2019 Volume 42 Issue 12 Pages 2024-2037
2019 Volume 42 Issue 12 Pages 2024-2037
Assays using lysate reagents prepared from horseshoe crab hemocyte extract (limulus amoebocyte lysate, LAL) are commonly and widely used to detect and measure endotoxin in parenteral drugs and medical devices. However, lysate reagents suffer from lot-to-lot variations leading to possible fluctuations in testing. Also, this continued usage of lysate reagents leads to the possible decline of the horseshoe crab population. Recently, a new recombinant chromogenic reagent, PyroSmart, consisting of three recombinant factors was introduced to the market. There are now three recombinant products; two with recombinant factor C reagents and PyroSmart with the complete recombinant LAL system. We evaluated the applicability of the reagent to the harmonized bacterial endotoxins test in the United States, European and Japanese pharmacopeias. The recombinant product showed equivalent potency of thirteen endotoxins from different bacterial strains to conventional chromogenic lysate reagents as long as their assay modes are identical. All analytical characteristics or assay parameters of the reagent satisfied the acceptance criteria which are set for the use for the bacterial endotoxins test filed in the pharmacopeias. All of 109 parenteral drugs tested can be measured with PyroSmart within respective maximum allowable dilutions. The lot-to-lot variation in recovery of endotoxin added in the parenteral drugs for PyroSmart was equal to or less than those of six limulus lysate reagents. In conclusion, the present study suggests that the recombinant reagent, PyroSmart, provide a good alternative to the LAL reagents with better lot-to-lot variation.
Parenteral drugs are strictly managed by pharmacopeias in all regions, including the United States, the European Union, and Japan, to ensure their quality and to avoid any health hazards. In particular, the level of pyrogen must be controlled as it potentially causes life-threating septic shock.1–5) Bacterial endotoxin, (also called lipopolysaccharide; LPS), of Gram-negative bacteria is the pyrogen of main concern to the parenteral drugs in pharmaceutical factories as it is a common contaminant and shows powerful pyrogenicity.1,2)
Bacterial endotoxins are detected and quantified by lysate reagents, which are prepared from amoebocyte extracts (limulus amoebocyte lysate; LAL) from a natural resource; horseshoe crab.6–8) Lysate reagents are composed of three protease zymogens, factor C, factor B, and the proclotting enzyme, as well as a coagulogen to form the cascade reaction.9) The test using limulus lysate reagents is called Bacterial Endotoxins Test (BET), and is filed in the pharmacopeias.10–12)
The reliance of the lysate reagents on a natural resource implies three issues. The first is the potential decrease in the population of the horseshoe crab due to harvesting by commercial fishing to bait traps for eels and conch.13–15) The second is the lot-to-lot variation of limulus lysate reagents possibly due to geographical and seasonal differences.16–18) The third is a nonspecific reaction derived from an alternative factor G pathway in LAL, reacting with (1 → 3)-β-D-glucan.8,9) This factor G pathway may lead to a false-positive test result. To address these issues, some products have been marketed, including PyroGene and EndoZyme, which use recombinant factor C alone and a fluorogenic substrate.19,20) PyroSmart is characterized by three recombinant factors including factor C, factor B and the proclotting enzyme and a chromogenic substrate.21)
Several reports regarding evaluations of PyroGene have been published.20,22,23) In these evaluations the equivalency of the endotoxin levels in some parenteral drugs by PyroGene and the current limulus lysate reagents was discussed. However, equivalency between all currently available recombinant reagent products and the existing limulus lysate reagents in measuring endotoxin levels in the many parenteral drugs listed in pharmacopeias has not been fully investigated.
In this report, the potencies of various rough and smooth forms of endotoxins from different bacterial strains using PyroSmart were compared against six lysate reagents. In addition, we assessed the performance of PyroSmart’s analytical characteristics. Based on the results obtained, the applicability of the PyroSmart to BET is shown. The equivalency of measuring endotoxin with PyroSmart and current limulus lysate reagents is discussed.
Reference standard endotoxin of the U.S. Pharmacopeia (USPRSE) and the Japanese Pharmacopoeia (JPRSE) was purchased from the U.S. Pharmaceutical Convention (MD, U.S.A.) and the Pharmaceutical and Medical Device Regulatory Science Society of Japan (PMRJ; Osaka, Japan), respectively. Endotoxins from Escherichia coli O55:B5, E. coli O111:B4, Salmonella minnesota R595 Re, S. typhimurium were purchased from List Biological Laboratory, Inc. (CA, U.S.A.). Endotoxins from E. coli O127:B8 and Shigella flexneri were from BD Difco (MI, U.S.A.). Endotoxins from E. coli O128:B12, E. coli J5, E. coli F583 Rd2, S. typhosa and Pseudomonas aeruginosa 10 (purified by gel filtration) were from Sigma-Aldrich (MO, U.S.A.). Pachyman, (1 → 3)-β-D-glucan (BG), was prepared from Poria cocos.13) Water for injection purchased from Otsuka Pharmaceutical Factory, Inc. (Tokushima, Japan) was used as water for the BET. Kinetic-QCL and PyroGene were purchased from Lonza (MD, U.S.A.), EndoZyme and EndoZyme II were purchased from bioMérieux (Munich, Germany), Limulus ES-II and Limulus Color KY Test were obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan), Endochrome-K was purchased from Charles River Laboratories (MA, U.S.A.) and Pyrochrome with Glucashield buffer, Endospecy ES-50M and PyroSmart were obtained from Seikagaku Corporation (Tokyo, Japan).
Endotoxin AssayMeasurements of endotoxins with the lysate reagents, Kinetic-QCL, Limulus ES-II, Limulus Color KY Test, Endochrome-K, Endospecy ES-50M and Pyrochrome reconstituted with Glucashield buffer, and also the recombinant reagents, PyroGene, EndoZyme and PyroSmart, were performed according to their Instruction for Use (IFU). Limulus ES-II is turbidimetric, PyroGene and EndoZyme are fluorogenic, and all the other reagents are chromogenic reagents. Both rate and onset time assays are the method to measure the rate of color development of the reaction mixture and to measure the time (onset time) needed to reach a predetermined absorbance, respectively. To calculate the kinetics, an onset time assay was used for Kinetic-QCL, Limulus ES-II, Limulus Color KY Test, Endochrome-K and Pyrochrome, and rate assay for Endospecy ES-50M, and endpoint assays were employed for PyroGene and EndoZyme, and both rate and onset time assays were recorded for PyroSmart.
Potencies of Various Endotoxins with Lysate Reagents and Recombinant ReagentsUSPRSE was used as reference standard and was diluted to prepare a series of standard solutions of 0.1, 0.05, 0.025, 0.0125, and 0.00625 endotoxin unit (EU)/mL and a series of standard solutions of 50, 5, 0.5, 0.05 and 0.005 EU/mL for rate and onset time assay mode, respectively. Endotoxins derived from Escherichia coli O55:B5, E.coli O111:B4, E.coli O127:B8, E.coli O128:B12, E.coli J5, Shigella flexneri and Salmonella enterica, were dissolved into water. The endotoxins from E. coli F583 Rd2, S. minnesota R595 Re, S. typhrimrium, S. typhosa and Pseudomonas aeruginosa 10, were dissolved into 0.1%(v/v) triethylamine (TEA). Subsequently, the dissolved endotoxins were diluted with water for BET to obtain a two-fold or ten-fold dilution series for tests in the endotoxin assays with various reagents. The potency, EU/ng, of each dilution of endotoxins was calculated by dividing EU/mL by concentration, ng/mL at each dilution of endotoxins. The mean potencies of endotoxins were calculated by averaging the potencies of endotoxin dilutions within the range of the standard solutions.
Assessments of the Analytical Characteristics of the ReagentWe evaluated PyroSmart with respect to analytical characteristics, accuracy, precision, specificity, quantitation limit, linearity, and range, which are required to be evaluated in quantitative tests for impurities’ content in the ICH Q2 guideline.24) We used both rate and onset time assay modes to assess the analytical characteristics of the recombinant chromogenic reagent. The acceptance criteria described in the Guideline on Bioanalytical Method (Ligand Binding Assay) Validation in Pharmaceutical Development,25) which is used to evaluate measuring methods of analytes in a given biological matrix was referred since the ICH Q2 guideline as well as the Japanese Pharmacopoeia (JP), United States Pharmacopeia (USP), European Pharmacopoeia (EP), and their related guidelines do not describe any acceptance criteria on repeatability, intermediate precision, reproducibility, and quantitation limit. The specificity was assessed based on the reactivity of the reagent with BG, which is well-known as a false positive substance to lysate reagents. We assessed these analytical characteristics except for specificity by changing three factors, analyst, equipment, and reagent lot. In practice, two analysts used two microplate readers and three lots of the reagent to conduct independently a total of 12 measurements.
Linearity—For the rate assay, a series of JPRSE dilutions of 0.1, 0.05, 0.025, 0.0125, and 0.00625 EU/mL were prepared and then measured in six replicates with the reagent. For the onset time assay, a series of USPRSE dilutions of 50, 5, 0.5, 0.05, and 0.005 EU /mL were prepared and then measured in eight replicates with the reagent. According to the regression analysis, the correlation coefficients for the rate and onset time assay modes were determined based on the results of twelve measurements.
Accuracy—Accuracy was assessed by calculating the recoveries of endotoxin based on the average of concentrations of endotoxin that had been measured in six replicates and eight replicates according to the rate and onset time methods, respectively, at each concentration of endotoxin on the regression line in the twelve measurements.
Repeatability—The coefficient of variation (CV) was calculated based on the concentration of endotoxin that was measured at each endotoxin concentration on the regression line in the twelve measurements described in the section of linearity assessment according to the rate and onset time assay, respectively.
Intermediate precision—The standard deviation (SD) was calculated based on twelve measurements in the section of linearity assessment in the rate assay mode, while the SD was calculated from each logarithmically converted value of the endotoxin concentration on the regression line in the twelve measurements described in the section of linearity assessment in the onset time mode. The 90% confidence interval (CI) for CV was calculated according to the following equation: [(n − 1) SD2/χ2 (n − 1, 0.05) ≤ σ2 ≤ (n − 1) SD2/χ2 (n − 1, 0.95)], where “σ” expresses the population standard deviation.
Reproducibility—Reproducibility was assessed in the onset time assay mode using a total of eighteen measured concentrations of endotoxin in two different laboratories consisting of the twelve measurements described in the section for linearity assessment and further six measurements in another institution, where one analyst measured three lots of the reagent with one microplate reader at two different days. Subsequently, SD was calculated from the abovementioned all eighteen measurements, and the 90% CI for the CV was calculated as described above. Furthermore, the eighteen endotoxin concentrations were converted logarithmically, and two-way repeated measures ANOVA was made (variable 1: two institutions, variable 2: five concentrations of endotoxin).
Range—The range is the interval between the upper and lower concentration of endotoxin in the sample for which it has been demonstrated that the analytical procedure has acceptable levels of accuracy, precision (repeatability and intermediate precision), and linearity.24)
Quantification limit (QL)—In the rate assay mode QL was calculated based on the slope of the standard curve around QL and on SD of the absorbance change rates in the water for BET as the blank sample: (QL = 10 SD/slope). A series of JPRSE dilutions of 0.01, 0.005, 0.0025 and 0.00125 EU/mL were prepared. The series of JPRSE dilutions and blank sample were measured in five and ten replicates, respectively. Three measurements with three PyroSmart lots—a total of nine measurements—were conducted. The 95% CI for the mean of the QLs was calculated. In the onset time, QL was determined as a minimum concentration where the acceptance criteria were satisfied for both accuracy and precision among endotoxin concentrations described above.
Specificity—To verify interference by (1 → 3)-β-D-glucan (BG), two series of USPRSE dilutions (0.1, 0.05, 0.025 and 0.0125 EU/mL) with and without the addition of pachyman as BG (5 µg/mL)26) were measured in three replicates, and the mean of the endotoxin concentrations were calculated. Regression analysis was conducted on measured endotoxin concentrations with and without BG by using three lots of PyroSmart.
Recovery of JPRSE added into various parenteral drugs was also assessed as one item of specificity. Endotoxin was added to the previously diluted drugs as well as water for the BET setting their endotoxin concentration as the middle point of standard solutions. The recoveries of the endotoxin added to the diluted drugs were calculated from the concentration found in the endotoxin-added drugs after subtracting the endotoxin concentration found in the drugs without addition. Diluted drugs under this test are judged to be free from interfering factors when the calculated recoveries of the endotoxins added to diluted drugs are within 50 and 200%. Minimum dilution factors are expressed as the Non-Interfering-Dilution (NID) where the drugs show neither inhibition nor enhancement in the test. In onset time assay, measurement time and threshold absorbance (onset OD) were optimized to appropriately measure endotoxins in the drugs.
The equivalence of product performance between a recombinant chromogenic reagent, PyroSmart, and limulus lysate reagents was evaluated from potencies of the endotoxins from thirteen strains of Gram-negative bacteria using either the rate or the onset time assay. These data are shown in Fig. 1. All potencies of thirteen endotoxins with PyroSmart fell in the range between 50% of minimum and 200% of maximum of those tested with the three limulus lysate reagents. The endotoxins that were dissolved initially in TEA, specifically from E. coli strain F583 Rd2 and the S. minnesota strain R595 Re which has a short-sugar chain structure (rough form), showed relatively large reagent-to-reagent variability when compared with other endotoxins. On the other hand, the relative potencies of JPRSE with a long-sugar chain structure (smooth form) which had been well purified and dissolved in water for the BET were almost equivalent using PyroSmart and limulus lysate reagents. All of the CVs which were calculated from the potencies of endotoxins with the three lots of PyroSmart were below 10%, except for Pseudomonas aeruginosa 10 (12.7%).

Reactivity is expressed as EU/ng using USP reference standard endotoxin (RSE) as a reference. Error bars indicate standard deviation in the results of 3 lots.
In addition, the potencies of endotoxins measured with two kinds of reagents were compared in regression analysis. The analysis was done after potencies were transformed logarithmically in nine combinations (combinations A to F, between PyroSmart and lysate reagents; combinations G to I, among lysate reagents; Table 1, Supplementary Fig. 1). In the case of combination A (PyroSmart and Endospecy; both in the rate assay mode) and combination E (PyroSmart and Kinetic-QCL; both in the onset time assay mode) the 95% CI of the slope included “1,” and the 95% CI for the y-intercept included “0,” indicating that the potencies of thirteen endotoxins that were measured with PyroSmart and lysate reagents in combinations A and E are statistically equivalent. In addition, the correlation coefficients in the combinations A and E were 0.936 and 0.947, respectively, showing good correlation in the two combinations.
| Combination | X Axis | Y Axis | Slope | Y-Intercept | Correlation coefficient | ||||
|---|---|---|---|---|---|---|---|---|---|
| Slope | 95% CI | Y-Intercept | 95% CI | ||||||
| Lower limit | Upper limit | Lower limit | Upper limit | ||||||
| A | Endospecy ES-50M1),† | PyroSmart1),† | 0.968 | 0.726 | 1.210 | 0.142 | −0.057 | 0.340 | 0.936 |
| B | Kinetic-QCL1),‡ | PyroSmart1),† | 0.767 | 0.574 | 0.961 | 0.016 | −0.214 | 0.246 | 0.935 |
| C | Limulus ES-II2),‡ | PyroSmart1),† | 0.889 | 0.444 | 1.333 | 0.102 | −0.304 | 0.508 | 0.799 |
| D | Endospecy ES-50M1),† | PyroSmart1),‡ | 1.463 | 1.059 | 1.866 | −0.050 | −0.382 | 0.282 | 0.923 |
| E | Kinetic-QCL1),‡ | PyroSmart1),‡ | 1.191 | 0.924 | 1.458 | −0.274 | −0.591 | 0.043 | 0.947 |
| F | Limulus ES-II2),‡ | PyroSmart1),‡ | 1.277 | 0.527 | 2.026 | −0.054 | −0.738 | 0.630 | 0.749 |
| G | Endospecy ES-50M1),† | Kinetic-QCL1),‡ | 1.217 | 1.000 | 1.434 | 0.197 | 0.018 | 0.375 | 0.966 |
| H | Endospecy ES-50M1),† | Limulus ES-II2),‡ | 0.845 | 0.588 | 1.102 | 0.225 | 0.013 | 0.436 | 0.909 |
| I | Kinetic-QCL1),‡ | Limulus ES-II2),‡ | 0.611 | 0.337 | 0.885 | 0.179 | −0.146 | 0.504 | 0.828 |
United States Pharmacopoeia Reference Standard Endotoxin was used as reference. 1) chromogenic reagent; 2) turbidimetric reagent; †: rate assay mode; ‡: onset time assay mode; CI: confidence interval.
Table 2 shows the comparison of the acceptance criteria to the results generated by PyroSmart.
| Analytical characteristics | Results | Acceptance criteria | ||||
|---|---|---|---|---|---|---|
| Rate assay mode | Onset time assay mode | |||||
| 1 | Linearity (correlation coefficient: absolute value) | 0.00625–0.1 EU/mL | 0.005–50 EU/mL | |r| ≥ 0.980 | ||
| 0.999 (minimum) | 1.000 | |||||
| 1.000 (maximum) | ||||||
| 2 | Accuracy (recovery) | EU/mL | Min–Max (%) | EU/mL | Min–Max (%) | |
| 0.00625 | 72.7–99.4 | 0.005 | 94.7–103.6 | 50–200% | ||
| 0.0125 | 94.2–101.3 | 0.05 | 91.6–103.1 | |||
| 0.025 | 100.0–105.6 | 0.5 | 99.1–108.2 | |||
| 0.05 | 100.3–105.4 | 5 | 100.1–109.4 | |||
| 0.1 | 98.5–99.8 | 50 | 92.4–99.3 | |||
| 3 | Precision | |||||
| 3–1 Repeatability (CV) | EU/mL | Min–Max (%) | EU/mL | Min–Max (%) | ||
| 0.00625 | 3.1–11.0 | 0.005 | 5.9–15.7 | CV ≤25% at lowest conc. | ||
| 0.0125 | 1.4–7.2 | 0.05 | 5.7–14.9 | CV ≤20% at the other conc. | ||
| 0.025 | 1.5–6.5 | 0.5 | 6.4–11.4 | |||
| 0.05 | 1.4–5.4 | 5 | 3.3–14.8 | |||
| 0.1 | 0.6–4.3 | 50 | 3.1–14.2 | |||
| 3–2 Intermediate precision (90% CI for CV) | EU/mL | Lower limit–Upper limit (%) | EU/mL | Lower limit–Upper limit (%) | ||
| 0.00625 | 7.3–15.2 | 0.005 | 0.4–0.7 | CV ≤25% at lowest conc. | ||
| 0.0125 | 1.4–2.9 | 0.05 | 0.7–1.5 | CV ≤20% at the other conc. | ||
| 0.025 | 1.2–2.5 | 0.5 | 2.9–6.1 | |||
| 0.05 | 1.3–2.6 | 5 | 1.2–2.5 | |||
| 0.1 | 0.3–0.7 | 50 | 0.4–0.8 | |||
| 3–3 Reproducibility (90% CI for CV) | Not assessed. | EU/mL | Lower limit–Upper limit (%) | |||
| 0.005 | 0.6–1.1 | CV ≤25% at lowest conc. | ||||
| 0.05 | 1.0–1.8 | CV ≤20% at the other conc. | ||||
| 0.5 | 4.0–7.1 | |||||
| 5 | 1.0–1.8 | |||||
| 50 | 0.5–0.9 | |||||
| 4 | Range | 0.00625–0.1 EU/mL | 0.005–50 EU/mL | Precision, accuracy and linearity are to be suitable level. | ||
| 5 | Quantitation limit | 95% CI | at 0.005 EU/mL | The lowest concentration of Et can be quantitatively determined with suitable precision and accuracy. | ||
| Lower limit-upper limit | Accuracy: 94.7–103.6% | |||||
| 0.0008–0.0016 EU/mL | Repeatability: 5.9–15.7% | |||||
| 6 | Specificity (reactivity with BG) USPRSE was used. | Results from the regression analysis (95% CI) Intercept: −0.133 to 0.126 Slope: 0.991–1.029 | — | Not reactive to BG | ||
Japanese Pharmacopoeia Reference Standard Endotoxin was used for rate assay mode except for specificity, and United States Pharmacopeia Reference Standard Endotoxin (USPRSE) was used for onset time assay mode. EU, endotoxin unit; CV, coefficient of variation; CI, confidence interval; BG, (1→3)-β-D-glucan.
Linearity—Typical standard curves for endotoxin quantitation obtained with PyroSmart in the rate and onset time assays are shown in Fig. 2. Both in the rate and onset time assays, the correlation coefficients calculated from all standard curves satisfied the acceptance criteria (|r| ≥ 0.980) that are described in the preparatory test of the BET.

Absorbance change rate (mAbs/min) obtained according to the rate assay mode (Panel A) or the onset time (second) obtained according to onset time mode (Panel B) are plotted against concentration of JP or USP reference standard endotoxin (RSE). Error bars show standard deviation in six replicates (Panel A) and eight replicates (Panel B) measurements.
Accuracy—In either the rate or onset time assay, the accuracy value, namely recovery, of added endotoxin satisfied the acceptance criteria for the test of interfering factors as described in the BET, i.e., 50–200%.
Precision—Repeatability, Intermediate precision and Reproducibility all satisfied their acceptance criteria as shown in Table 2.
Range—The range was found to be 0.00625–0.1 EU/mL for the rate assay and 0.005–50 EU/mL for the onset time method.
Quantitation limit—In the rate assay mode, the mean of the quantitation limit was 0.0012 EU/mL. The upper limit of the 95% CI for the endotoxin concentrations in the blank sample was 0.0002 EU/mL, while the lower limit of the 95% CI for the JPRSE 0.00125 EU/mL was 0.00126 EU/mL. Therefore, the ranges of endotoxin concentrations of in the blank sample and JPRSE did not overlap. Using onset time, both accuracy and precision at 0.005 EU/mL of endotoxin satisfied the acceptance criteria, indicating the lower limit of quantitation for the onset time method is 0.005 EU/mL.
Reactivity to (1 → 3)-β-D-glucan (BG)—Regression analysis for the results of three lots with and without the addition of BG was conducted (Fig. 3). The 95% CI for the y-intercept and the slope of the regression formula with the three lots contained “0” and “1,” respectively, indicating there is no statistical difference between the endotoxin concentrations with and without BG.
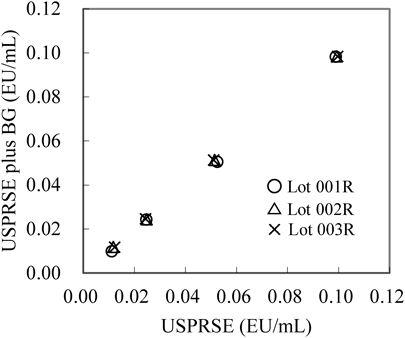
Regression analysis was done as described in Materials and Methods.
Ability to assess endotoxin in the presence of injectable drugs—We examined the interference to the test by examining the recovery of endotoxins added to 109 out of 118 available injectable drugs listed in the monographs of Japanese Pharmacopoeia. In both the rate and onset time assays, all minimum dilution factors expressed as NIDs of the 109 injectable drugs, which were measured with PyroSmart, were smaller than the MVDs of respective drugs (Table 3). NIDs of 109 injectable drugs which were measured with three lysate reagents were also smaller than their MVDs.
| ID | Parenteral drug | Stock solution conc. | Release limit | NID (upper line)/MVD (lower line) | ||||
|---|---|---|---|---|---|---|---|---|
| PyroSmart | Endospecy | Kinetic-QCL | Limulus ES-II | |||||
| Rate | Onset time | Rate | Onset time | Onset time | ||||
| 1 | Aciclovir injection | 25 mg/mL | 0.5 EU/mg | 2 | 8 | 4 | 8 | 8 |
| 2000 | 2500 | 2000 | 2500 | 1600 | ||||
| 2 | Ascorbic acid injection | 100 mg/mL | 0.15 EU/mg | 4 | 2 | 4 | 8 | 4 |
| 2400 | 3000 | 2400 | 3000 | 1920 | ||||
| 3 | Aztreonam for injection | 333 mg/mL | 0.10 EU/mg1) | 32 | 64 | 16 | 128 | 32 |
| 5333 | 6667 | 5333 | 6667 | 4267 | ||||
| 4 | Atropine sulfate injection | 0.5 mg/mL | 75 EU/mg | 1 | 1 | 1 | 4 | 1 |
| 6000 | 7500 | 6000 | 7500 | 4800 | ||||
| 5 | Amikacin sulfate injection | 100 mg/mL | 0.50 EU/mg1) | 16 | 16 | 2 | 64 | 4 |
| 8000 | 10000 | 8000 | 10000 | 6400 | ||||
| 6 | Amikacin sulfate for injection | 50 mg/mL | 0.50 EU/mg1) | 16 | 16 | 1 | 64 | 2 |
| 4000 | 5000 | 4000 | 5000 | 3200 | ||||
| 7 | Aminophylline injection | 25 mg/mL | 0.6 EU/mg | 16 | 16 | 8 | 4 | 8 |
| 2400 | 3000 | 2400 | 3000 | 1920 | ||||
| 8 | Amphotericin b for injection | 4.2 mg/mL | 3.0 EU/mg1) | 8 | 8‡ | 4 | 32 | 64 |
| 2000 | 2500 | 2000 | 2500 | 1600 | ||||
| 9 | L-Arginine hydrochloride injection | 100 mg/mL | 0.50 EU/mg1) | 8 | 8 | 4 | 16 | 4 |
| 80 | 100 | 80 | 100 | 64 | ||||
| 10 | Alprostadil injection | 0.005 mg/mL | 10 EU/mL | 8 | 256 | 16 | 1 | 256 |
| 1600 | 2000 | 1600 | 2000 | 1280 | ||||
| 11 | Arbekacin sulfate injection | 50 mg/mL | 0.50 EU/mg1) | 4 | 4‡ | 2 | 32 | 2 |
| 4000 | 5000 | 4000 | 5000 | 3200 | ||||
| 12 | Alendronate sodium injection | 2.5 mg/mL | 119 EU/mg | 2 | 2 | 2 | 16 | 8 |
| 47600 | 59500 | 47600 | 59500 | 38080 | ||||
| 13 | Ampicillin sodium for injection | 250 mg/mL | 0.075 EU/mg1) | 8 | 32‡ | 8 | 16 | 32 |
| 3000 | 3750 | 3000 | 3750 | 2400 | ||||
| 14 | Sodium iotalamate injection | 66.8 % | 3.4 EU/mL | 32 | 64 | 16 | 32 | 32 |
| 544 | 680 | 544 | 680 | 435 | ||||
| 15 | Isepamicin sulfate injection | 200 mg/mL | 0.50 EU/mg1) | 16 | 32 | 4 | 128 | 8 |
| 16000 | 20000 | 16000 | 20000 | 12800 | ||||
| 16 | Isoniazid injection | 50 mg/mL | 0.50 EU/mg | 4 | 4 | 4 | 4 | 4 |
| 4000 | 5000 | 4000 | 5000 | 3200 | ||||
| 17 | Idarubicin hydrochloride† | 8.9 EU/mg1) | ||||||
| 18 | Idarubicin hydrochloride for injection | 1 mg/mL | 8.9 EU/mg1) | 128 | 512 | 128 | 16 | 8 |
| 1424 | 1780 | 1424 | 1780 | 1139 | ||||
| 19 | Imipenem and cilastatin sodium for injection | 25 mg/mL | 0.25 EU/mg1) | 8 | 16 | 1 | 4 | 16 |
| 1000 | 1250 | 1000 | 1250 | 800 | ||||
| 20 | Indigocarmine injection | 4 mg/mL | 7.5 EU/mg | 4 | 16 | 4 | 16 | 32 |
| 4800 | 6000 | 4800 | 6000 | 3840 | ||||
| 21 | Insulin human (genetic recombination) | 100 unit2)/mL | 0.8 EU/unit2) | 4 | 2 | 2 | 8 | 2 |
| 12800 | 16000 | 12800 | 16000 | 10240 | ||||
| 22 | Edrophonium chloride injection | 1 mg/mL | 15 EU/mg | 8 | 8 | 4 | 8 | 4 |
| 2400 | 3000 | 2400 | 3000 | 1920 | ||||
| 23 | Ephedrine hydrochloride injection | 40 mg/mL | 7.5 EU/mg | 8 | 20 | 4 | 8 | 8 |
| 48000 | 60000 | 48000 | 60000 | 38400 | ||||
| 24 | Ergometrine maleate injection | 0.2 mg/mL | 1500 EU/mg | 1 | 2 | 1 | 32 | 1 |
| 48000 | 60000 | 48000 | 60000 | 38400 | ||||
| 25 | Calcium chloride injection | 55.5 mg/mL | 0.30 EU/mg | 32 | 32 | 64 | 32 | 16 |
| 2664 | 3330 | 2664 | 3330 | 2131 | ||||
| 26 | 10% Sodium chloride injection | 10 % | 3.6 EU/mL | 2 | 2 | 4 | 16 | 2 |
| 576 | 720 | 576 | 720 | 461 | ||||
| 27 | Oxytocin injection | 1 unit/mL | 10 EU/unit | 2 | 16 | 2 | 8 | 2 |
| 1600 | 2000 | 1600 | 2000 | 1280 | ||||
| 28 | Ozagrel sodium for injection | 10 mg/mL | 3.7 EU/mg | 1 | 1 | 2 | 2 | 4 |
| 5920 | 7400 | 5920 | 7400 | 4736 | ||||
| 29 | Fructose injection | 20 % | 0.5 EU/mL | 4 | 4 | 2 | 4 | 2 |
| 80 | 100 | 80 | 100 | 64 | ||||
| 30 | Xylitol injection | 5 % | 0.50 EU/mL | 1 | 1 | 1 | 1 | 1 |
| 80 | 100 | 80 | 100 | 64 | ||||
| 31 | Sodium citrate injection for transfusion | 100 mg/mL | 5.6 EU/mL | 1 | 1 | 4 | 64 | 4 |
| 896 | 1120 | 896 | 1120 | 717 | ||||
| 32 | Clindamycin phosphate injection | 150 mg/mL | 0.1 EU/mg1) | 128 | 64 | 16 | 32 | 32 |
| 2400 | 3000 | 2400 | 3000 | 1920 | ||||
| 33 | Chlorpheniramine maleate injection | 2 mg/mL | 8.8 EU/mg | 2 | 4 | 2 | 8 | 4 |
| 2816 | 3520 | 2816 | 3520 | 2253 | ||||
| 34 | Cyanocobalamin injection | 1000 µg /mL | 0.30 EU/µg | 1 | 2 | 1 | 4 | 2 |
| 48000 | 60000 | 48000 | 60000 | 38400 | ||||
| 35 | Digoxin injection | 0.25 mg/mL | 200 EU/mg | 16 | 32 | 16 | 8 | 32 |
| 8000 | 10000 | 8000 | 10000 | 6400 | ||||
| 36 | Dimorpholamine injection3) | 15 mg/mL | 5.0 EU/mg | 16 | 64 | 16 | 16 | 8 |
| 12000 | 15000 | 12000 | 15000 | 9600 | ||||
| 37 | Water for injection† | — | 0.25 EU/mL | |||||
| 38 | Water for injection in containers | — | 0.25 EU/mL | 1 | 1 | 1 | 1 | 1 |
| — | — | — | — | — | ||||
| 39 | Suxamethonium chloride injection | 22 mg/mL | 2.0 EU/mg | 1 | 2 | 1 | 8 | 1 |
| 7040 | 8800 | 7040 | 8800 | 5632 | ||||
| 40 | Suxamethonium chloride for injection | 100 mg/mL | 1.5 EU/mg | 8 | 8 | 4 | 16 | 8 |
| 24000 | 30000 | 24000 | 30000 | 19200 | ||||
| 41 | Streptomycin sulfate for injection | 333 mg/mL | 0.10 EU/mg | 32 | 128 | 8 | 1024 | 16 |
| 5333 | 6667 | 5333 | 6667 | 4267 | ||||
| 42 | Serum gonadotrophin† | 0.1 EU/unit | ||||||
| 43 | Serum gonadotrophin for injection | 200 unit/mL | 0.1 EU/unit | 164) | 1 | 164) | 1 | 164) |
| 3200 | 4000 | 3200 | 4000 | 2520 | ||||
| 44 | Human menopausal gonadotrophin | 75 Unit5)/mL | 0.66 EU/unit5,6) | 164) | 2 | 44) | 1 | 44) |
| 7920 | 9900 | 7920 | 9900 | 6336 | ||||
| 45 | Human chorionic gonadotrophin† | 0.03 EU/unit | ||||||
| 46 | Human chorionic gonadotrophin for injection | 200 mg/mL | 0.03 EU/unit | 1 | 1 | 1 | 1 | 1 |
| 960 | 1200 | 960 | 1200 | 768 | ||||
| 47 | Isotonic sodium chloride solution | — | 0.50 EU/mL | 1 | 1 | 1 | 2 | 1 |
| 80 | 100 | 80 | 100 | 64 | ||||
| 48 | Cefazolin sodium hydrate† | 0.10 EU/mg1) | ||||||
| 49 | Cefazolin sodium for injection | 333 mg/mL | 0.05 EU/mg1) | 32 | 64 | 32 | 16 | 32 |
| 2667 | 3333 | 2667 | 3333 | 2133 | ||||
| 50 | Cefepime dihydrochloride hydrate† | 0.04 EU/mg1) | ||||||
| 51 | Cefepime dihydrochloride hydrate for injection | 50 mg/mL | 0.06 EU/mg1) | 8 | 16 | 4 | 32 | 8 |
| 480 | 600 | 480 | 600 | 384 | ||||
| 52 | Cefozopran hydrochloride† | 0.05 EU/mg1) | ||||||
| 53 | Cefozopran hydrochloride for injection | 100 mg/mL | 0.05 EU/mg1) | 16 | 64 | 8 | 128 | 16 |
| 800 | 1000 | 800 | 1000 | 640 | ||||
| 54 | Cefotiam hydrochloride for injection | 83.3 mg/mL | 0.125 EU/mg | 16 | 16 | 8 | 64 | 32 |
| 1667 | 2083 | 1667 | 2083 | 1333 | ||||
| 55 | Ceftazidime for injection | 200 mg/mL | 0.067 EU/mg1) | 32 | 64 | 8 | 64 | 16 |
| 2144 | 2680 | 2144 | 2680 | 1715 | ||||
| 56 | Cefpirome sulfate | 50 mg/mL | 0.10 EU/mg | 16 | 32 | 8 | 32 | 8 |
| 800 | 1000 | 800 | 1000 | 640 | ||||
| 57 | Cefmetazole sodium for injection | 400 mg/mL | 0.06 EU/mg | 32 | 32 | 64 | 64 | 64 |
| 3840 | 4800 | 3840 | 4800 | 3072 | ||||
| 58 | Celmoleukin (genetic recombination) | 40 Unit7)/mL | 100 EU/mL | 1 | 2‡ | 1 | 64 | 2 |
| 16000 | 20000 | 16000 | 20000 | 12800 | ||||
| 59 | Tazobactam8) | 450 mg/mL | 0.04 EU/mg1) | 128 | 128 | 64 | 64 | 128 |
| 2880 | 3600 | 2880 | 3600 | 2304 | ||||
| 60 | Sodium bicarbonate injection | 0.7 mg/mL | 5.0 EU/mEq | 4 | 4 | 4 | 8 | 8 |
| 560 | 700 | 560 | 700 | 448 | ||||
| 61 | Thiamylal sodium for injection | 25 mg/mL | 1.0 EU/mg | 32 | 16 | 16 | 16 | 16 |
| 4000 | 5000 | 4000 | 5000 | 3200 | ||||
| 62 | Thiamine chloride hydrochloride injection | 50 mg/mL | 6.0 EU/mg | 8 | 16‡ | 4 | 128 | 32 |
| 48000 | 60000 | 48000 | 60000 | 38400 | ||||
| 63 | Thiopental sodium for injection | 25 mg/mL | 0.30 EU/mg | 32 | 16 | 16 | 128 | 32 |
| 1200 | 1500 | 1200 | 1500 | 960 | ||||
| 64 | Sodium thiosulfate hydrate | 100 mg/mL | 0.01 EU/mg | 8 | 16 | 2 | 32 | 4 |
| 160 | 200 | 160 | 200 | 128 | ||||
| 65 | Teicoplanin | 66.7 mg/mL | 0.75 EU/mg1) | 512 | 1024‡ | 512 | 512 | 256 |
| 8000 | 10000 | 8000 | 10000 | 6400 | ||||
| 66 | Dextran 40 | 0.1 g/mL | 2.5 EU/g | 1 | 2 | 1 | 4 | 4 |
| 40 | 50 | 40 | 50 | 32 | ||||
| 67 | Dextran 40 injection9) | 100 mg/mL | 0.50 EU/mL | 1 | 1 | 1 | 64 | 4 |
| 80 | 100 | 80 | 100 | 64 | ||||
| 68 | Deslanoside injection | 0.2 mg/mL | 500 EU/mg | 4 | 8 | 4 | 2 | 8 |
| 16000 | 20000 | 16000 | 20000 | 12800 | ||||
| 69 | Teceleukin (genetic recombination)† | 5 EU/mg10) | ||||||
| 70 | Teceleukin for injection (genetic recombination) | 35 Unit7)/mL | 5 EU/35 unit7) | 2 | 2 | 2 | 8 | 2 |
| 28000 | 35000 | 28000 | 35000 | 22400 | ||||
| 71 | Dehydrocholic acid injection | 100 mg/mL | 0.30 EU/mg | 64 | 64 | 32 | 32 | 64 |
| 4800 | 6000 | 4800 | 6000 | 3840 | ||||
| 72 | Doxorubicin hydrochloride for injection | 10 mg/mL | 2.50 EU/mg1) | 256 | 512‡ | 32 | 32 | 64 |
| 4000 | 5000 | 4000 | 5000 | 3200 | ||||
| 73 | Dopamine hydrochloride injection | 20 mg/mL | 4.2 EU/mg | 8 | 4 | 2 | 4 | 4 |
| 13440 | 16800 | 13440 | 16800 | 10752 | ||||
| 74 | Tobramycin injection | 60 mg/mL | 0.50 EU/mg1) | 32 | 32‡ | 4 | 512 | 8 |
| 4800 | 6000 | 4800 | 6000 | 3840 | ||||
| 75 | Tranexamic acid injection | 50 mg/mL | 0.12 EU/mg | 2 | 4 | 2 | 2 | 2 |
| 960 | 1200 | 960 | 1200 | 768 | ||||
| 76 | Nicardipine hydrochloride injection | 1 mg/mL | 8.33 EU/mg | 16 | 16 | 16 | 8 | 8 |
| 1333 | 1666 | 1333 | 1666 | 1066 | ||||
| 77 | Nicotinic acid injection | 50 mg/mL | 3.0 EU/mg | 8 | 8 | 8 | 16 | 16 |
| 24000 | 30000 | 24000 | 30000 | 19200 | ||||
| 78 | Neostigmine methylsulfate injection | 0.5 mg/mL | 5 EU/mg | 1 | 1 | 1 | 2 | 1 |
| 400 | 500 | 400 | 500 | 320 | ||||
| 79 | Noradrenaline injection | 1 mg/mL | 300 EU/mg | 2 | 2 | 2 | 4 | 4 |
| 48000 | 60000 | 48000 | 60000 | 38400 | ||||
| 80 | Sucrose | 100 mg/mL | 0.25 EU/mg | 44) | 1 | 84) | 1 | 1 |
| 4000 | 5000 | 4000 | 5000 | 3200 | ||||
| 81 | Vasopressin injection | 20 unit/mL | 15 EU/unit11) | 4 | 32 | 4 | 32 | 4 |
| 48000 | 60000 | 48000 | 60000 | 38400 | ||||
| 82 | Panipenem | 25 mg/mL | 0.15 EU/mg1) | 16 | 16 | 8 | 8 | 8 |
| 600 | 750 | 600 | 750 | 480 | ||||
| 83 | Papaverine hydrochloride injection | 40 mg/mL | 6.0 EU/mg | 256 | 1024 | 64 | 128 | 64 |
| 38400 | 48000 | 38400 | 48000 | 30720 | ||||
| 84 | Vancomycin hydrochloride injection | 100 mg/mL | 0.25 EU/mg | 128 | 512 | 64 | 128 | 128 |
| 4000 | 5000 | 4000 | 5000 | 3200 | ||||
| 85 | Hydralazine hydrochloride injection | 20 mg/mL | 5.0 EU/mg | 16 | 16‡ | 8 | 32 | 16 |
| 16000 | 20000 | 16000 | 20000 | 12800 | ||||
| 86 | Piperacillin hydrate† | 0.07 EU/mg1) | ||||||
| 87 | Piperacillin sodium for injection | 200 mg/mL | 0.04 EU/mg1) | 64 | 128 | 64 | 32 | 64 |
| 1280 | 1600 | 1280 | 1600 | 1024 | ||||
| 88 | Pyridoxine hydrochloride injection | 10 mg/mL | 3.0 EU/mg | 4 | 8 | 2 | 128 | 16 |
| 4800 | 6000 | 4800 | 6000 | 3840 | ||||
| 89 | Vinblastine sulfate for injection | 2 mg/mL | 10 EU/mg | 16 | 32 | 8 | 8 | 2 |
| 3200 | 4000 | 3200 | 4000 | 2560 | ||||
| 90 | Famotidine injection | 10 mg/mL | 15 EU/mg | 8 | 16‡ | 8 | 16 | 16 |
| 24000 | 30000 | 24000 | 30000 | 19200 | ||||
| 91 | Famotidine for injection | 10 mg/mL | 15 EU/mg | 4 | 8 | 2 | 16 | 8 |
| 24000 | 30000 | 24000 | 30000 | 19200 | ||||
| 92 | Phenolsulfonphthalein injection | 6 mg/mL | 7.5 EU/mg | 16 | 128‡ | 16 | 64 | 64 |
| 80 | 100 | 80 | 100 | 64 | ||||
| 93 | Glucose injection | 50 % | 0.50 EU/mL | 16 | 16 | 8 | 32 | 4 |
| 80 | 100 | 80 | 100 | 64 | ||||
| 94 | Prednisolone succinate for injection | 10 mg/mL | 2.4 EU/mg12) | 16 | 16 | 16 | 8 | 32 |
| 3840 | 4800 | 3840 | 4800 | 3072 | ||||
| 95 | Procain hydrochloride injection | 20 mg/mL | 0.02 EU/mg | 8 | 32 | 4 | 16 | 8 |
| 64 | 80 | 64 | 80 | 51 | ||||
| 96 | Procainamide hydrochloride injection | 100 mg/mL | 0.30 EU/mg | 32 | 64 | 16 | 32 | 32 |
| 4800 | 6000 | 4800 | 6000 | 3840 | ||||
| 97 | Furosemide injection | 10 mg/mL | 1.25 EU/mg | 4 | 4 | 8 | 8 | 8 |
| 2000 | 2500 | 2000 | 2500 | 1600 | ||||
| 98 | Protamine sulfate injection | 10 mg/mL | 6.0 EU/mg | 256 | 1024 | 4 | 4096 | 8 |
| 9600 | 12000 | 9600 | 12000 | 7680 | ||||
| 99 | Flomoxef sodium for injection | 250 mg/mL | 0.025 EU/mg1) | 32 | 64 | 16 | 32 | 32 |
| 1000 | 1250 | 1000 | 1250 | 800 | ||||
| 100 | Pethidine hydrochloride injection | 50 mg/mL | 6.0 EU/mg | 32 | 32‡ | 16 | 32 | 64 |
| 48000 | 60000 | 48000 | 60000 | 38400 | ||||
| 101 | Heparin calcium | 25000 Unit13)/mL | 0.003 EU/unit13) | 2000 | 8000 | 32 | 10000 | 64 |
| 12000 | 15000 | 12000 | 15000 | 9600 | ||||
| 102 | Heparin sodium injection | 1000 unit/mL | 0.0030 EU/unit | 64 | 128‡ | 2 | 512 | 2 |
| 480 | 600 | 480 | 600 | 384 | ||||
| 103 | Peplomycin sulfate injection | 1 mg/mL | 1.5 EU/mg1) | 2 | 2 | 4 | 1 | 4 |
| 240 | 300 | 240 | 300 | 192 | ||||
| 104 | Benzylpenicillin potassium for injection | 100000 unit/mL | 0.000125 EU/unit | 8 | 16 | 8 | 4 | 8 |
| 2000 | 2500 | 2000 | 2500 | 1600 | ||||
| 105 | Fosfomycin sodium for injection | 100 mg/mL | 0.025 EU/mg1) | 1 | 2 | 2 | 32 | 2 |
| 400 | 500 | 400 | 500 | 320 | ||||
| 106 | Mitomycin c for injection | 2 mg/mL | 10 EU/mg1) | 2 | 4 | 2 | 8 | 2 |
| 3200 | 4000 | 3200 | 4000 | 2520 | ||||
| 107 | D-Mannitol injection | 20 % | 0.50 EU/mL | 1 | 2 | 1 | 1 | 2 |
| 80 | 100 | 80 | 100 | 64 | ||||
| 108 | Minocycline hydrochloride for injection | 50 mg/mL | 1.25 EU/mg1) | 2000 | 8000 | 512 | 2048 | 128 |
| 10000 | 12500 | 10000 | 12500 | 8000 | ||||
| 109 | Mepivacain hydrochlorideInjection | 20 mg/mL | 0.6 EU/mg | 8 | 32 | 4 | 8 | 8 |
| 1920 | 2400 | 1920 | 2400 | 1536 | ||||
| 110 | Meropenem for injection | 50 mg/mL | 0.12 EU/mg1) | 16 | 16 | 8 | 8 | 16 |
| 960 | 1200 | 960 | 1200 | 768 | ||||
| 111 | Morphine hydrochloride injection | 10 mg/mL | 1.5 EU/mg | 4 | 8‡ | 2 | 16 | 4 |
| 2400 | 3000 | 2400 | 3000 | 1920 | ||||
| 112 | Lidocain injection | 20 mg/mL | 1.0 EU/mg | 8 | 32 | 4 | 8 | 4 |
| 3200 | 4000 | 3200 | 4000 | 2560 | ||||
| 113 | Riboflavin sodium phosphate injection | 10 mg/mL | 10 EU/mg | 16 | 8 | 16 | 32 | 8 |
| 16000 | 20000 | 16000 | 20000 | 12800 | ||||
| 114 | Magnesium sulfate injection | 60.2 mg/mL | 0.09 EU/mg | 8 | 32 | 1 | 16 | 2 |
| 867 | 1084 | 867 | 1084 | 694 | ||||
| 115 | Ringer’s solution | — | 0.50 EU/mL | 1 | 1 | 1 | 2 | 1 |
| 80 | 100 | 80 | 100 | 64 | ||||
| 116 | Lincomycin hydrochloride injection | 300 mg/mL | 0.50 EU/mg1) | 32 | 128 | 32 | 64 | 32 |
| 24000 | 30000 | 24000 | 30000 | 19200 | ||||
| 117 | Levallorphan tartrate injection | 1 mg/mL | 150 EU/mg | 2 | 8 | 2 | 16 | 2 |
| 24000 | 30000 | 24000 | 30000 | 19200 | ||||
| 118 | Roxatidine acetate hydrochloride for injection | 15 mg/mL | 4.0 EU/mg | 4 | 32 | 2 | 16 | 4 |
| 9600 | 12000 | 9600 | 12000 | 7680 | ||||
1) Potency; 2) Insulin unit; 3) Performed the test at 0.15 (w/v) %; 4) Because of its contamination, the sample was diluted to the extent where the measured value entered the range of the standard curve; 5) Follicle-stimulating hormone unit; 6) Performed the test by dissolving 75 unit of follicle-stimulating hormone per mL of water for Bacterial Endotoxins Test; 7) × 10000 units; 8) Tazobactam sodium/piperacillin sodium for injection (reference); 9) Low-molecular-weight dextran-added lactated Ringer’s solution; 10) Per 1 mg of protein;11) Vasopressin unit; 12) Amounts corresponding to prednisolone; 13) Heparin unit; MVD, maximum valid dilution. †: Drug substance that was not available for us. ‡: 60-min incubation.
Potencies of endotoxins—The potencies of three typical types of endotoxins were measured with the three recombinant reagents including PyroSmart and six lysate reagents produced from the horseshoe crab (Fig. 4). There was no significant difference based on F-test and t-test (p = 0.05) between the recombinant reagents (generating four data sets) and lysate reagents (generating six data sets).

Reactivity is expressed as EU/ng using USP reference standard endotoxin (RSE) as a reference. Error bars indicate standard deviation in the results of three lots. Two lots of EndoZyme and one lot of Endozyme II were used for the study.
Figure 4 also shows variations in the potencies as error bars with each reagent between the three lots. In a few cases lot-to-lot variances (CV; >20%) in the potencies were recognized; P. aeruginosa 10 with Endochrome-K and S. minnesota R595 Re with PyroGene and Endochrome-K. In the case of EndoZyme, only two lots were available and one lot of Endozyme II was used instead.
Recoveries of endotoxin added to parenteral drugs—The recoveries of endotoxins added into twenty seven parenteral drugs, which were evenly selected according to the wide range of susceptibility to interfering factors, were measured with three kinds of recombinant reagents and five different lysate reagents. The minimum dilution factors, NIDs, are shown in Table 4.
| (A) Recombinant reagents | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Parenteral drugs | NID (upper line) / MVD (lower line) | ||||||||||||||
| PyroSmart | PyroGene | EndoZyme | ||||||||||||||
| Rate | Onset time | Endpoint | Endpoint | |||||||||||||
| Lot | ||||||||||||||||
| 1st. | 2nd. | 3rd. | 1st. | 2nd. | 3rd. | 1st. | 2nd. | 3rd. | 1st. | 2nd. | 3rd. | |||||
| 1 | Aminophylline injection | 16 | 16 | 16 | 32 | 16 | 32 | 4 | 8 | 8 | Not tested | |||||
| 2400 | 3000 | 3000 | 3000 | |||||||||||||
| 2 | Alprostadil injection | 8 | 8 | 8 | 256* | 128* | 128* | 256 | 512 | 256 | 128 | 128 | 128 | |||
| 1600 | 2000 | 2000 | 2000 | |||||||||||||
| 3 | Idarubicin hydrochloride for injection | 256 | 256 | 256 | 512 | 512 | 512 | 128 | 128 | 128 | 32 | 32 | 32 | |||
| 1424 | 1780 | 1780 | 1780 | |||||||||||||
| 4 | Calcium chloride injection | 16 | 16 | 16 | 32 | 64 | 32 | 128 | 128 | 64 | 128 | 64 | 128 | |||
| 2664 | 3330 | 3330 | 3330 | |||||||||||||
| 5 | 10% Sodium chloride injection | 2 | 2 | 2 | 4 | 4 | 4 | 8 | 4 | 8 | 4 | 4 | 4 | |||
| 576 | 720 | 720 | 720 | |||||||||||||
| 6 | Xylitol injection | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| 80 | 100 | 100 | 100 | |||||||||||||
| 7 | Sodium citrate injection for transfusion | 2 | 2 | 1 | 1 | 1 | 1 | 64 | 64 | 128 | 128 | 128 | 128 | |||
| 896 | 1120 | 1120 | 1120 | |||||||||||||
| 8 | Clindamycin phosphate injection | 128 | 128 | 128 | 64 | 128 | 64 | 16 | 16 | 16 | 32 | 32 | 32 | |||
| 2400 | 3000 | 3000 | 3000 | |||||||||||||
| 9 | Digoxin injection | 16 | 16 | 16 | 16 | 16 | 16 | 8 | 8 | 4 | 8 | 8 | 8 | |||
| 8000 | 10000 | 10000 | 10000 | |||||||||||||
| 10 | Dimorpholamine injection | 16 | 16 | 16 | 32* | 16* | 32* | 8 | 4 | 8 | 8 | 8 | 8 | |||
| 12000 | 15000 | 15000 | 15000 | |||||||||||||
| 11 | Isotonic sodium chloride solution | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| 80 | 100 | 100 | 100 | |||||||||||||
| 12 | Cefazolin sodium for injection | 32 | 32 | 32 | 128 | 64 | 64 | 128 | 256 | 128 | 64 | 64 | 64 | |||
| 2667 | 3333 | 3333 | 3333 | |||||||||||||
| 13 | Cefmetazole sodium for injection | 32 | 32 | 32 | 64 | 32 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | |||
| 3840 | 4800 | 4800 | 4800 | |||||||||||||
| 14 | Tazobactam | 128 | 128 | 128 | 256 | 128 | 256 | 64 | 64 | 64 | 64 | 64 | 64 | |||
| 2880 | 3600 | 3600 | 3600 | |||||||||||||
| 15 | Sodium bicarbonate injection | 8 | 8 | 8 | 8 | 4 | 8 | 16 | 16 | 16 | 8 | 8 | 8 | |||
| 560 | 700 | 700 | 700 | |||||||||||||
| 16 | Teicoplanin | 512 | 512 | 512 | 1024* | 1024* | 1024* | 1024 | 1024 | 2048 | 2048 | 2048 | 1024 | |||
| 8000 | 10000 | 10000 | 10000 | |||||||||||||
| 17 | Dehydrocholic acid injection | 64 | 32 | 64 | 64 | 64 | 64 | 32 | 32 | 32 | 32 | 64 | 32 | |||
| 4800 | 6000 | 6000 | 6000 | |||||||||||||
| 18 | Doxorubicin hydrochloride for injection | 1024 | 512 | 1024 | 512* | 512* | 512* | 512 | 512 | 512 | 256 | 256 | 256 | |||
| 4000 | 5000 | 5000 | 5000 | |||||||||||||
| 19 | Nicardipine hydrochloride injection | 16 | 16 | 16 | 16 | 16 | 32 | 64 | 64 | 64 | 32 | 32 | 32 | |||
| 1333 | 1666 | 1666 | 1666 | |||||||||||||
| 20 | Nicotinic acid injection | 32 | 64 | 64 | 32 | 32 | 64 | 16 | 16 | 16 | 8 | 16 | 16 | |||
| 24000 | 30000 | 30000 | 30000 | |||||||||||||
| 21 | Papaverine hydrochloride injection | 256 | 256 | 256 | 1024 | 512 | 512 | 256 | 128 | 128 | 256 | 256 | 256 | |||
| 38400 | 48000 | 48000 | 48000 | |||||||||||||
| 22 | Vancomycin hydrochloride injection | 256 | 64 | 128 | 512 | 256 | 512 | 64 | 128 | 128 | 64 | 64 | 64 | |||
| 4000 | 5000 | 5000 | 5000 | |||||||||||||
| 23 | Piperacillin sodium for injection | 64 | 64 | 64 | 128 | 128 | 128 | 32 | 32 | 32 | 32 | 32 | 64 | |||
| 1280 | 1600 | 1600 | 1600 | |||||||||||||
| 24 | Famotidine injection | 8 | 8 | 8 | 16 | 16 | 8 | 4 | 4 | 4 | 16 | 16 | 8 | |||
| 24000 | 30000 | 30000 | 30000 | |||||||||||||
| 25 | Glucose injection | 8 | 4 | 4 | 8 | 8 | 16 | 4 | 4 | 4 | 4 | 8 | 8 | |||
| 80 | 100 | 100 | 100 | |||||||||||||
| 26 | Heparin calcium | 1000 | 2000 | 1000 | 4000* | 1000* | 2000* | >30000 | >30000 | >30000 | >30000 | >30000 | >30000 | |||
| 12000 | 15000 | 15000 | 15000 | |||||||||||||
| 27 | D-Mannitol injection | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | |||
| 80 | 100 | 100 | 100 | |||||||||||||
| (B) Lysate reagents | ||||||||||||||||
| ID | Parenteral drugs | NID (upper line)/MVD (lower line) | ||||||||||||||
| Endospecy ES-50M | Pyrochrome | Kinetic-QCL | Limulus ES-II | Limulus color KY test | ||||||||||||
| Rate | Onset time | Onset time | Onset time | Onset time | ||||||||||||
| Lot | ||||||||||||||||
| 1st. | 2nd. | 3rd. | 1st. | 2nd. | 3rd. | 1st. | 2nd. | 3rd. | 1st. | 2nd. | 3rd. | 1st. | 2nd. | 3rd. | ||
| 1 | Aminophylline injection | 8 | 8 | 8 | 16 | 8 | 8 | 4 | 4 | 4 | 8 | 8 | 8 | 8 | 8 | 8 |
| 2400 | 3000 | 3000 | 1920 | 30000 | ||||||||||||
| 2 | Alprostadil injection | 16 | 16 | 16 | 32 | 256 | 128 | 4 | 8 | 8 | 256 | 256 | 256 | 256 | 256 | 256 |
| 1600 | 2000 | 2000 | 1280 | 20000 | ||||||||||||
| 3 | Idarubicin hydrochloride for injection | 64 | 256 | 64 | 128 | 64 | 128 | 32 | 32 | 32 | 32 | 512 | 512 | 32 | 32 | 128 |
| 1424 | 1780 | 1780 | 1139 | 17800 | ||||||||||||
| 4 | Calcium chloride injection | 32 | 64 | 32 | 16 | 16 | 16 | 256 | 128 | 128 | 4 | 16 | 16 | 8 | 8 | 8 |
| 2664 | 3330 | 3330 | 2131 | 33300 | ||||||||||||
| 5 | 10% Sodium chloride injection | 4 | 4 | 4 | 2 | 2 | 2 | 16 | 16 | 16 | 4 | 2 | 4 | 1 | 1 | 1 |
| 576 | 720 | 720 | 461 | 7200 | ||||||||||||
| 6 | Xylitol injection | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 80 | 100 | 100 | 64 | 1000 | ||||||||||||
| 7 | Sodium citrate injection for transfusion | 4 | 8 | 8 | 8 | 8 | 8 | 64 | 128 | 64 | 4 | 4 | 4 | 1 | 1 | 1 |
| 896 | 1120 | 1120 | 717 | 11200 | ||||||||||||
| 8 | Clindamycin phosphate injection | 32 | 32 | 32 | 16 | 16 | 16 | 32 | 32 | 32 | 32 | 64 | 32 | 64 | 64 | 64 |
| 2400 | 3000 | 3000 | 1920 | 30000 | ||||||||||||
| 9 | Digoxin injection | 16 | 16 | 16 | 16 | 16 | 16 | 8 | 8 | 8 | 16 | 16 | 16 | 16 | 16 | 16 |
| 8000 | 10000 | 10000 | 6400 | 100000 | ||||||||||||
| 10 | Dimorpholamine injection | 8 | 8 | 8 | 16 | 8 | 16 | 16 | 16 | 16 | 8 | 8 | 16 | 4 | 8 | 16 |
| 12000 | 15000 | 15000 | 9600 | 150000 | ||||||||||||
| 11 | Isotonic sodium chloride solution | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| 80 | 100 | 100 | 64 | 1000 | ||||||||||||
| 12 | Cefazolin sodium for injection | 32 | 32 | 32 | 16 | 16 | 16 | 128 | 128 | 64 | 32 | 32 | 32 | 32 | 16 | 16 |
| 2667 | 3333 | 3333 | 2133 | 33330 | ||||||||||||
| 13 | Cefmetazole sodium for injection | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 128 | 128 | 64 | 64 | 16 | 32 | 32 | 32 |
| 3840 | 4800 | 4800 | 3072 | 48000 | ||||||||||||
| 14 | Tazobactam | 128 | 128 | 128 | 256 | 64 | 64 | 64 | 64 | 64 | 64 | 128 | 64 | 32 | 32 | 32 |
| 2880 | 3600 | 3600 | 2304 | 36000 | ||||||||||||
| 15 | Sodium bicarbonate injection | 4 | 8 | 4 | 4 | 4 | 8 | 8 | 16 | 16 | 8 | 8 | 8 | 8 | 4 | 4 |
| 560 | 700 | 700 | 448 | 7000 | ||||||||||||
| 16 | Teicoplanin | 512 | 512 | 512 | 1024 | 512 | 512 | 1024 | 512 | 512 | 256 | 256 | 256 | 1024 | 1024 | 1024 |
| 8000 | 10000 | 10000 | 6400 | 100000 | ||||||||||||
| 17 | Dehydrocholic acid injection | 32 | 32 | 32 | 64 | 32 | 32 | 32 | 32 | 32 | 64 | 64 | 64 | 32 | 32 | 32 |
| 4800 | 6000 | 6000 | 3840 | 60000 | ||||||||||||
| 18 | Doxorubicin hydrochloride for injection | 64 | 128 | 4 | 2048 | 16 | 4 | 128 | 64 | 64 | 32 | 2048 | 1024 | 128 | 16 | 32 |
| 4000 | 5000 | 5000 | 3200 | 50000 | ||||||||||||
| 19 | Nicardipine hydrochloride injection | 8 | 16 | 16 | 32 | 32 | 32 | 16 | 8 | 8 | 16 | 128 | 64 | 16 | 32 | 32 |
| 1333 | 1666 | 1666 | 1066 | 16660 | ||||||||||||
| 20 | Nicotinic acid injection | 32 | 64 | 64 | 16 | 16 | 64 | 16 | 16 | 16 | 16 | 32 | 16 | 64 | 64 | 32 |
| 24000 | 30000 | 30000 | 19200 | 300000 | ||||||||||||
| 21 | Papaverine hydrochloride injection | 64 | 64 | 64 | 512 | 256 | 512 | 128 | 128 | 128 | 64 | 1024 | 512 | 64 | 128 | 128 |
| 38400 | 48000 | 48000 | 30720 | 480000 | ||||||||||||
| 22 | Vancomycin hydrochloride injection | 32 | 64 | 32 | 128 | 256 | 256 | 128 | 128 | 128 | 64 | 128 | 128 | 64 | 64 | 64 |
| 4000 | 5000 | 5000 | 3200 | 50000 | ||||||||||||
| 23 | Piperacillin sodium for injection | 64 | 128 | 64 | 64 | 32 | 64 | 32 | 32 | 64 | 64 | 64 | 64 | 16 | 32 | 32 |
| 1280 | 1600 | 1600 | 1024 | 16000 | ||||||||||||
| 24 | Famotidine injection | 8 | 4 | 4 | 16 | 8 | 8 | 8 | 8 | 8 | 16 | 8 | 8 | 16 | 2 | 8 |
| 24000 | 30000 | 30000 | 19200 | 300000 | ||||||||||||
| 25 | Glucose injection | 4 | 8 | 4 | 8 | 8 | 8 | 4 | 8 | 8 | 4 | 4 | 4 | 4 | 4 | 4 |
| 80 | 100 | 100 | 64 | 1000 | ||||||||||||
| 26 | Heparin calcium | 64 | 64 | 64 | 8 | 8 | 8 | 10000 | 8000 | 8000 | 16 | 8 | 8 | 16 | 1024 | 1024 |
| 12000 | 15000 | 15000 | 9600 | 150000 | ||||||||||||
| 27 | D-Mannitol injection | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 |
| 80 | 100 | 100 | 64 | 1000 | ||||||||||||
* Sixty minutes assay was performed. Shadows indicate that the difference in the NID among 3 lots is equal to or more than eight-fold.
The variation in the NIDs within the three recombinant reagents is similar to that within five lysate reagents. All three recombinant reagents show little lot-to-lot variations of endotoxin-recoveries in all twenty-seven drugs. In contrast, four out of five lysate reagents showed lot-to-lot variations equal to or larger than 8-fold in seven of twenty-seven drugs examined.
As reported in literature,22,27–32) endotoxins from different bacterial strains showed highly varying potencies. This is due to their differing structures of the lipid A moiety and polysaccharide length (1.87 EU/ng of E. coli J5 versus 118 EU/ng of E. coli F583 Rd2) (Fig. 1).
The variance of the potency of each endotoxin among lysate reagents is maximally 5.5-fold and minimally 1.7-fold. On the other hand, all potencies of the thirteen endotoxins when tested with PyroSmart fell in the range between 50 of minimum and 200% of maximum of those with the three lysate reagents. In addition, potencies except for that of S. typhimurium obtained with PyroSmart fell in the range of the mean+/−3SD of the potencies with the three lysate reagents, suggesting again that the reactivity of PyroSmart to each endotoxin is almost equal to those of the three lysate reagents. Figure 4 also shows no significant differences in reactivities of the three recombinant reagents and the six lysate reagents.
All analytical characteristics in method validation of the PyroSmart satisfied the acceptance criteria (Table 2). Thus, the recombinant chromogenic reagent, PyroSmart can be applicable as an alternative to the BET in the pharmacopeias.
All minimum dilution factors expressed as NIDs of the 109 injectable drugs measured with PyroSmart were smaller than the MVDs of respective drugs (Table 3). In further experiment, the three recombinant reagents showed a similar susceptibility to interference in 27 drugs as the five lysate reagents (Table 4). Moreover, the lot-to-lot variation of susceptibility in the assays with the recombinant reagents was equal to or smaller than those with lysate reagents. Of the three recombinant reagents, only PyroSmart was able to detect endotoxin in the drug, Heparin Calcium, which strongly inhibited the detection by other recombinant reagents. The difference in susceptibility to the interference derived from Heparin Calcium between PyroSmart and the two recombinant factor C reagents may be attributable to the components, other than factor C, contained in PyroSmart. Heparin is known to interact with factors C and B,33,34) therefore Heparin Calcium may reduce the quantity of recombinant factor C available for endotoxin in PyroGene and EndoZyme also in PyroSmart. However factor B is also reported to be involved in endotoxin recognition as well as in signal transduction from the upstream factor C.35) Thus, the presence of factor B in PyroSmart may mitigate the strong inhibition by Heparin Calcium.
It is also reported that the recombinant factor C in PyroSmart produced from mammalian cells is less susceptible to interference by some substances than the recombinant products only containing factor C from insect cells or factor C purified from horseshoe crab.21)
In conclusion, an endotoxin assay using recombinant reagents can be an effective alternative assay for the Bacterial Endotoxins Test in the pharmacopeias. It would resolve the inconsistencies in test results caused by lot-to-lot variance of the lysate reagents and contribute to the conservation of the horseshoe crab.
This work was supported in part by a grant “Study of pharmaceutical and medical device regulatory science” from the Health Labour Sciences Research Grant and “Research on Regulatory Harmonization and Evaluation of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics” from the Japan Agency for Medical Research and Development, AMED.
Ogura, Mizumura, Aketagawa and Oda are employees of Seikagaku Corporation. Muroi and Tanamoto declare no conflict of interest.
The online version of this article contains supplementary materials.