2019 Volume 42 Issue 3 Pages 475-480
2019 Volume 42 Issue 3 Pages 475-480
Human papillomaviruses (HPVs), such as HPV 16 and HPV 18 are related to cervical cancer. Therefore, it is important to inhibit HPV-positive cervical cancer for treating cervical cancer. This study is aiming at investigating the proposed molecular mechanism, which underlies the antineoplastic potential of the aqueous extract of juglone of HPV-positive cervical cancer cells. According to the results, it is showed that, juglone prohibited HPV positive cervical cancer cells’ growth through dose-dependent way. Nevertheless, when pin 1 was knocked down, the proliferation inhibition reduced. The detection of apoptosis and cell cycle also illustrated that juglone influenced HPV positive cells. Western blot expressed the influence mechanism that it affected the B-cell lymphoma 2 (Bcl-2) family and later activated the Caspase-depended apoptosis way. It is contributable for this study to understand the mechanism of inhibiting HPV positive cells by juglone and it also provides an effective strategy for the application of it in the future.
Cervical cancer is the second of high incidence malignant tumors of women.1) The incidence of cervical cancer is related to human papillomavirus (HPV), which has some HPV subgroups, such as HPV 16 and HPV 18, referred to as Subgroup 2 with high risks.2) The mechanism is that, the HR-HPVs with high risks encoded E 6 and E 7 viral oncoproteins, can disturb with p53-tumor suppressor proteins & retinoblastoma respectively, and cause abnormal proliferation of cells.3)
Juglone is a natural product with the chemical structure of 5-hydroxy-1, 4-naphthoqulnone, and it is extracted from leaves, roots, nut-hulls, wood and bark of Juglans mandshurica MAXIM. Its structure is similar to the structure of plumbagin.4) Earlier research showed that Juglone demonstrates allelopathic activities besides its antiviral, antibacterial and antifungal activities. At present, several studies showed that juglone had strong in vitro cytotoxic effect on human tumor cell lines, which includes HL-60 (leukaemia), human gastric cancer SGC-7901 cells, MDA-MB-435 (melanoma), human prostate cancer LNCaP cells, human glioblastoma U251 cells and human lung cancer cell lines.5–10)
The antitumor effect of juglone can be realized through various ways, such as the induction of DNA damage,11) reducing p53 protein level by inhibiting transcription,12) inducing cell death13) and own-regulating androgen receptor (AR) expression.8) However, it still remains unknown that the in-vitro mechanism of juglone including tumor cells apoptosis .
Juglone’s antioxidant and cytotoxic activity in HeLa cells14) was reported by us previously. Nevertheless, it still remains unknown that the in vitro mechanism of juglone including tumor cells apoptosis. The putative molecular mechanism of the aqueous extract of juglone in cervical cancer was investigated by us in this study. Our research showed that juglone inhibits cervical cancer cells growth by facilitating cell apoptosis, and meanwhile, reduce the expression of Pin 1 in HPV positive cells shall rescue the apoptosis which was induced by juglone, and it is suggested that the potential influence of juglone in cervical cancer treatment.
RPMI-1640 culture medium, Cell lysis buffer for Western (Invitrogen Corp., U.S.A.); Juglone was bought from Sigma-Aldrich Corp. (St. Louis, MO, U.S.A.) with the purity of 97%; Lipofectamine 2000 (Invitrogen); Rabbit anti-human Pin 1, Bax, pro-Caspase-3, poly(ADP-ribose)-polymerase (PARP), pro-Caspase-8, pro-Caspase-9, and β-actin antibody (Epotomics Inc., U.S.A.); fetal bovine serum, pancreatin (Grand Island, NY, U.S.A.); Annexin V-fluoresceine isothiocyanate (FITC) Apoptosis Detection Kit and propidium iodide (Sigma-Aldrich).
Cell CultureCell lines of human cervical carcinoma, HeLa (HPV18 positive), Caski (HPV16 positive), and C33A (HPV18/16 negative) sourced from Cell Bank of Wuhan Institute of Biochemistry and Cell Biology (Wuhan of China). All of the cells were cultured in RMPI-1640, which were supplemented with 10% fetal bovine serum at 37°C with 5% CO2 in a humidified incubator. These cells were obtained after trypsinization (0.02% ethylenediaminetetraacetic acid (EDTA) and 0.025% trypsin) and they were washed for twice using phosphate buffered saline (PBS). The cells were sub-cultured while the cell density reached about 80% confluence, and then juglone was added to the cells after incubation for 24 h.
Assays of Cell ViabilityAbout 1 × 104 cells were plated/well in 96 well microtiter plates with overnight growth, and they were treated using various concentrations of juglone for 24 h in order to test the effects of Juglone on cell viability. Viability of cell was analyzed by means of MTS assay per instructions (Promega) of manufacturer. Optical absorbance was tested by a microplate reader (Apollo LB 9110; Berthold Technologies GmbH, Germany) at 492 nm.
RNA’s InterferencePin 1 short hairpin RNA (shRNA) (5′-CCA CCG TCA CAC AGT ATT TAT-3′) was described15) previously. Caski cells were infected with lentiviruses expressing Pin1 shRNA for establishing stable Pin1 knockdown cell lines. By using 0.5 mg/mL puromycin, stably knockdown cells were chosen.
Morphological Observation on Nuclear ChangeCervical cancer cells (1 × 104 cells/mL) were treated with juglone at 37°C for 24 h with designated concentration, and the nuclear morphological change was evaluated by Hoechst 33258. After 24 h, the cells were observed through phasecontrast microscopy by an Olympus model Bx61/Bx62 inverted microscope, which was equipped with a MCA-85001 camera (America).The Hoechst dye was executed at 340 nm and fluorescence emission was filtered by a 510 nm barrier filter.
Cell Cycle Analysis through Flow CytometryAbout 1 × 104 cells/well was cultured in six well plates, which had been incubated for the whole night, and then treated with apigenin with different concentrations. Later, cells were washed for twice with PBS 24 h, and then then they were collected and fixed at 4°C in 75% ethanol for whole night. Then, cells were washed for twice with PBS, re-suspended in propidium iodide (PI) solution (50 µg/mL) which contains ribonuclease (RNase) A, and incubated at room temperature for 30 min. Cell cycle was analyzed by the BD LSRII Flow Cytometer System with FACSDiva Software (BD Bioscience, Franklin Lakes, NJ, U.S.A.). Data were analyzed using ModFit LT 3.2 software (Verity Software House, Topsham, ME, U.S.A.).
Analysis of Cell Apoptosis through Flow CytometryCells were collected from six well plates and then washed after apigenin treatment with PBS 48 h. Then, cells were incubated in mixed binding buffer (including annexin V-FITC and PI) in the dark for 15 min in accordance with the instructions of manufacturer. It was analyzed by the BD LSRII Flow Cytometer System with FACSDiva Software (BD Bioscience) within 1 h. The percentage of apoptotic cells was quantified by FACSDiva software.
Analysis of Western BlotSamples and cells were dissolved in lysis buffer (0.5% Triton X-100, 50 mM Tris, pH 7.4, 2 mM EDTA, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS) and 1% Nonidet P-40 and). Whole protein was extracted through centrifugation (14000 × g) at 4°C for 15 min. Proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, U.S.A.) blocked in 5% bovine serum albumin (BSA). Then, the membrane was probed for the whole night in blocking buffer at 4°C with primary antibodies before washing in TBST (0.02 M Tris pH 7.6, 0.8% NaCl, 0.1% Tween-20) and incubated in TBST for 1 h at RT with secondary antibodies (1 : 10000). After re-washing in TBST, the chemiluminescence liquid (Millipore) was put and fluorescence was obtained on photographic film (Kodak, Tokyo, Japan).
Statistical AnalysisData are expressed according to the mean ± standard deviation. Statistical analysis was executed between various treatment groups and controls through analyzing variance in one way. Data were analyzed with SPSS software (Version 18.0; SPSS Inc., Chicago, IL, U.S.A.). p < 0.05 was considered as indication of statistically significant difference.
The influences of Juglone on growth kinetics in Caski, HeLa and C33A cells were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay through measure cell viability. After culturing juglone for 24 h, both Caski and HeLa cells’ viability reduced by means of dose dependent manner from 10 to 100 µM significantly. Nevertheless, it was observed that C33A (Figs. 1A–C) had also decrease of viability but lower than another cell lines. In the same way, both Caski and HeLa cells’ viability dropped dramatically by means of time dependent from 24 to 72 h (Fig. 1D) through culturing only with 20 µM juglone. According to the results, compared to negative cells, proliferation rates of juglone-treated HPV positive cells were reduced.
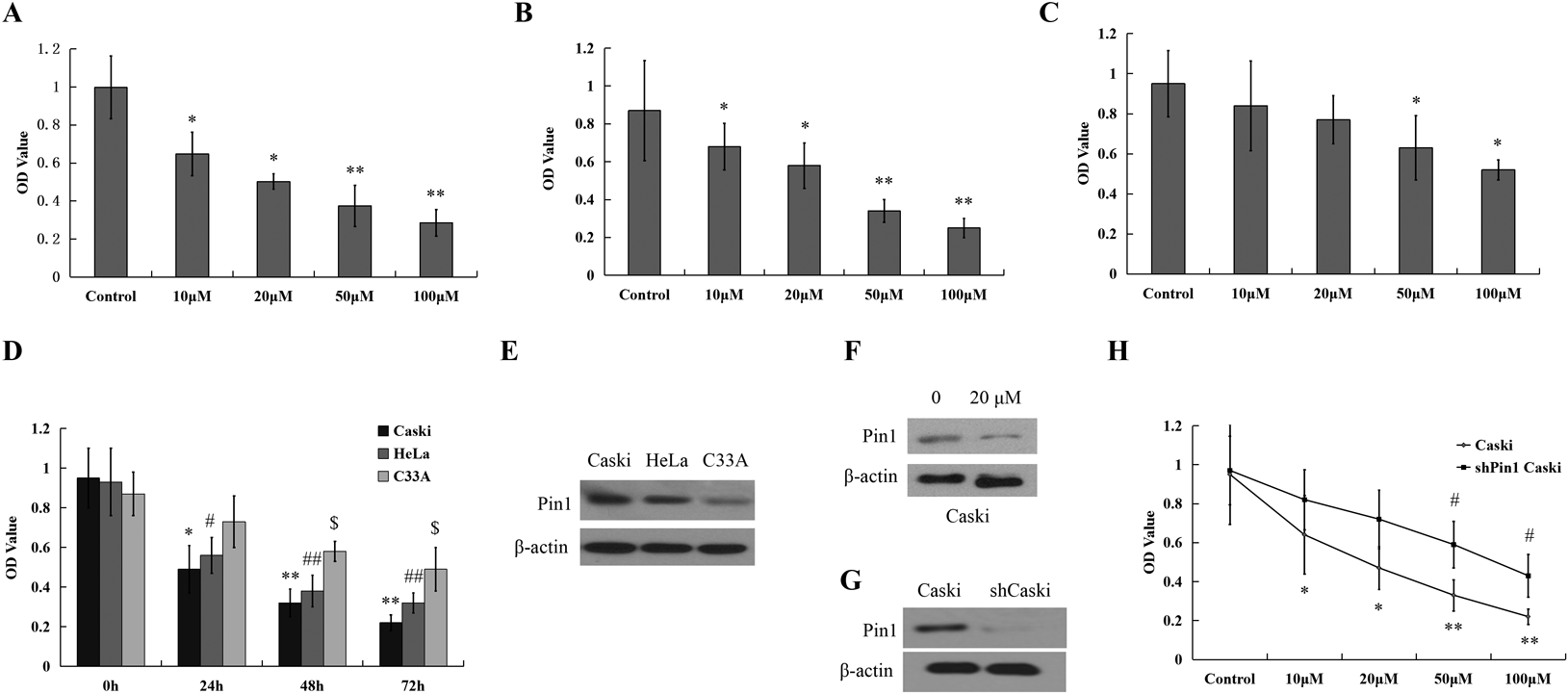
Cervical cancer cells were seeded in 96-well plates and cultured with juglone for different time. Cell viability was determined by MTT assay. Values are means ± standard deviation (S.D.) of five experiments. * p < 0.05 and ** p < 0.01, compared with untreated group. (A): HeLa; (B): Caski; (C): C33A; (D): Cervical cancer cells were treated with 20 µM of juglone for 24, 48, 72 h. *#$ p < 0.05 and **## p < 0.01, compared with control group; (E): Expression Pin1 in different cell lines; (F): Effect expression of Pin1 by juglone; (G): Caski cells were transfected with shPin 1; (H): Cells and trandfected cells were treated with different concentrations of juglone for 24 h. *# p < 0.05 and ** p < 0.01, compared with untreated group.
Pin 1 is a highly conserved and essential mitotic regulator involved in phosphorylation signaling.16–18) It is further involved in several events about pivotal oncogenic cellular, like angiogenesis, cell proliferation and tumor metastasis.19) Compared with scrambled shRNA control cells, pin 1 expression in Caski cells was stably knocked down by validated pin 1 shRNA lentiviruses for investigating the importance of the death of Pin 1 in juglone-induced cell, which caused effective pin 1 knockdown (Fig. 1E). Then Pin 1 knockdown cells were treated for 24 h with different concentration of juglone. It was suggested according to the results that pin 1 knockdown decreased the ability of juglone to induce cell death of Caski cells (Fig. 1F) drastically.
Triggers G2/M Phase Cell Cycle Arrest of Juglone through ATM SignalingCompared with control cells, expected cell cycle arrest in G2/M phase was observed in Caski cells treated with 20 µM juglone. Analysis of cell cycle from 3 independent experiments expressed that the G2/M phase-arrested population increased to 38.97% in 20 µM juglone treated cells, up from 17.38% in controls (Figs. 2A, B). Treatment also reduced the population of G1 phase.

(A): Caski and C33A cultured with 20 µM juglone for 24 h and then cell cycle detected through FCM (B): Different cell cycle according (A); (C): Expression levels of protein realted cell cycle by Western blot when treated with 20 µM juglone. * p < 0.05 and ** p < 0.01, compared with untreated group.
Ataxia telangiectasia, mutated/ATM and Rad3-related (ATM/ATR) signaling significantly regulated DNA damage-induced G2/M phase arrest. ATM could activate major downstream effectors such as Phosphorylated Chk2 (p-Chk2), phosphorylated Cdc2 (p-Cdc2 on tyrosine 15) and phosphorylated Cdc25c (p-Cdc25c on serine 216). Protein electrophoresis results showed that, after cultured with 20 µM juglone for 24 h, ATM expression showed no statistical difference among Caski cells, with phosphorylated ATM (p-ATM) significantly increased, and little differences between ATM and p-ATM in C33A cells (Fig. 2C). Accordingly, it was confirmed by our results that, DNA damage-induced G2/M phase arrest activated ATM-mediated cell signaling in HPV positive cells by juglone.
Effect of Juglone-Induced Morphologic Change of CellCervical cancer cells were incubated with 20 µM juglone. Nuclei were kept with Hoechst 33258, a kind of cellpermeable blue fluorescent DNA dye for detecting nuclear condensation & fragmentation, apoptosis’ characteristics and nuclear condensation % was also counted (Fig. 3A). It was observed that controlling Caski cells had normal nuclear morphology. In contrast, the condensation of nuclei characteristic of apoptotic cells were viewed in Caski cells cultured for 24 h with 20 µM of juglone. Furthermore, compared with the control group, after C33A cells had been also cultured in the same way, the nuclear morphology has little change.

(A): Caski and C33A cultured with 20 µM juglone for 24 h and then Nuclei change stained with Hoechst 33258 deteced through fluorescence microscope; (B): Caski and C33A cultured with 20 µM juglone for 24 h and then cell apoptosis detected by FCM with AnnexinV-FITC and PI double labeling. (C): Quantitative analysis of data the lower right quadrant. Data were showed as mean ± S.D., n = 3, ** p < 0.01 and * p < 0.05 vs. control.
The cells were kept with Annexin V-FITC/PI and analyzed by flow cytometry subsequently for further quantifying the apoptosis of cervical cancer cells induced by juglone. According to Figs. 3B and C, apoptotic cells’ quantity increased after treating for 24 h with 20 µM juglone. The ratio of early apoptotic cells [(Annexin V+/PI−) cells, LR] increased from 3.32 (untreated) to 12.23% (20 µM) in Caski cells significantly, and increased from 3.03 to 6.45% in C33A cells slightly.
Juglone Promoted Apoptosis through Up-Regulating Apoptosis-Related Signaling PathwaysBcl-2-associated protein (Bax) and B-cell lymphoma 2 (Bcl-2) played a promoting role in cell apoptosis through mitochondrial permabiliztion, so they are considered as the p53-dependent apoptotic markers; Therefore, protein electrophoresis was used to observe Bax and Bcl-2 proteins’ expression in Caski and C33A cells after treating with juglone for investigating whether juglone cause cell apoptosis by mitochondrial way. After treating with juglone for 24 h, the protein level of pro-apoptotic factor Bax increased, meanwhile, Bcl-2 protein level decreased without little difference in C33A cells (Fig. 4A). It was suggested that, junglone can regulate the functional disorder of apoptosis markers of Bax and Bcl-2 induced mitochondrial membrane potential, which caused the potential release of cytochrome c (Fig. 4B).

(A) and (B): Total protein extracts were prepared after applied for 24 h, and cultured with primer antibodies to Bcl-2, Bax, cytoplasmic and mitochondrial CytC. (C): Expression of pro-Caspases-3, cleaved-Caspase-3 and cleaved-PARP after juglone applied for 24 h.
Cytoplasmic and mitochondrial fractions were executed and analyzed using Western blot for testing whether the cytochrome c release was referred in the juglone-induced apoptosis or not. According to the results, it was known that juglone treatment caused significant increase in the level of cytoplasmic cytochrome c and a concomitant decrease of mitochondrial cytochrome c in Caski cells (Fig. 4C).
Juglone Induced Apoptosis by Activation of Caspase-3 and PARP CleavageThe expression levels of cleaved Caspase-3, pro-Caspase-3 and PARP were assessed for further investigating the mechanism of juglone-induced apoptosis in Caski cells. Analysis of Western blot showed that treatment with juglone caused the activation of caspase-3 expression, but the level of pro-Caspase-3 protein decreased and the level of the activated 17-kDa subunit increased. Additionally, although juglone treatment caused cleavage of PARP, the level of the 89-kDa cleaved subunit also increased (Fig. 4C). It was suggested by these results that juglone may induce apoptosis through activating the caspase pathway.
In this study, juglone—a plumbagin extracted from the Juglans mandshurica indicated anti-cancer activity for human cervical cancer cells by inducing the apoptosis in HPV positive cells.
At present, natural products are considered as potential therapeutic agents, for example: matrine could inhibit cell cycle,20) camptothecin could block cells proliferation,21) and podophyllotoxin could induce cells apoptosis.22) Accordingly, juglone has been extensively applied as chemotherapeutic agent in Chinese herbal medicine for various tumors, such as leukaemia,5) melanoma,6) gastric cancer,7) and pancreatic cancer23) through activating caspase pathway and the increase of reactive oxygen species (ROS).24) At present, it is found that juglone could prohibit cell proliferation and induce apoptosis of in-vitro HeLa cells.14) But, whether it has cytotoxic effect on other cervical cancer cells is still unknown.
In this study of us, it was observed that juglone markedly inhibited the proliferation of HeLa and Caski cells by dose-dependent means, but little for C33A cells. As it was described in previous studies, juglone specifically inhibited the prolyl isomerase Pin 1.25) Later, we supposed that if the inhibition of juglone on HPV positive cells was related to the expression of Pin 1. Thus, we knocked down pin 1 gene in Caski cells and found that the proliferation inhibition had been reduced.
Confirming whether the decrease of cells viability is crucial for apoptosis, since the induction of apoptosis of cancer cells is a main indicator of anticancer effects.26) Thus, fluorescence microscopy was applied for observing the morphological changes in the Caski cells and C33A cells, at the same time the extent of apoptosis was also quantified through flow cytometric analysis. The prominent characteristics of apoptosis and the number of apoptotic cells increased only in Caski cells after juglone culturing.
Bax and Bcl-2 were considered as apoptosis markers, which could change mitochondrial permabilization, cause release of cytochrome c, therefore initiating apoptosis.27,28) The mitochondrial pathway mentioned above is important for inducing cancer cell apoptosis during chemotherapy, but the Bcl-2 family members and cytochrome c are vital. The Bcl-2 family members regulated mitochondrial release of cytochrome c mainly through regulating Bax and Bcl-2 proteins levels and the ratio of Bax/Bcl-229,30) increased. It was showed in previous studies that cell apoptosis was accelerated by Bax trans-located to the mitochondria. After treating Caski cells with juglone, Bax was found to be trans-located to the mitochondria, and caused the release of cytochrome c from mitochondria to the cytosol.31,32) At present, it is also observed by us in the study that, in Caski cells cultured with juglone, Bax expression was up-regulated, Bcl-2 expression reduced and Bax/Bcl-2 ratio was also improved. Therefore, it can be concluded that, cell apoptosis process induced by juglone was related to BcL-2 family.
After releasing into the cytoplasm from the mitochondria, cytochrome c, apoptotic protease activating factor 1 (APAF1), and a Caspase-9 precursor form an apoptosome that activates Caspase-9.33) Then, the activated Caspase-9 cleaved and activated Caspase-3 and PARP and induced apoptosis finally.34) In the present study, it was found that, juglone-induced Caski cell apoptosis was accompanied by significant release of cytochrome c from the mitochondria to the cytoplasm. The expression of Caspase-3 and its substrate, PARP, were analyzed for investigating its downstream apoptotic signal transduction process. According to the results that juglone reduced pro-Caspase-3 expression of Caski cells and elevated the expression of the activated 17-kDa cleavage fragment and 89-kDa PARP cleavage fragment by juglone concentration-dependent way significantly. It indicates that Caspase-3 is activated during Caski cell apoptosis and promoted cell apoptosis by acting on its substrate, PARP. But, further studies are needed for identifying the precise underlying mechanisms of related signaling pathway.
Generally, it was suggested by our results that juglone can induce apoptosis mediated by inhibiting Pin 1 expression and by inducing intrinsic pathways in HPV positive cervical cancer effectively. Juglone triggered apoptosis through up-regulating Bax, down regulation of Bcl-2 and Bax translocation with mitochondrial release of cytochrome c into the cytosol and activation of effector Caspase-3. But this effect was obviously in cervical cancer cell lines expressing HPV. All of the phenomenon induced by juglone may be in relation to the expression of Pin 1, the further mechanism is needed in the future.
At present, the anti-tumor effect of Jjuglone is limited to in vitro studies and a small number of animal studies, while the clinical studies have not yet been on processing. There are huge difference between the pharmacological effects and drug effects in vitro, so further research on juglone will focus on the development and clinical application as a new anti-tumor drugs.
This work was supported by Science and Technology Department of Jilin Provincial (20130101157JC) and Education Department of Jilin Province (JJKH20191062KJ) jointly. The authors thank Dr. An Jian (Mayo Clinic University, U.S.A.) gratefully for his expert advice and critical reading of the manuscript.
The authors declare no conflict of interest.