide protective effects against the PM10-induced inflammatory response in the skin and skin morphological alterations.

The skins were treated with concentrations of 2FL (10 µg/mL) for 24 h following PM10 (50 µg/mL) treatment. H&E staining (A–C) was performed on the RHE and the epidermal thickness (D) was quantified by photo-analysis. The stained skin sections were observed and photographed under a light microscope. Magnification, ×200. ## p < 0.01 indicates a significant difference from the control. ** p < 0.01 indicates a significant difference from the PM10-treated control.
It has been recognized that various environmental factors affect human health. Among these factors, the concern over air pollutants has been increasing over the last decade, and WHO has proposed guidelines for limiting PM concentrations.2,26) Recent reports indicate that PM exposure exerts negative effects on human skin as well as lung tissue and the cardiovascular system. PM exposure is associated with skin inflammation and premature skin aging. In a prior study, PM exposure resulted in the generation of ROS, leading to mitochondrial damage and induction of pro-inflammatory cytokine expression in human keratinocytes.8,27) PM also carries organic chemicals known as PAHs, which are highly lipophilic and easily penetrate the skin, where they subsequently act as ligands for AhR.28,29) Benzo[a]pyrene, one of the type of PAHs, induces IL-8 production in human epidermal keratinocytes. In addition, IL-8 in the exposure to benzo[a]pyrene is expressed in inflammatory acne vulgaris skin more than normal skin.2,30)
There are several factors that respond to environmental changes in human cells. The protein level and activities of HIF-1α are regulated upon an O2-dependent post-translational modification, resulting in proteasomal degradation after exposure to increased O2 concentrations or various chemicals, such as Cobalt chloride (CoCl2) and o-phenanthroline.31,32) Accumulating evidence demonstrates that hypoxia induces inflammatory responses that are associated with the increase in HIF-1α stabilization. CoCl2, as a hypoxia mimicking agent, enhanced the overexpression of pro-inflammatory cytokines, such as IL-6, IL-8 and IL-1β, in response to hypoxic conditions.17,25) In a recent study, it was reported that dioxin (TCDD) induced HIF-1α stabilization mediated by AhR and ROS-dependent activation of PI3K/Akt pathway in human trophoblastic cells.13) Taken together, these studies suggest that there is a correlation between HIF-1α and PM, which is also a known ligand for AhR.
2FL, a major constituent of the HMO, offers a protective effect against infectious diseases. Dietary 2FL significantly decreased the mRNA expression levels of pro-inflammatory cytokine-related genes, such as IL-1α, IL-1β, TNF-α and CXCL-10 in human intestinal cells.18–21) In our previous study, 2FL reduced the UVB-induced mRNA expression levels of IL-6, IL-8 and MMP-1 known as collagenase, in Hs68 fibroblasts (data not shown). Together with our results, we hypothesized that 2FL may have an inhibitory effect on the PM10-induced inflammatory response in human keratinocytes.
In this study, we first evaluated whether PM10 induced the mRNA expression of pro-inflammatory cytokines and HIF-1α. The results showed that the mRNA expression levels of IL-6, IL-8, IL-1α and IL-1β were increased upon PM10 treatment in a dose-dependent manner (Fig. 1). In addition, PM10 also increased HIF-1α protein levels (Fig. 3A) similar to that observed with CoCl2 treatment (data not shown). As shown Fig. 3D, PM10-induced HIF-1α accumulation is mainly caused by increasing stabilization of HIF-1α. These results reveal that PM10 may act as a hypoxia-mimicking chemical (similar to CoCl2) in the regulation of HIF-1α degradation. Furthermore, to determine the inhibitory effects of 2FL on PM10-induced pro-inflammatory cytokines, HaCaT keratinocytes were treated with PM10 in the presence and absence of 2FL. The elevated mRNA expression levels of IL-6, IL-8, IL-1α and IL-1β were significantly decreased by 2FL treatment (Fig. 2). Moreover, PM10-induced HIF-1α protein accumulation was also inhibited by 2FL treatment (Fig. 3B). These results provide evidence that 2FL acts as an inhibitor of the inflammatory responses caused by PM stimulation in skin cells.
Accumulating studies demonstrate that the synthesis and induction of HIF-1α are stimulated through the activation of the PI3K/Akt pathway. Sun et al. reported that treatment of HaCaT keratinocytes with CoCl2, which induces a hypoxia-like condition, increased the cytotoxicity and inflammatory response via the accumulation of HIF-1α through the PI3K/Akt pathway.16) In the present study, 2FL treatment decreased PM10-induced stabilization and expression of HIF-1α and downregulated the phosphorylation of PI3K and Akt, similar to LY294002 (Figs. 3, 4B). As shown in Fig. 2, the treatment of 2FL (100 µg/mL) decreased the PM10 induced the pro-inflammatory cytokine gene expression, such as IL-6 and IL-8, up to non-treated control as well as suppressed the PI3K/Akt/HIF1 pathway shown in Figs. 3B and 4B. However, the mRNA expressions of IL-1α and IL-1β were still remains. Although it is well known that the effect of PM10 on inflammatory response mediated by PI3K/Akt/HIF1 axis, however, it is also reported that PM exposure increased pro-inflammatory cytokines, IL-1β, by regulating the Toll-like receptors (TLRs), leucine-rich repeat protein 3 (NLRP3) inflammasome and nuclear factor-κ-gene binding (NF-κB).33,34) Therefore, we assume that IL-1α and IL-1β gene expressions may partially inhibited with 2FL treatment through suppressing PI3K/Akt/HIF1 pathway and other pathways remained unaffected. Next, we evaluated the suppressive effect of 2FL on PM10 exposure using the RHE model.35,36) PM10 was shown to cause epidermal alterations, including thickening the cornified layer and thinning of the epidermis in the RHE model.
However, 2FL restored the thickness of the epidermis and the integrity of the cornified layer following PM10 exposure in the RHE model (Fig. 5). These results suggest that 2FL rescues the proliferation and abnormal differentiation processes in PM10-exposed keratinocytes, thereby alleviating conditions of the epidermal layer. Based on these results, we suggest further studies to evaluate the effects of 2FL on skin barrier function, including epidermal differentiation and stratum corneum hydration.
Taken together, a PM10-induced hypoxia mimicking environment resulted in an increase in the mRNA expression levels of pro-inflammatory cytokines, whereas 2FL treatment reduced IL-6, IL-8, IL-1α and IL-1β mRNA expression by inhibiting HIF-1α accumulation through the downregulation of the PI3K/Akt pathway. Moreover, 2FL treatment alleviated the PM10-induced skin epidermal alterations in the RHE model. Based on these results, 2FL can be used to alleviate skin inflammation and irritation by urban air pollution. However, further detailed investigation via a clinical trial with 2FL would help to evaluate its potential cosmeceutical effects.
The authors declare no conflict of interest.