2019 Volume 42 Issue 10 Pages 1733-1740
2019 Volume 42 Issue 10 Pages 1733-1740
The aim of this study was to clarify the relationship between chemotherapy-induced mucositis and endogenous glucagon-like peptide-2 (GLP-2) dynamics in the small intestine following treatment with either methotrexate or 5-fluorouracil. Rats were injected intraperitoneally with a single dose of either 50 mg/kg methotrexate or 100 mg/kg 5-fluorouracil. At 24 and 72 h after drug administration, ileal tissues and plasma were used to investigate GLP-2 dynamics. Administration of methotrexate caused moderate but not significant intestinal injury within 72 h, while administration of 5-fluorouracil caused severe injury in a time-dependent manner. Methotrexate significantly increased proglucagon mRNA expression and the number of anti-GLP-2 antibody-positive cells in the ileal tissue at 24 h after administration. Methotrexate also significantly induced GLP-2 receptor, insulin-like growth factor-1 (IGF-1), and transforming growth factor-β2 (TGF-β2) mRNA expression in ileal tissue. In contrast, 5-fluorouracil significantly inhibited proglucagon, GLP-2 receptor, IGF-1, and TGF-β2 mRNA expression as well as the number of anti-GLP-2 antibody-positive cells. Methotrexate slightly increased dipeptidyl peptidase IV (DPP-4) mRNA expression, whereas 5-fluorouracil significantly increased DPP-4 mRNA expression. These results suggest that potentiation of endogenous GLP-2 dynamics by methotrexate is associated with a mechanism that preserves gastrointestinal mucosal integrity at a moderate level.
Chemotherapy-induced mucositis in the gastrointestinal tract is one of the major adverse events of anticancer drug administration. Because severely impaired absorption and diarrhea can directly affect a patient’s QOL, countermeasures for mucositis are essential to ensure patient tolerance and improve the rate of success of chemotherapy. Chemotherapy-induced gastrointestinal mucositis is initiated by acute inflammatory mucosal damage, generation of reactive oxygen species,1) and the production of cytokines that lead to epithelial cell apoptosis.2) This is followed by the wound-healing phase with restoration and proliferation of the epithelium and barrier function.3,4) The degree of gastrointestinal injury differs according to the type of anticancer drug administered. Anticancer drugs, such as cisplatin and 5-fluorouracil, are known to cause severe gastrointestinal injury.
A recent study showed that the intestinal tolerance of chemotherapy-induced gastrointestinal injury is partly related to intestinal hormones such as glucagon-like peptide (GLP)-2. Proglucagon undergoes post-translational cleavage in the enteroendocrine L-cells that exist in the intestinal mucosal epithelium to form GLP-1 and GLP-2. GLP-2 is subsequently rapidly metabolized (within a few minutes) by dipeptidyl peptidase IV (DPP-4). Intestinal GLP-2 has multiple physiologic actions that are involved in maintaining homeostasis, including proliferation of crypt cells, barrier function, nutrient absorption, reduction of epithelial apoptosis, motility, anti-inflammation, and blood flow.5) These effects of GLP-2 are mainly manifested through the GLP-2 receptor in the subepithelial myofibroblasts. Insulin-like growth factor-1 (IGF-1) has been identified as the common mediator of multiple enterotrophic hormones. Intestine-derived IGF-1 is an essential mediator of GLP-2-induced intestinotrophic actions, such as inducing crypt cell proliferation.6) Transforming growth factor-β (TGF-β) has also been shown to be an important tool for demonstrating the function of GLP-2 in the small intestine.7) There are three homologous TGF-β isoforms in mammals: TGF-β1, TGF-β2, and TGF-β3. Recent studies demonstrated that TGF-β2 plays an important role in the maintenance of intestinal epithelial cells.8,9)
Methotrexate, a folate antagonist, is widely used for the treatment of various malignancies as well as autoimmune diseases. Generally, methotrexate-induced gastrointestinal mucositis is a moderately severe but common side effect in routine clinical use.10) We previously reported that the administration of methotrexate at a dose of 50 mg/kg in rats caused significant kaolin intake, which is indicative of emesis in rats, and hyperplasia of the enterochromaffin cells in the ileal tissue.11,12) Although a 50-mg/kg dose of methotrexate in rats is nearly equivalent to the dosage that associated with an increased risk of emesis in humans, there was no severe histologic injury due to the methotrexate observed in the ileal tissue at 96 h after the initial administration.11) Therefore, we hypothesized that the dynamic properties of endogenous GLP-2 in the small intestine are associated with tolerance to methotrexate-induced gastrointestinal injury. To evaluate this hypothesis, in the present study we compared the effect of methotrexate and 5-fluorouracil administration on endogenous GLP-2 dynamics in the rat small intestine.
Methotrexate and 5-fluorouracil were obtained from Pfizer Co., Ltd. (Tokyo, Japan) and Kyowa Hakko Kirin Co., Ltd. (Tokyo, Japan), respectively. All other reagents used in this study were of special grade and purchased from local suppliers unless otherwise noted.
AnimalsMale Wistar rats weighing 180–200 g were purchased from Japan SLC, Inc. (Shizuoka, Japan). All animals were housed under constant conditions at room temperature of 22 ± 2°C and humidity of 50 ± 10% with a regular 12-h light–dark cycle (8:00–20:00, 20:00–8:00, respectively) and free access to water and food. The animal experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals by the Animal Research Committee of Health Sciences University of Hokkaido.
Rats were injected intraperitoneally with a single dose of 50 mg/kg methotrexate, 100 mg/kg 5-fluorouracil, or physiological saline (control). At 24 and 72 h after the administration of the drugs/saline control, rats were killed by exsanguination under light anesthesia using isoflurane. Ileal tissues were dissected in approximately 3-cm-long segments and frozen rapidly in liquid nitrogen and stored until further analysis. Fresh ileal tissues were also fixed with 4% paraformaldehyde in 0.1 M phosphate buffer and embedded in paraffin for immunohistochemical analysis. Blood samples were collected into plastic tubes containing 500 KIU aprotinin and ethylenediaminetetraacetic acid (EDTA) disodium. After preparing plasma from blood samples by centrifugation at 1600 × g for 20 min, GLP-2 and IGF-1 plasma levels were measured.
Immunohistochemical AnalysisThe expression and localization of GLP-2 in rat ileum were analyzed by immunohistochemistry. After deparaffinization, the specimens were treated with methanol containing 0.3% hydrogen peroxidase to suppress endogenous peroxidase activity and allowed to react with anti-GLP-2 polyclonal antibody (Bioss Antibodies, Woburm, MA, U.S.A.) diluted in antibody diluent (Dako, Carpinteria, CA, U.S.A.) at 4°C overnight. Incubation with antibody diluent alone served as the negative control. Sections were then incubated with anti-rabbit immunoglobulin G antibody labeled with Histofine Simple Stain MAX PO (Multi) peroxidase (Nichirei Corp., Tokyo, Japan). The reaction products were visualized with 3,3′-diaminobenzidine tetrahydrochloride (Nichirei Corp.). The number of anti-GLP-2 antibody-positive cells was counted under a light microscope. Only the antibody-positive spindle cells located in the epithelial cell line of the villus and crypt were judged to be L-cells. The cells located within the width of 20 villi were counted in two different fields of view and a mean count was determined for each specimen. The expression and localization of myeloperoxidase in the rat ileum were also analyzed by immunohistochemistry, as previously described.13)
RNA Extraction and RT-PCRMessenger RNA expression was quantified by real-time RT-PCR using a 7500 Fast Real-Time PCR system (Life Technologies, Applied Biosystems, Foster City, CA, U.S.A.) and PrimeScript™ One Step RT-PCR kit (TaKaRa Bio, Shiga, Japan), as previously described.14) The following sense and antisense primers, respectively, were used: proglucagon, 5′-AAC AAC ATT GCC AAA CGT CA-3′ and 5′-CAA TGA ATT CCT TTG CTG CC-3′; GLP-2 receptor, 5′-CGG ATT CTG GAA ATT CTT C-3′ and 5′-CCC CAG GAA CCG GAA ATT CTT C-3′; IGF-1, 5′-AAG CCT ACA AAG TCA GCT CG-3′ and 5′-GGT CTT GTT TCC TGC ACT TC-3′; DPP-4, 5′-TCC CAA CTC CAG AGG ACA AC-3′ and 5′-CAG GGT TTG GAG TCT GAG-3′; TGF-β1, 5′-TGG CGT TAC CTT GGT AAC C-3′ and 5′-CGT GTT GAG CCC TTT CCA G-3′; TGF-β2, 5′-ATC GAT GGC ACC TCC ACA TAT G-3′ and 5′-GCG AAG GCA GCA ATT ATG CTG-3′; TGF-β3, 5′-AAG CGC ACA GAG CAG AGA ATC-3′ and 5′-AGT GTC AGT GAC ATC GAA G 3′; β-catenin, 5′-GCC AAG TGG GTG GCA TAG A-3′ and 5′-TCC CTG TCA CCA GCA CGA A-3′; the exchange protein activated by cAMP 1 (Epac1), 5′-GTG TTG GTG AAG GTC AAT TCT G-3′ and 5′-CCA CAC CAC GGG CAT C-3′; Epac2, 5′-TGT TAA AGT GTC TGA GAC CAG CA-3′ and 5′-AAA GGC TGT CCC AAT TCC CAG-3′; and glyceraldehyde 3-phosphate dehydrogenase, 5′-ATG TTC CAG TAT GAC TCC ACT CAC G-3′ and 5′-GAA GAC ACC AGT AGA CTC CAC GAC A-3′. The PCR products were calculated relative to glyceraldehyde 3-phosphate dehydrogenase.
Measurement of Plasma GLP-2 and IGF-1 LevelsBlood samples were collected into tubes containing EDTA and a protease inhibitor cocktail (BD™ P700; Becton Dickinson and Company, Tokyo, Japan) and then centrifuged to obtain plasma. GLP-2 and IGF-1 plasma concentrations were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits for GLP-2 (AssayMax GLP-2 ELISA Kit; Assaypro LLC, Charles, MO, U.S.A.) and IGF-1 (Quantikine ELISA; R&D Systems, Minneapolis, MN, U.S.A.). Measureable ranges were defined as 0.28–72 ng/mL for GLP-2 and 30–2000 pg/mL for IGF-1. Intra- and inter-assay precisions for each substrate were less than 9%.
Statistical AnalysisStatistical analysis of the results was carried out using the F-test followed by Student’s t-test or Welch’s t-test. p Values less than 0.05 were considered significant.
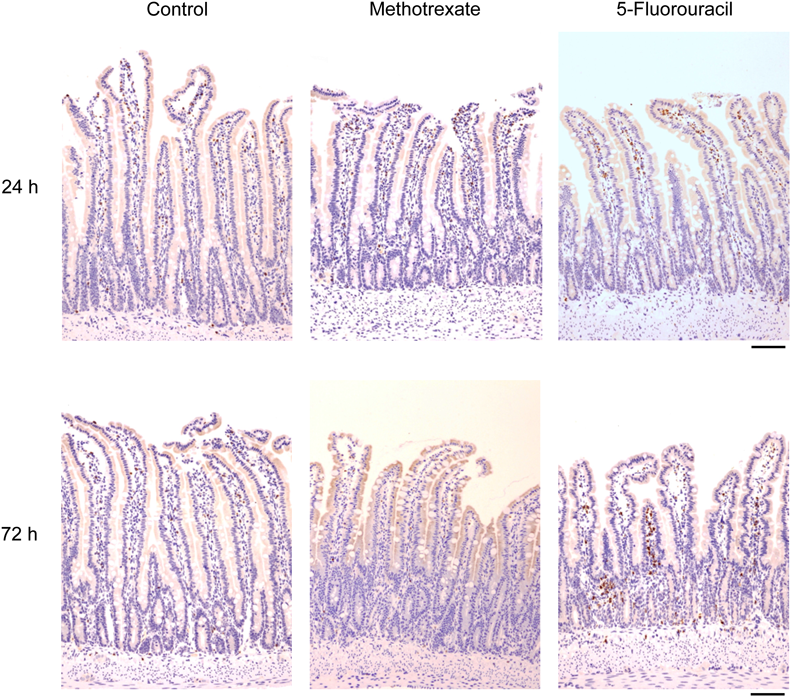
Methotrexate had no effect on the morphologic changes at 24 h after a single administration (Fig. 1). Although methotrexate caused moderate mucosal injury that was characterized by atrophy of the villus and disturbance of the epithelial line arrangement and architecture at 72 h after a single administration, histologic injury with an inflammatory response characterized by edema, hyperemia, or leukocyte infiltration was not clearly documented (Fig. 1). By contrast, 5-fluorouracil caused dramatic morphologic changes in a time-dependent manner (Fig. 1). Indeed, 5-fluorouracil caused severe regional inflammation that was characterized by edema, hyperemia, or leukocyte infiltration, along with disturbances of the epithelial architecture in the ileal mucosa at 72 h after a single administration (Fig. 1).

At 24 or 72 h after a single intraperitoneal administration of saline (control), 50 mg/kg methotrexate, or 100 mg/kg 5-fluorouracil, ileal tissues were isolated and fixed with 4% paraformaldehyde and embedded in paraffin. Scale bars = 100 µm. (Color figure can be accessed in the online version.)
Immunohistochemical analysis of the expression of myeloperoxidase, an enzyme marker for inflammatory cells, demonstrated that methotrexate had no effect on myeloperoxidase expression at 24 or 72 h after a single administration (Fig. 2). In contrast, 5-fluorouracil increased myeloperoxidase expression within 24 h after the administration of a single dose, and then further enhanced expression in a time-dependent manner for up to 72 h (Fig. 2). Since clear differences in intestinal morphology and injury were observed at 24 h after the administration of methotrexate and 5-fluorouracil, we focused on clarifying the effect of these drugs on GLP-2 dynamics at 24 h after initial administration.
At 24 or 72 h after the administration of saline (control), 50 mg/kg methotrexate, or 100 mg/kg 5-fluorouracil, ileal tissues were isolated and fixed with 4% paraformaldehyde for immunohistochemical examination with an anti-myeloperoxidase antibody. Scale bars = 100 µm. (Color figure can be accessed in the online version.)
The effects of methotrexate and 5-fluorouracil on the number of cells that express GLP-2 in the ileal mucosa were determined by immunohistochemical analysis. As shown in Figs. 3A, B, almost all of the GLP-2-expressing cells were localized in the epithelial cell line and crypts. The number of GLP-2-expressing cells was significantly increased at 24 h after a single administration of methotrexate (Figs. 3A, C), whereas it was significantly inhibited after a single administration of 5-fluorouracil (Figs. 3B, D). In addition, proglucagon mRNA expression was increased by methotrexate whereas it was significantly inhibited by 5-fluorouracil (Figs. 4A, B). In contrast to the observed change in GLP-2 dynamics in the ileal tissue, the plasma GLP-2 concentration was not significantly affected by either methotrexate or 5-fluorouracil (Figs. 4C, D).

A and B: At 24 h after the administration of saline (control), 50 mg/kg methotrexate, or 100 mg/kg 5-fluorouracil, ileal tissues were isolated and fixed with 4% paraformaldehyde for immunohistochemical examination using an anti-GLP-2 antibody. Arrows indicate GLP-2-expressing cells in the ileal mucosa. Magnification of the square with a dotted line is shown at the top left of each photo. Scale bars = 100 µm. C and D: Each column represents the mean ± standard error (S.E.) (n = 5 each for C and 6 each for D). Statistical analysis of the results was carried out using Welch’s t-test. * p < 0.05 versus control. (Color figure can be accessed in the online version.)

Each column represents the mean ± S.E. (n = 6 each for A and C; n = 6 for control and 5 for 5-fluorouracil for B and D). Statistical analysis of the results was carried out using either Student’s t-test (A–C) or Welch’s t-test (D). * p < 0.05 versus control.
We then investigated GLP-2 receptor mRNA expression in the ileal tissue. The mRNA expression was increased by methotrexate but significantly inhibited by 5-fluorouracil (Figs. 5A, B). Although there was a tendency toward increased DPP-4 mRNA expression in the ileal tissue at 24 h after methotrexate administration, this increase was not statistically significant (p < 0.1; Fig. 5C). DPP-4 mRNA expression was significantly increased at 24 h after 5-fluorouracil administration (Fig. 5D).

Each column represents the mean ± S.E. (n = 5 each for A; n = 5 for control and 6 for 5-fluorouracil for B and D; n = 6 for control and 5 for methotrexate for C). Statistical analysis of the results was carried out using Student’s t-test. * p < 0.05 versus control.
We also investigated the effects of methotrexate and 5-fluorouracil on several major factors associated with the expression of GLP-2. IGF-1 mRNA expression was increased by methotrexate but was significantly inhibited by 5-fluorouracil (Figs. 6A, B). Methotrexate had no effect on the plasma IGF-1 concentration (Fig. 6C), whereas 5-fluorouracil significantly reduced plasma IGF-1 (Fig. 6D).

Each column represents the mean ± S.E. (n = 6 each for A, B, and C; n = 5 each for D). Statistical analysis of the results was carried out using either Student’s t-test (A and B) or Welch’s t-test (C and D). ** p < 0.01, * p < 0.05 versus control.
Methotrexate significantly increased TGF-β2 but not TGF-β1 or TGF-β3 mRNA expression (Figs. 7A, C, E). Administration of 5-fluorouracil significantly increased TGF-β1 mRNA expression, whereas it significantly inhibited TGF-β2 mRNA expression (Figs. 7B, D); 5-fluorouracil also had no effect on TGF-β3 mRNA expression (Fig. 7F).

Each column represents the mean ± S.E. (n = 6 each for A, C, and E; n = 6 for control and 5 for 5-fluorouracil for B, D, and F). Statistical analysis of the results was carried out using either Student’s t-test (A–E) or Welch’s t-test (F). * p < 0.05 versus control.
Methotrexate increased β-catenin mRNA expression (Fig. 8A), whereas 5-fluorouracil had no significant effect (Fig. 8B).

Each column represents the mean ± S.E. (n = 6 each for A; n = 6 for control and 5 for 5-fluorouracil for B). Statistical analysis of the results was carried out using either Student’s t-test (A) or Welch’s t-test (B). * p < 0.05 versus control.
Methotrexate significantly increased the exchange protein activated by cAMP 1 (Epac1) and Epac2 mRNA expression (Figs. 9A, C). 5-Fluorouracil had no effect on these expression (Figs. 9B, D).

Each column represents the mean ± S.E. (n = 6 each for A, C; n = 6 for control and 5 for 5-fluorouracil for B, D). Statistical analysis of the results was carried out using either Student’s t-test (A and D) or Welch’s t-test (B and C). * p < 0.05 versus control.
In this study we demonstrated that methotrexate potentiated GLP-2 dynamics in the rat small intestine at 24 h after a single administration. Although methotrexate caused slight morphologic change in the ileal tissue in a time-dependent manner, there was no severe histologic injury due to methotrexate, which is consistent with our previous findings.11) In contrast to the present results, consecutive administration of methotrexate at even lower dose is generally found to cause severe intestinal injury in rats.15–17) Hirotani et al.15) demonstrates that methotrexate administered orally for 6 consecutive days at a dose of 5.0 mg/kg/d significantly causes body and mucosal weight loss but increases plasma GLP-2 level. Therefore, it is possible that a consecutive administration has a cumulative effect to cause more severe intestinal injury than a single administration. Furthermore, the fact that plasma GLP-2 concentration did not alter under our experimental conditions is consistent with the suggestion that the circulating levels of GLP-2 may reflect in the pathological states of injured intestine by Hirotani et al.15) On the other hand, a 100 mg/kg dose of 5-fluorouracil caused significant mucositis in a time-dependent manner. The dose of 5-fluorouracil used in the present study was rather low, considering that a 400-mg/kg dose is usually chosen when attempting to produce 5-fluorouracil-induced mucositis in rats.18,19) We also confirmed that a 100-mg/kg dose of 5-fluorouracil significantly reduced body weight, which implied that there was a decrease in the QOL (data not shown), although no obvious diarrhea was observed within 72 h after administration of 5-fluorouracil.
Kissow et al.18) demonstrated that an analog of GLP-2 significantly inhibited the mucositis induced by 5-fluorouracil administration in rats. This GLP-2 analog has also been reported to counteract the mucositis caused by chronic cisplatin treatment in mouse gastric fundus.20) Furthermore, the combination of biguanide, which causes secretion of both GLP-1 and GLP-2 from L-cells, and a DPP-4 inhibitor was shown to prevent the 5-fluorouracil-induced reduction of rat small intestine weight due to enhancement of the endogenous GLP-2 level.21)
We also found that 5-fluorouracil dramatically reduced GLP-2 expression in the rat intestine, which supports the results reported by Kissow et al.18) Furthermore, we showed that DPP-4 mRNA expression was significantly potentiated by 5-fluorouracil. However, since methotrexate also tended to increases DPP-4 mRNA expression, further studies are required to determine whether alteration of DPP-4 mRNA expression in the intestine affects the endogenous GLP-2 availability. In contrast, methotrexate significantly potentiated GLP-2 protein and mRNA expression at 24 h. Since we determined the GLP-2 protein in the present study based on the number of anti-GLP-2 antibody-positive cells, our results suggest that methotrexate increases the number of L-cells. Our previous studies also showed that methotrexate increased the number of enterochromaffin cells.11,12) Like enterochromaffin cells, L-cells originate from pluripotent stem cells in the depths of crypts, and migrate upward from the crypts onto the surfaces of the villi.22) Considering the present results with our previous report,12) our results indicate that methotrexate caused a transient stimulation of intestinal stem cells to generate fully differentiated cell types within 24 h after administration. The mechanisms and mediators by which methotrexate induces hyperplasia of L-cells in the small intestine remain to be clarified. Because neither methotrexate nor 5-fluorouracil had any significant effect on the plasma GLP-2 concentration, it appears that these anticancer drugs affect GLP-2 dynamics at a local site.
GLP-2 receptor is located in gut enteroendocrine cells in the small bowel epithelium and enteric neurons in humans as well as in rats.23,24) GLP-2 receptor mRNA expression was significantly increased by methotrexate but significantly decreased by 5-fluorouracil, suggesting that administration of these anticancer drugs has an effect downstream of the GLP-2 receptor. It has also been reported that GLP-2 receptor mRNA expression correlates with endogenous GLP-2 levels in mice.25) GLP-2 was shown to increase IGF-1 mRNA expression and secretion in intestinal cultures along with increasing the expression of IGF-1 mRNA in the mouse small intestine in vivo.26)
In this study, we found that IGF-1 mRNA expression was increased by methotrexate, but decreased by 5-fluorouracil. Therefore, changes in the endogenous GLP-2 levels by anticancer drugs may directly affect the IGF-1 expression in the small intestine. Furthermore, we also observed decreased plasma IGF-1 levels in the 5-fluorouracil-treated rats. From a cancer treatment perspective, a reduction in 5-fluorouracil-induced IGF-1 in the blood may contribute to suppressing the growth of cancer cells. TGF-β is also known to be an essential factor for demonstrating the function of GLP-2 in the small intestine.7) For example, TGF-β has been reported to be a potent inhibitor of epithelial cell growth.27,28) Thus, it is able to minimize intestinal damage and facilitate regeneration after mucositis. Indeed, GLP-2-induced TGF-β and vascular endothelial growth factor have been reported to be involved in the migration of mucosal epithelial cells associated with mucosal regeneration.7) Although Bulut et al.7) showed that GLP-2 treatment increased TGF-β1 protein and mRNA expression in human colonic fibroblasts (CCD-18Co cells), we showed that 5-fluorouracil, but not methotrexate, had a significant effect on TGF-β1 mRNA expression. Because 5-fluorouracil-induced intestinal injury has already been shown at 24 h, the upregulation of TGF-β1 might be caused by the accumulation of thrombocytes and immunocytes responding to inflammation because TGF-β1 induces the repair process in conjunction with inflammation.
Recent studies have shown that TGF-β2 has a more important role than TGF-β1 in the maintenance of intestinal epithelial cells.8,9) We demonstrated that TGF-β2 mRNA expression is correlated with GLP-2 dynamics in the intestine. This may, at least in part, contribute to upregulation by methotrexate of the turnover of the small intestinal epithelial cells. In addition, we demonstrated that β-catenin mRNA expression was upregulated by methotrexate administration. It is well known that the Wnt/β-catenin signaling pathway plays a pivotal role in the development and homeostatic self-renewal of stem cells, including gastrointestinal tissue.29,30) Ben-Lulu et al.8) showed that supplementation of TGF-β2 in rats elevated the intestinal β-catenin protein levels. Moreover, IGF-1 that is activated by GLP-2 is known to activate β-catenin signaling.31) Therefore, upregulation of both IGF-1 and TGF-β2 by endogenous GLP-2 may activate the Wnt/β-catenin signaling pathway in the methotrexate-treated rat intestine.
Multiple mechanisms are involved in the pharmacologic effect of methotrexate, including folate antagonism and adenosine signaling potentiation. When used to treat rheumatoid arthritis, the pharmacologic mechanism of methotrexate is thought to involve an increase in the extracellular adenosine levels due to blocking of 5-aminoimidazole-4-carboxamide, which then blocks adenosine deaminase.32) Furthermore, activation of the adenosine A2A receptor by increased adenosine causes the accumulation of intracellular cAMP. Subsequently, this leads to the activation of enzymes such as protein kinase A and Epac1/2. Proglucagon gene transcription in L-cells is stimulated by the protein kinase A pathway through a cAMP response element.33) Lotfi et al.34) reported that Epac2 was expressed in intestinal L-cells. Furthermore, Epac signaling has been shown to be involved in glucagon production and secretion.35,36) Since we found that methotrexate significantly increased Epac1/2 mRNA expression in rat small intestine, it is speculated that A2A receptor-cAMP-Epac signaling has a role in the upregulation of intestinal GLP-2 pharmacodynamics by methotrexate. Further studies will be needed to clarify the precise mechanisms involved.
In conclusion, methotrexate potentiated GLP-2 dynamics in rat small intestine at 24 h after a single administration. Potentiation of endogenous GLP-2 dynamics by methotrexate may have a role in the tolerance to gastrointestinal injury by counteracting the mucosal injury induced by methotrexate itself.
The authors declare no conflict of interest.