2019 Volume 42 Issue 2 Pages 158-163
2019 Volume 42 Issue 2 Pages 158-163
Ghrelin is a circulating peptide hormone, which involved in promoting feeding and regulating energy metabolism in human and rodents. Abnormal synovial hyperplasia is the most important pathologic hallmark of rheumatoid arthritis (RA), which is characterised by tumor-like expansion. Existing studies indicated that there may exist some relation between the decreased ghrelin and the abnormally proliferating synovial cells in RA. Therefore, the aim of this study is to explore the apoptotic effects of ghrelin on MH7A synovial cells in vitro. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to evaluate the effects of ghrelin on the viability of MH7A cells. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick-end labeling (TUNEL) and flow cytometry were used to test the apoptotic effects of ghrelin. At last, Western blot and real-time PCR were performed to explore the expression of caspases-8, -9, and -3 after the treatment of ghrelin. MTT experiments showed that ghrelin could inhibit viability of MH7A cells. The results of flow cytometry and TUNEL showed that ghrelin could induce apoptosis of MH7A synovial cells. Western blot showed that expression of cleaved-caspases-8, -9, and -3 were increased in ghrelin stimulation group compared with the control group, while expression of pro-caspases-8, -9, and -3 had no significant difference. In mRNA levels, ghrelin can decrease pro-caspases-8, -9, and -3 mRNA expression, which confirmed the results of protein levels. Then these apoptotic effects were significantly reversed by [D-Lys3] GHRP-6 (ghrelin receptor antagonist). This study found that ghrelin can induce apoptosis of MH7A cells through caspase signaling pathways.
Rheumatoid arthritis (RA) is a chronic systemic autoimmune inflammatory disease mainly characterized by synovial hyperplasia, inducing joint destruction, functional disability.1) The etiology and pathogenesis of RA remain uncertain, but an increasing number of studies have found that fibroblast-like synoviocyte (FLS) played an important role in cell over-proliferation and inflammation.2) The imbalance of apoptosis and over-proliferation of FLS may contribute to synovial hyperplasia, which is similar to tumor cells.3) Apoptosis induction of FLS is suggested as a potential therapeutic strategy for RA.
Ghrelin is an important circulating peptide hormone consisting of 28 amino acids, which is mainly produced in the stomach during fasting, and then secreted into the systemic circulation.4) Growth hormone secretagogue receptor 1a (GHSR1a) is thought to be a biologically relevant receptor for ghrelin. With the accumulating studies of ghrelin and GHSR1a, they are found expressing in many other tissues (brain, lung, liver and cardiovascular) except gastrointestinal tissue. And the ghrelin system has been found to participate in the modulation of series of physiological functions, specifically food intake, endocrine actions, energy homeostasis, inflammatory processes, apoptosis, as well as in the development of several types of cancers.5) It has been reported that ghrelin can inhibit the growth of ovarian cancer cells through the extracellular signal-regulated kinase (ERK) pathway.6,7) Previous studies by investigators at our laboratory reported that serum ghrelin levels decreased in patients with active RA,8,9) so we hypothesized that there may exist some relation between the decreased ghrelin and the abnormally proliferating synovial cells.
Gram-negative bacterial lipopolysaccharide (LPS) is released during bacterial lysis, accumulating evidence shows that LPS exerts a direct influence on tumor cell proliferation, invasion and metastasis in vitro and in vivo. In addition, it has been reported that systemic injection of LPS could reduce the levels of plasma ghrelin in rat.10,11) The aim of this study is to explore the apoptotic effects of ghrelin on LPS induced rheumatoid arthritis fibroblast-Like synoviocyte MH7A cells in vitro. This would not only get a further understanding of RA pathogenesis, but also provide a new therapy strategy for RA.
MH7A cells were isolated from intra-articular synovial tissues of the knee joints from RA patients, and were obtained from Riken Cell Bank (Saitama, Japan).12) MH7A cells were incubated in modified RPMI-1640 (Hyclone, U.S.A.) with 10% heat-inactivated fetal bovine serum (Sijiqing, China), 100 units/mL of penicillin and 100 µg/mL of streptomycin (Solarbio, China), in an atmosphere of 5% CO2 in humidified air at 37°C.
ImmunofluorescenceMH7A cells was seeded onto microscopy cover glasses in 6-well tissue culture plates at a density of 1 × 105 cells/well. After 32 h culture, the culture medium was removed, and the cells were washed with phosphate buffered saline (PBS) and fixed with 4% paraformaldehyde for 30 min at room temperature. The fixed cells were permeabilized with 0.3% Triton X-100 for 30 min, stained with monoclonal mouse anti-ghrelin antibody (1 : 100 dilution in PBS, Abcam, U.K.) or rabbit anti-ghrelin (1 : 100 dilution in PBS, Abcam), washed, incubated with a 488-anti-mouse secondary antibody (1 : 200; Invitrogen, U.S.A.) and a Cy3-anti-rabbit for 1 h at room temperature. After incubated with 4′,6-diamidino-2-phenylindole (DAPI) to visualize nuclei, the sections were immediately examined with an Olympus IX71 fluorescence microscope (Olympus Optical, Tokyo, Japan) and images were captured with a CCD spot camera.
Cell-Viability AssayThe 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was carried out to measure cytotoxic effects of LPS and ghrelin on MH7A cells. A total of 100 µL cells (1.0 × 104 cells/well) were seeded into a 96-well, incubated overnight, and then treated with LPS and ghrelin at various concentrations for 24 h. Besides, some cells were pre-treated with [D-Lys3]-GHRP-6 (ghrelin receptor antagonist) (1 ng/mL) for 1 h. and then co-incubated with ghrelin (0.1 ng/mL) for 24 h. Twenty microliters of MTT reagent (5.0 mg/mL) was added to each well and incubated in the dark at 37°C for 4 h. The supernatant was aspirated and 200 µL of dimethyl sulfoxide (DMSO) added to dissolve the purple formazan precipitates. The absorbance at 490 nm was obtained using a microtiter plate reader (Tecan Sunrise, Switzerland). All experiments were repeated at least three times, and all samples were performed in triplicate. Depending on the results of this step, we can get an optimal stimulus concentration.
Apoptosis by Flow CytometryMH7A cells (5 × 105 cells) were seeded onto 30 cm dishes, and cultured overnight prior to assays. After the incubation, cells were divided into 3 groups: control, LPS (100 µg/mL), and ghrelin (0.1 ng/mL). Besides, some cells were pre-treated with [D-Lys3]-GHRP-6 (1 ng/mL) for 1 h. and then co-incubated with ghrelin (0.1 ng/mL) for 24 h. The concentrations were based on results of the MTT assay. After 24 h treatment, MH7A cells were analyzed using AnnexinV-fluorescein isothiocyanate/propidium iodide (FITC/PI) apoptosis detection kit in a flow cytometer (FACSCalibur system, Becton & Dickson, San Jose, CA, U.S.A.). The assay was carried out according to the manufacturer’s instructions in the assay kit.
Apoptosis by Terminal Deoxynucleotide Transferase (TdT)-Mediated Deoxyuridine Triphosphate (dUTP)-Biotin Nick End Labeling (TUNEL)Besides, we also used a TUNEL kit (Roche, Mannheim, Germany) to detect apoptotic cells. MH7A cells, which were seeded onto on glass coverslips, were treated with LPS (100 µg/mL) for 24 h, ghrelin (0.1 ng/mL) for 24 h. Besides, some cells were pre-treated with [D-Lys3]-GHRP-6 (1 ng/mL) for 1 h. and then co-incubated with ghrelin (0.1 ng/mL) for 24 h. Then they were fixed in 4% buffered formaldehyde for 1 h. After treating with 1% hydrogen peroxide (H2O2) for 30 min, MH7A cells were incubated with TdT and dUTP for 1.5 h at 37°C. Then, a 50 µL Converter-peroxidase (POD) was added to the samples for 40 min at 37°C. The samples were subsequently stained with 3,3′-diaminobenzidine (DAB) for 5 min. Counter-staining with haematoxylin was to identify the non-apoptotic cells. TUNEL-positive apoptotic cells exhibited brown nuclear staining. The number of apoptotic cells of MH7A was semi quantitatively assessed using light microscopy (4 different random high-powered fields, 400× magnification).
Western Blot AnalysisMH7A cells were lysed in RIPA lysis buffer (Beyotime, China), and protein content was determined using a protein assay reagent (Beyotime) with bovine serum albumin (BSA) as a standard. Each sample was resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and then transferred to 0.45 mm nitrocellulose membranes. After blocking with 5% nonfat milk (Biosharp, China), membranes were then incubated with primary antibodies against caspase 3 (1 : 500, Santa, U.S.A.), caspase 8 (1 : 1000, Abcam), caspase 9 (1 : 1000, Cell Signaling Technology, U.S.A.) at 4°C overnight. After washing the membranes with Tris buffered saline (TBS) for 5 times, appropriate Horseradish peroxidase (HRP)-conjugated goat anti-mouse and goat anti-rabbit secondary antibody (1 : 1000, Proteintech, China) was added for 1h. Proteins were detected by enhanced chemiluminescence (ECL) (Bio-Rad, U.S.A.) reagent. The protein level quantification was performed by Image Lab.
Quantitative RT-PCRFor the determination of mRNA expression of the target genes, total RNA was extracted using TRIzol reagent (Invitrogen). cDNA was prepared by reverse transcription using the cDNA synthesis kit (TaKaRa, Japan) according to the manufacturer’s protocol. Real-time PCR was performed in a LightCycler instrument (Bio-Rad CFX, U.S.A.) with the SYBRgreen mastermix kit (TaKaRa). The house keeping gene β-actin was used to normalization. Data analysis was carried out using the Bio-Rad CFX Manager Software Version 3.0 (Bio-Rad CFX). The results were analyzed using the comparative Ct method (also known as the 2−△△Ct method).13) All experiments were conducted at least three times. Humans pecific primers were as follows: β-actin (forward: 5′-TGG CAC CCA GCA CAA TGA A-3′; and reverse: 5′-CTA AGT CAT AGT CCG CCT AGA AGC A-3′); procaspase 3 (forward: 5′-GAC TCT GGA ATA TCC CTG GAC AAC A-3′; and reverse: 5′-AGG TTT GCT GCA TCG ACA TCT G-3′); procaspase 8 (forward: 5′-CGG ACT CTC CAA GAG AAC AGG-3′; and reverse: 5′-TCA AAG GTC GTG GTC AAA GCC-3′); procaspase 9 (forward: 5′-CAC CCA GAC CAG TGG ACA TT-3′; and reverse: 5′-CCT TCT GCT TGA CCT CCA TCT T-3′).
Statistical AnalysesStatistical analysis was conducted using GraphPad Prism 6.0. Data were presented as mean ± standard error of mean (S.E.M). Significant differences between each group was analyzed by one-way ANOVA analysis followed by a Tukey test. p < 0.05 was considered significant.
Immuno-fluorescence was performed to observe the localization of ghrelin and its receptor, which showed that they were expressed in cytoplasmic as well as membrane localization of MH7A synovial cells (Fig. 1).

Scale bar: 50 µm. (Color figure can be accessed in the online version.)
In this study, we used the MTT assay to verify our optimal concentration, which would be used in the subsequent experiments. Figure 2 showed that LPS can increase the cell viability of MH7A in a dose-dependent manner, 100 µg/mL is the best stimulus concentration with an increasing of 24.17% cell viability compared with control (p < 0.0001). Compared with control group, ghrelin in 0.1 ng/mL can significantly decrease cell viability (100.60 vs. 83.75%, p < 0.01); ghrelin coincubated with LPS can reduce the increasing of cell viability induced by LPS (124.17 vs. 103.78, p < 0.0001).

Data present the mean ± S.E.M. of three independent experiments. * p<0.05 versus control group; *** p<0.001 versus control group; **** p<0.0001 versus control group.
To investigate the cellular apoptosis induced by LPS and ghrelin, the MH7A cells were labeled with Annexin V-FITC/PI and analyzed by flow cytometry (Fig. 3). Both LPS (22.10 ± 2.30%) and ghrelin (18.17 ± 1.63%, p < 0.001) induced obviously apoptosis when compared with the control group (5.00 ± 0.40, p < 0.01), while there was no significant difference between LPS treatment group and ghrelin treatment group (p > 0.05).
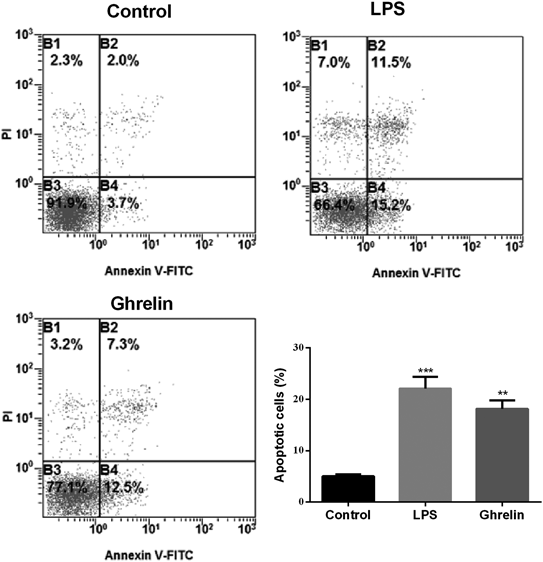
Data present the mean ± S.E.M. of three independent experiments. ** p<0.01 versus control group; *** p<0.001 versus control group.
As shown in Fig. 4, the number of TUNEL-positive apoptotic cells was significantly increased in ghrelin-induced cells of 24h (14.15 ± 0.78%) when compared to control group (8.32 ± 0.43%), p < 0.0001. The treatment of LPS resulted in a increasing of TUNEL-positive cells with 11.95 ± 0.36% (p < 0.01). The positive apoptotic cells of ghrelin treatment group were significantly higher than LPS treatment group (p < 0.001).
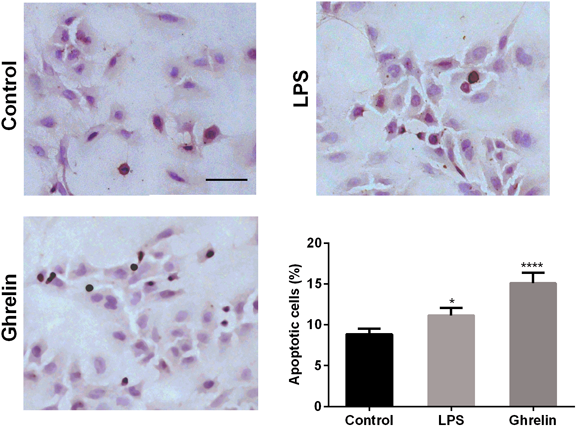
Apoptotic cells were stained brown nuclear. Scale bar: 50 µm. The data present the mean ± S.E.M. of three independent experiments. * p<0.05 versus control group; **** p<0.0001 versus control group. (Color figure can be accessed in the online version.)
First of all, we evaluated the effect of ghrelin on MH7A cells by examining the appearance of cleaved caspases-8, -9, and -3 after 24h stimulation. As shown in Fig. 5, LPS and ghrelin can significantly increase the production of cleaved caspases-8, -9, and -3 (p < 0.05), suggesting that all of the three can lead to the apoptosis of MH7A cells (Figs. 5a–c). This result was consisted with the result of flow cytometry and TUNEL. As for mRNA expression of pro-caspases-8, -9, and -3, there was no obvious difference between LPS stimulation group and control group, while ghrelin or ghrelin together with LPS significantly decreased the level of pro-caspases-8, -9, and -3 (Figs. 5d–f). The reduced in pro-caspase of mRNA was consisted with the increased of cleaved caspases-8, -9, and -3 in protein level.
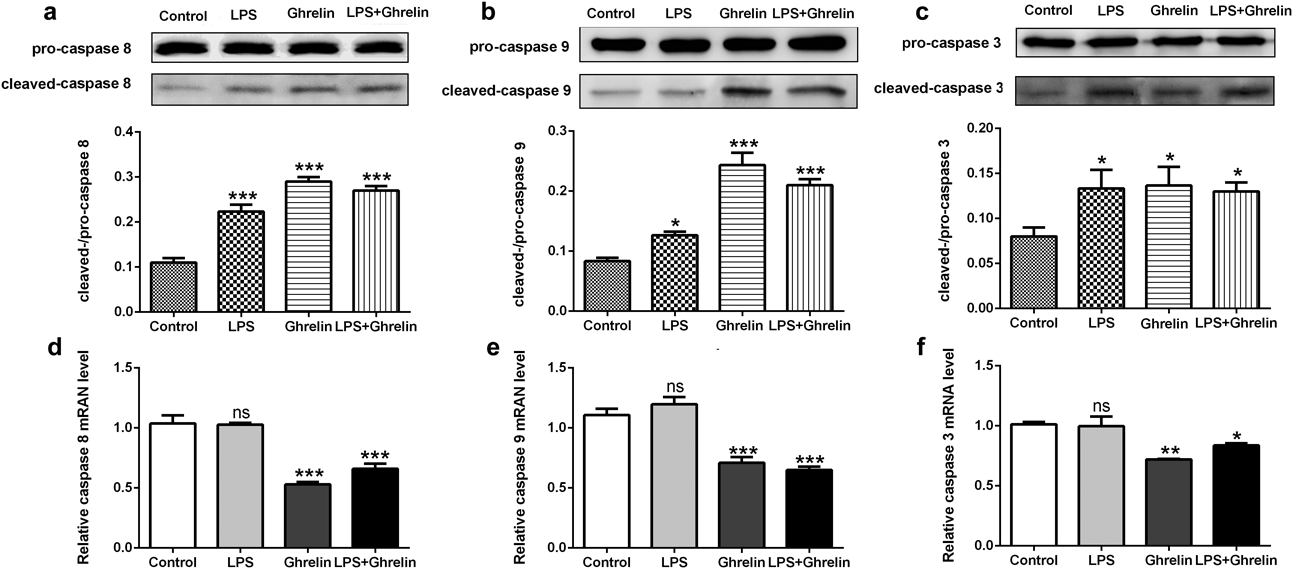
(a) Expression of cleaved/pro-caspase 8 in protein level; (b) Expression of cleaved/pro-caspase 9 in protein level; (c) Expression of cleaved/pro-caspase 3 in protein level; (d) Relative expression of pro-caspase 8 in mRNA level; (e) Relative expression of pro-caspase 9 in mRNA level; (f) Relative expression of pro-caspase 3 in mRNA level. * p<0.05 versus control group; ** p<0.01 versus control group; *** p<0.001 versus control group.
MH7A cells were pretreatment with [D-Lys3] GHRP-6 for 1 h, and then co-incubated with ghrelin (0.1 ng/mL) for 24 h. As showing in Fig. 6, [D-Lys3] GHRP-6 could fully block the apoptosis induced by ghrelin in MTT assay (Fig. 6a, control vs. ghrelin+ [D-Lys3] GHRP-6, p > 0.05), flow cytometry (Fig. 6b, control vs. ghrelin+ [D-Lys3] GHRP-6, p > 0.05), and TUNEL (Fig. 6c, control vs. ghrelin+ [D-Lys3] GHRP-6, p > 0.05). Besides, there were no significant differences between the control group and ghrelin+ [D-Lys3] GHRP-6 in the expression of cleaved caspases-8, -9, and -3 (Figs. 6d1–d3, p > 0.05). Above all, these results suggested that apoptotic effect of ghrelin is involved in the ghrelin receptor system.

Data is presented as mean ± S.E.M. Differences between groups were analyzed by one-way ANOVA followed by a Tukey test. (a) MTT assay; (b) Apoptosis by flow cytometry; (c) Apoptosis by TUNEL; (d1) Expression of cleaved/pro-caspase 8 in protein level; (d2) Expression of cleaved/pro-caspase 9 in protein level; (d3) Expression of cleaved/pro-caspase 3 in protein level. *** p<0.001 versus control group; ns p > 0.05 versus control group.
RA is a chronic inflammatory disease. Abnormal synovial hyperplasia is the most important pathologic hallmark of RA, which is characterised by tumor-like expansion. Synovial cells aggressively invade into the cartilage and bones cause direct dysfunction of joints. Hence, inhibiting proliferation and inducing apoptosis of RA-FLS is an efficient therapeutic means for RA. Besides, the decreased of RA-FLS can reduce the production of pro-inflammatory cytokines.14) Ghrelin, a 28-amino acid peptide, is mainly secreted by the stomach. Existing studies indicated that ghrelin may participate in the pathogenesis of RA. Serum levels of ghrelin in RA patients were varied by the activity of RA and different treatment strategies.15–18) And systemic administration of ghrelin could decrease the inflammatory reaction and joint destruction of CIA mouse.19)
MTT experiments showed that ghrelin could inhibit the cell viability of MH7A cells. The results of flow cytometry and TUNEL showed that ghrelin could induce the apoptosis of MH7A synovial cells. These results were consistent with some previous evidences, which have reported that ghrelin can attenuate the growth of ovarian cancer cells through the ERK pathway.6,7) While in numerous normal tissues, ghrelin can stimulate cell proliferation and inhibit apoptosis. Such as, brain neurons of mouse,20) renal tubular epithelial cells NRK-52E cell,21) liver of mouse,22) mesenchymal stem cells in ischemic heart,23) islet cells,24) spermatogoniac cell of mouse.25) Besides, ghrelin neither inhibited nor induced neutrophil apoptosis.26)
There had been a lot of related reports about ghrelin interaction with LPS in different tissues and cells, and most previous studies found that ghrelin protected cells against LPS induced apoptosis, such as mouse dopaminergic neurones cell,27) alveolar macrophages,28) and oligodendrocyte cells.29) Our results indicated that ghrelin can induce apoptosis of MH7A cells, while the effect of LPS for MH7A cells was puzzling. LPS not only increased cell viability of MH7A cells, but also induced cell apoptosis. The mechanism underlying is unknown, we guessed that was the interaction of its pro-apoptotic and pro-inflammatory effect. More mechanism studies are needed to explore this phenomenon.
As we all know, caspases-8 and -9 are essential molecule of extrinsic and intrinsic apoptotic pathways, respectively; and caspase-3 is the executor of these two pathways. The cleavaged subunit of these proteases would result in cell death.30) Caspase-3 is one of the most studied proteases, which can directly lead to the disintegration of apoptotic cells. Activation of this protease makes cells into an irreversible apoptosis pathway.31) The results of this studies indicated that the expression of cleaved-caspases-8, -9, and -3 were increased in ghrelin stimulation group compared with the control group in protein levels, while expression of pro-caspases-8, -9, and -3 had no significant difference. In mRNA levels, we found that ghrelin can decrease pro-caspases-8, -9, and -3 mRNA expression, which confirmed the results of protein level. Collectively, these results demonstrated that ghrelin promoted MH7A cells apoptosis by activating endogenous mitochondrial pathway and exogenous death receptor pathway. While the mechanism and relationships between ghrelin signaling and induction of caspase cascades are unknown, further researches should be conducted.
In conclusion, the present study found that ghrelin can induce apoptosis of MH7A cells through caspase signaling pathways. Although further studies are warranted to make clear the regulatory mechanism of ghrelin in RA, our studies provide a novel idea of ghrelin can induce synoviocyte apoptosis in RA patients, which may provide a new treatment strategy for RA.
This work was supported by National Natural Science Foundation of China (NSFC) of China (Nos. 81273280 and 81272176). We are greatly indebted to the technical guidance of laboratory technicians in the Department of Human Anatomy and Histology and Embryology, the Fourth Military Medical University.
The authors declare no conflict of interest.