2019 Volume 42 Issue 5 Pages 671-679
2019 Volume 42 Issue 5 Pages 671-679
Nicotiana glauca is a cosmopolitan shrub, used in medicine to treat swellings, wounds, sores and cancer. However, its users lack of knowledge of the adverse effects. We seek to evaluate the effects of lipid extracts from N. glauca on myoblasts, identifying the compounds which cause undesirable effects. Myoblasts are important in muscle homeostasis, thus a high death rate of them cause myopathies. We performed an ethanolic extraction from leaves of N. glauca and the extract was successively partitioned with hexane, chloroform and ethyl acetate. The effects of extracts in C2C12 cells were analysed by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL), Mitotracker and 4′,6-diamidino-2-phenylindole (DAPI) staining, Western blotting, real-time PCR and immunofluorescence assays. Caspase activity was studied. The fraction with the highest apoptotic effects was analysed by chromatography, NMR and GC-MS spectrometry were used to identify the apoptotic agent, after which its biological activity was evaluated. The extracts from N. glauca induced apoptosis in C2C12 cells involving caspase-3/7. We found that the extracts trigger a defence response in muscle through Akt and heat shock protein 27 (HSP27). We identified an apoptotic agent as palmitic acid. These data suggest that the use of N. glauca in hormone replacement therapy, or in other therapies affects skeletal muscle homeostasis, worsening the negative effects of the menopause. Thus, the relevance of this work lies in the fact that it is the first time that a report about the molecular mechanism responsible for the side effects of medicinal use of N. glauca, has been shown. Moreover the compound responsible for these effects has been identified.
Plant compounds, phytochemicals, serve as a source of medication. Almost 80% of present day drugs are derived from plants.1–3) Plants generate alkaloids, saponins, terpenoids, tannins, phenolic compounds and flavonoids, which have played a role in the maintenance of health since ancient times.4) However, plant extracts have been traditionally used without any knowledge of their side effects or of the molecular mechanisms responsible for their medicinal action.
Among the flowering plant families, Solanaceae has species of medicinal value.3,5) The Solanaceae Nicotiana glauca has been used to treat swellings, bruises, cuts, boils, inflamed throats, swollen glands and jaundice.6) Moreover, Solanaceae provide phytoestrogens.2,7) Phytoestrogens became the focus of interest as alternatives for hormone replacement therapy (HRT) or due to the therapeutic actions in carcinogenesis and atherosclerosis (Review in ref. 8). Although there is evidence that phytoestrogens can fulfil those actions, it remains to be proven: controlled interventional studies are lacking and side effects have not been evaluated.
Previously, we determined the contents of 17β-estradiol, estrone and progesterone-like molecules in Nicotiana glauca.9–11) These confirm that N. glauca could be used to ameliorate menopausal symptoms such as osteoporosis or sarcopenia.
Adult skeletal muscle increases its size and shows a remarkable adaptability to injury. However, skeletal muscle cells are postmitotic and cannot replicate. Therefore, any increase in myonuclear number required for the growth or repair of muscle depends on satellite cells, a pool of myogenic precursor cells.12,13) Satellite cells localise under the basement membrane, outside the plasma membrane of the muscle fibre. Their co-localisation with blood vessels14) places them in an optimal position to respond to intrinsic signals from both the muscle fibre itself and from the systemic environment. Thus, satellite cells are sensitive to external stimuli. Satellite cells exist in a quiescent state after birth and begin to proliferate in response to regulatory factors during development or injuries.15,16) Then, they are activated by either physiological or pathological stimuli, such as exercise or degenerative diseases. Satellite cells play a key role in sarcopenia associated with apoptosis by hormonal dysregulation (Review in ref. 17).
Plant extracts exert pharmacological and physiological functions, including apoptosis.18–20) These apoptotic effects have been preferentially attributed to phytosterols of the plant lipid extracts, preferentially21–23) or to the content of fatty acids.
N. glauca has ethnomedicinal uses and potential in HRT. Thus, the goal of this study was to investigate the effects of N. glauca on myoblasts, in relation to apoptosis as a cause of sarcopenia and other myopathies (Review in ref. 17). Therefore, we seeks to elucidate the putative side effects of the N. glauca extracts, identifying the compounds responsible for them.
The ability of lipid extracts from N. glauca to induce apoptosis in muscle cells was evaluated. Previously, we observed that H2O2 induced apoptosis in C2C12 cells.24) C2C12 cells were challenged by crude extracts during the times indicated and apoptotic events were investigated (Methods). The dye 4′,6-diamidino-2-phenylindole (DAPI) showed morphological changes typical of apoptosis such as nuclear fragmentation/condensation (pyknosis) after treatment with the crude extract from N. glauca, which represented close to 70% of the cultured cells in similar fashion as those treated with 0.5 mM H2O2. In addition, morphological changes and cellular redistribution of mitochondria could be detected in C2C12 cells treated with ethanolic extract from N. glauca and then stained with the mitochondrial probe MitoTracker (Methods). Thus, Fig. 1 shows that cells treated with vehicle (control) displays a uniform distribution of mitochondria through the cytosol; and the organelles present normal morphology. On the other hand, when apoptosis was induced with H2O2 or the crude extract, we observed reduced mitochondria size and characteristic clustering of the organelles around the nucleus (which represented almost 70% of the cells), events associated to apoptosis. To confirm those observations, we evaluated the effects of the crude extract from N. glauca on C2C12 cells by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assays (Fig. 2). We perform the same experimental conditions as before. Cells treated with the crude extract exhibited a large increase in DNA fragmentation (63 ± 9.09% of TUNEL positive cells above the control), similar to the values obtained with H2O2 treatment (Fig. 2).

C2C12 cells grown on coverslips as 60–70% confluent monolayers were treated (see below), stained with MitoTracker Red, and fixed with methanol as described under Materials and Methods Section. (CONTROL) Untreated cells. (CRUDE EXTRACT from N. glauca) Cells treated with crude extract (Methods) during 2 h. (H2O2) Cells treated with H2O2 0.5 mM during 2 h. Untreated cells present normal mitochondrial morphology and distribution throughout the entire cell distant to the nucleus or display ‘spiderweb’ mitochondria; but cells treated with the crude extract exhibit mitochondria clustered around the nucleus with condensed or pyknotic aspect as cells treated with H2O2. At least ten fields per slide and three independent cultures were examined. The content of palmitic acid in the crude extract is 4 µg. Representative photographs are shown. Magnification: 63X.
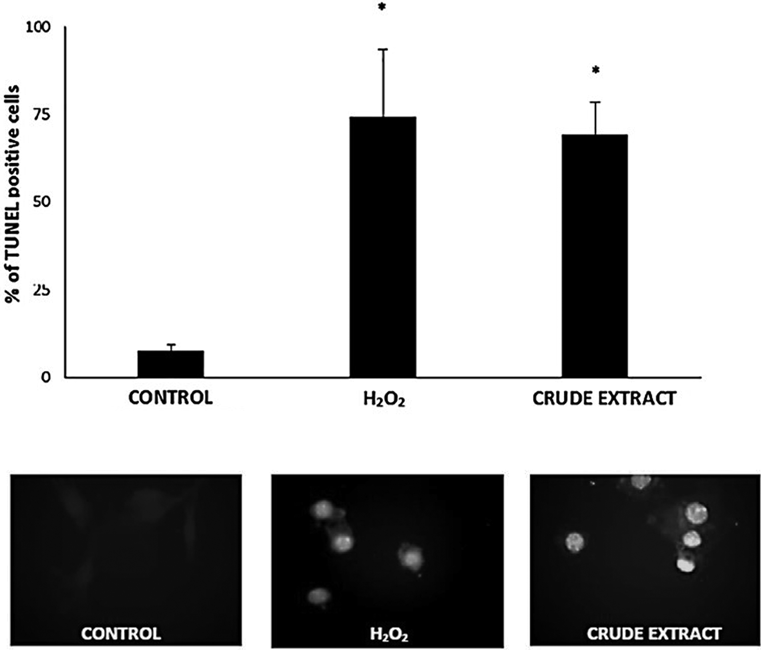
C2C12 cells untreated (CONTROL) or incubated with crude extract from N. glauca for 1 h (CRUDE EXTRACT) or with the apoptotic inducer H2O2 (0.5 mM, 2 h). Then, apoptosis was determined by TUNEL assays as described under Materials and Methods Section and expressed as the percentage of TUNEL positive cells in the coverslips. The content of palmitic acid in the crude extract is 4 µg. Each value represents the mean of three independent determinations ± S.D.; * p < 0.05 with respect to the control. Representative microscopy photographs of each condition are shown. Apoptotic cells (TUNEL positive) were identified by nuclei green fluorescence.
To determine the molecular mechanism activated by the extracts from N. glauca, we investigated the participation of caspases-3 and -7.
Within the caspase family, the effector caspases -3 and -7 orchestrate the destruction phase of apoptosis that results in the controlled dismantling of a range of proteins within the cell and its subsequent disposal.25) Moreover, one of the most evident and specific features of apoptosis is the degradation of DNA, driven by the activation of caspase-3,26) the central effector caspase, which makes it an attractive biomarker of apoptosis. To address whether the apoptotic action of the crude extract from N. glauca on C2C12 cells is exerted through caspases-3/7 activation, C2C12 cells were treated with ethanolic extract as before (Methods) and analysed with the Cell Event Caspase-3/7 reagent. As shown in Fig. 3, the crude extract induces the caspases-3/7 activation (86 ± 7.21% of caspase positive cells above the control) in a similar fashion as H2O2.
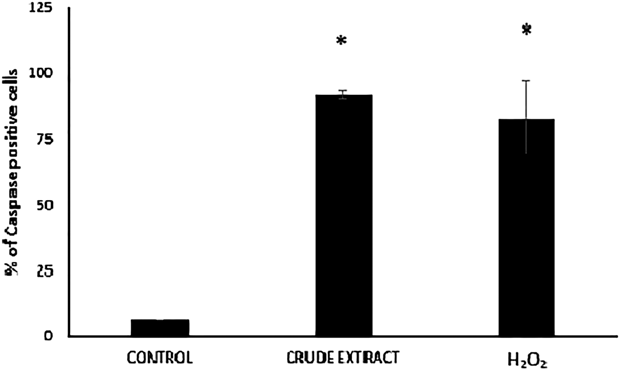
C2C12 cells grown on coverslips as 60–70% confluent monolayers were treated with the indicated stimuli as before and were analysed for caspase activation using Cell Event Caspase-3/7 detection probe (Methods). (CONTROL) untreated cells, (CRUDE EXTRACT) cells incubated with crude extract from N. glauca for 1 to 2 h, (H2O2) cells treated with H2O2 (0.5 mM, 1 to 2 h). Experiments were repeated at least three times with essentially identical results. Mean ± S.D.; * p < 0.05 with respect to the control.
With the aim of characterising the chemical structure of the apoptotic effectors present in the crude extract, an extraction procedure was carried out (Methods). Thus, the crude extract of N. glauca was successively partitioned with hexane, chloroform and ethyl acetate and each sub-extract was tested for apoptotic activity in C2C12 cells by TUNEL assays and caspase activation. With regard to caspase activation, the assay indicated that hexane and to a lesser extent chloroform sub-extracts (90 ± 4.6% and 55 ± 14% of caspase positive cells above the control, respectively) induce apoptosis, thereby activating caspases-3/7 (Fig. 4). Congruent with these data, TUNEL assays shown that hexane and to a lesser extent chloroform and ethyl acetate sub-extracts are able to induce apoptosis (98 ± 1.01%, 67 ± 18.38%, 44.6 ± 12.7% TUNEL positive cells respectively) (Fig. 4).
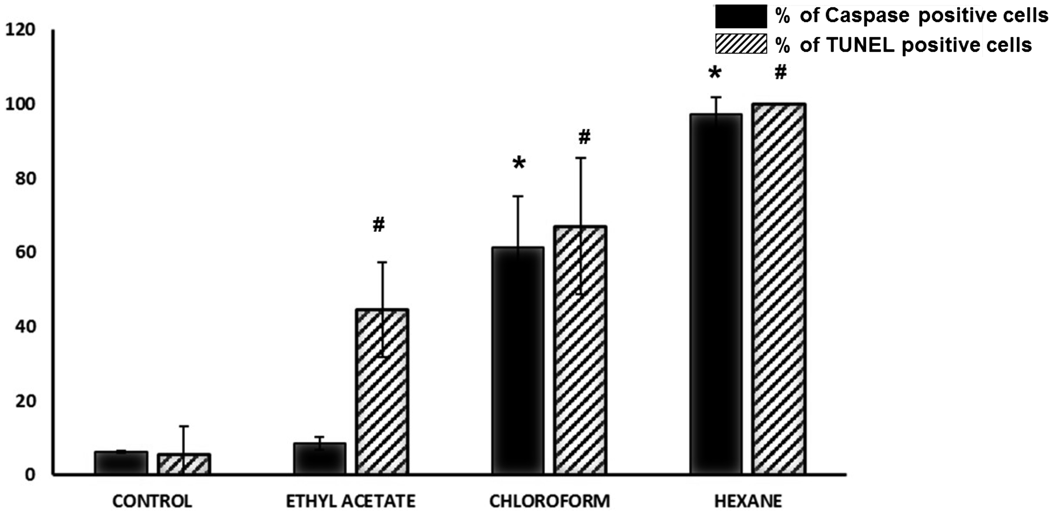
C2C12 cells were treated with the indicated stimuli (ETHYL ACETATE, CHLOROFORM or HEXANE) as before. CONTROL, untreated cells. Then, apoptosis was determined by TUNEL assays as described under Materials and Methods Section and expressed as the percentage of TUNEL positive cells in the coverslips. Likewise, other cultures were treated with sub-extracts as before and analysed for caspase activation using Cell Event Caspase-3/7 detection probe (Methods). Each value represents the mean of three independent determinations ± S.D.; * p < 0.05 with respect to the control. # p < 0.05 with respect to the control of TUNEL assay
Against potentially damaging stresses the cell activates its survival signalling pathways, as a first and rapid response that allows them to tolerate and/or recover from the damage incurred. As injury continues, such mechanisms are no longer sufficient. Thus, when the injurious signal persists and exceeds the mechanisms of survival, the net effect is the cell death. Among the cellular responses to stress, here we evaluated heat shock protein 27 (HSP27) and Akt phosphorylation levels in response to the lipid sub-extracts obtained from N. glauca crude extract. C2C12 cell cultures were incubated with each sub-extract during 30 min followed by measurement of HSP27 and Akt phosphorylation. As shown in Fig. 5 (A), Western blotting analysis using anti-phospho-Akt and anti-phospho-HSP27 antibodies revealed Akt and HSP27 activation (phosphorylation) in response to hexane, chloroform and ethyl acetate sub-extract treatments. Immunocytochemistry studies were congruent with the Western blotting results, (Fig. 5B).
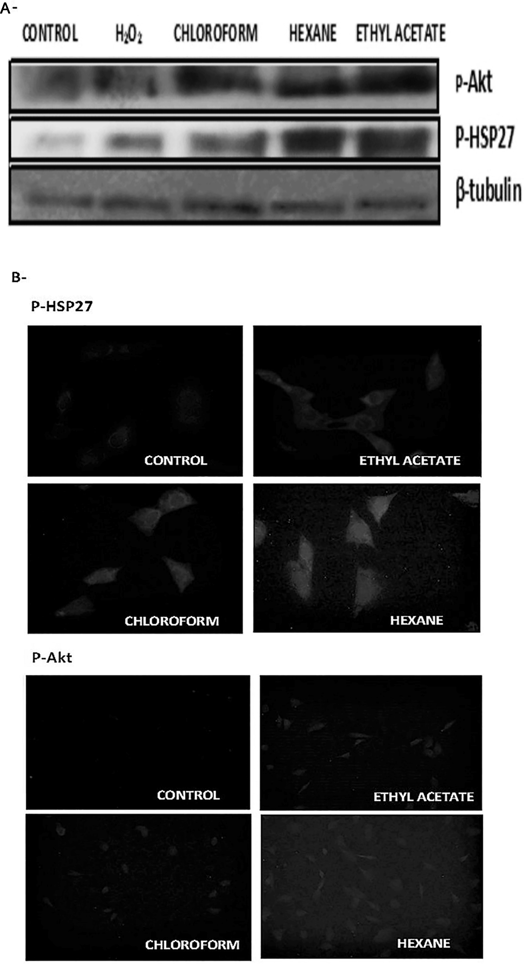
C2C12 cells were treated with the indicated stimuli (CHLOROFORM, HEXANE, ETHYL ACETATE or H2O2) as before. CONTROL, untreated cells. (A) Cell lysate proteins from each condition containing equivalent protein amounts (25 mg) were fractionated by SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes, and Western blotted with Phospho-Akt or Phospho-HSP27 antibodies as described in Materials and Methods Section. β-Tubulin levels are shown as protein loading control. Immunoblots representative are shown. (B) Fluorescence microscopy of p-HSP27 and p-Akt phosphorylation. HSP27 or Akt phosphorylated (green fluorescence) were stained by using anti-phospho-HSP27 or anti-phospho-Akt primary antibody, respectively and Alexa 488-conjugated secondary antibody. Experiments were repeated at least three times with essentially identical results (Magnification for p-Akt: 20x and for P-HSP27: 63x).
As described in the Methods, the ethanolic extract was successively partitioned with hexane, chloroform and ethyl acetate. Each extract obtained was tested for apoptosis. Of the three sub-extracts, the n-hexane sub-extract had the highest apoptotic ability (see Results, above). Thus, it was selected for further separation by chromatography, obtaining five fractions (F1–F5). Again, the fractions obtained were analysed for apoptosis by microscopy using mitotracker and DAPI staining to select the most apoptotic (F1: 13 ± 3.5%; F2: 59 ± 10%; F3: 89 ± 9.5%; F4: 70 ± 6.2%; F5: 38 ± 2.6%, TUNEL positive cells). This fraction (F3) was subjected to chromatography, yielding three sub-fractions (SF1–SF3), of which subfraction SF1 was the most apoptotic. This subfraction led to us obtaining palmitic acid in pure form (6.4 mg, 0.033% from the material plant, Fig. 6A). The structure of palmitic acid was confirmed using NMR and GC-MS spectrometry. The final residue of each phase was solubilised in isopropanol (60 µL) and stored at −20°C or used for the following assays. Next, we evaluated the effects of palmitic acid on C2C12 cells by DAPI and MitoTracker staining, and TUNEL and Caspase 3/7 assays. We performed the same experimental protocols/conditions as before. Cells treated with palmitic acid exhibited apoptotic morphology at the nuclear and mitochondrial level, a large increase in DNA fragmentation (74% ± 11.09 of TUNEL positive cells above the control) and caspase 3/7 activation (87% ± 7.6 of caspase positive cells above the control) (Fig. 6B).
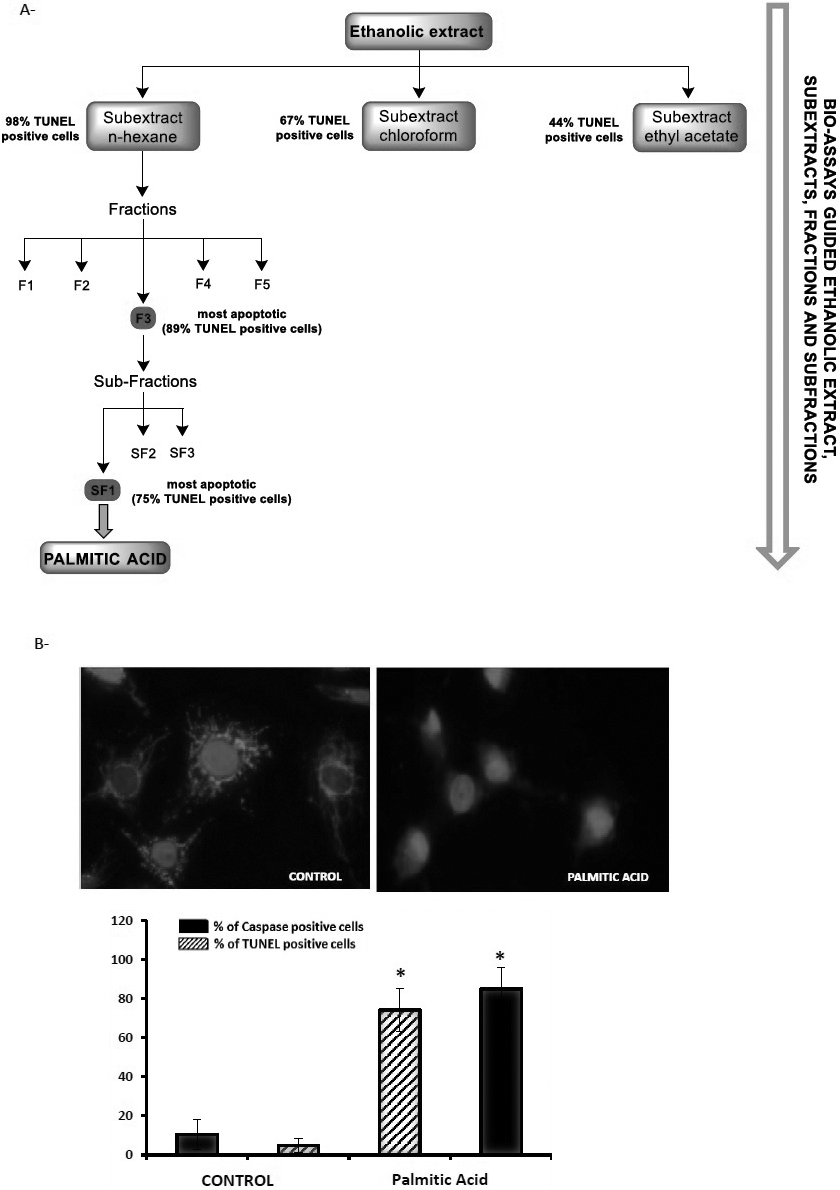
A) Partition and successive chromatographies of ethanolic extract from N. glauca led to obtain palmitic acid. B) C2C12 cells untreated (CONTROL) or incubated with palmitic acid from N. glauca for 1 h (PA) as it described in Methods. Morphology corresponding to apoptosis was detected with DAPI and Mitotracker staining, as before. Then, apoptosis was determined by TUNEL assays as described under Materials and Methods Section and expressed as the percentage of TUNEL positive cells in the coverslips. In addition, assays for caspase activation using Cell Event Caspase-3/7 detection probe (Methods) were performed. Each value represents the mean of three independent determinations ± S.D. * p < 0.05 with respect to the control. Apoptotic cells (TUNEL or Caspase 3/7 positive) were identified by nuclei green fluorescence in each respective assay.
Apoptosis is a highly conserved and tightly gene regulated process, in which cellular self-destruction occurs in a controlled manner, without damage to neighbouring cells.27) In order to evaluate the gene expression during palmitic acid-induced apoptosis, we analysed by quantitative PCR, the relative levels of a key genes (B-cell lymphoma 2 (Bcl-2) and p53 upregulated modulator of apoptosis (PUMA)) associated to apoptosis. Total RNA from cell cultures exposed to palmitic acid from N. glauca (Methods) was isolated and employed to determine the relative levels of mRNA expression. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was a reference gene.
It has been extensively reported that overexpression of the antiapoptotic member Bcl-2 of the Bcl-2 family induces the inhibition of apoptosis.27) We showed that Bcl-2 mRNA level was downregulated by palmitic acid (43% respect control, Fig. 7). In addition, PUMA has been shown to play an essential role in the induction of apoptosis.28) In keeping with its pro-apoptotic nature, PUMA is a potent BH3-only protein, with the capacity to bind to all known anti-apoptotic Bcl-2 proteins.29) Here, we observed that treatment with palmitic acid from N. glauca increased PUMA expression (325% above the control, Fig. 7). These results suggest that the palmitic acid negatively regulates Bcl-2 and upregulates PUMA mRNA transcript levels.
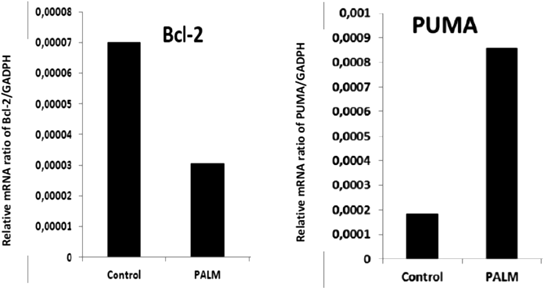
Cells were treated with palmitic acid (2.5 mg/5 mL isopropanol) obtained from N. glauca or DMEM without serum (C), as was indicated in Methods. Under the designated conditions, quantification by real-time PCR of Bcl-2 and PUMA mRNA transcript levels was carried out, which were normalized to the expression level of GAPDH. SYBR Green runs were performed on duplicate samples of cDNAs from three independent reverse transcription reactions. The comparative CT method was used for quantification.
N. glauca is used in traditional medicine.6) This Solanaceae provides a significant amount of phytoestrogens.7) Phytoestrogens are supplements and marketed as a natural alternative to HRT to overcome menopausal symptoms or to treat a range of health conditions. The risk of adverse effects, however, has not been studied. Moreover, the molecular mechanism underlying the medicinal effects of N. glauca is still unknown.
In this work, the murine skeletal muscle cells were treated with the ethanolic extract from N. glauca, with the lipid sub-extracts obtained from that or with H2O2 as a positive control of apoptosis. As a first approach we evaluated the effects of those treatments, seeing the cellular morphology using DAPI and mitotracker dyes. Cells exposed to the crude extract, to the hexane or to the chloroform sub-extracts showed typical apoptotic morphology (nuclear condensation/fragmentation, mitochondrial picnosis and nuclear clustering of the organelle) similar to H2O2 treatment. We found that the crude extract and sub-extracts of hexane and chloroform from N. glauca induced apoptosis in C2C12 cells. In addition, we confirm this observation by specific enzymatic TUNEL assays. Indeed, in accordance with the results from TUNEL assays, we found the activation of caspases when cells were treated with the hexane and chloroform sub-extracts, with the hexane sub-extract being the most potent. Instead, the ethyl acetate sub-extract was unable to induce higher levels of apoptosis. It has been fully demonstrated, as the key molecules required for the execution of apoptosis seem to be the caspases. Caspases cleave a variety of substrates and thereby cause the characteristic morphology of apoptotic cells. Our results suggest that molecules that are lipidic in nature, are concentrated upon preferentially in the hexane sub-extract, and to a lesser extend in the chloroform, are responsible for apoptosis.
We have also observed that short treatments times (30 min) with the sub-extracts from N. glauca induced the activation of a rapid defence response with Akt and HSP27 activation, as observed in previous works with H2O2 as the apoptotic inductor.30) This indicates that skeletal muscle cells sense an injury from those treatments. However, when we perform longer treatments (1 to 2 h) (data not shown), in which the expression levels of these proteins could increase, cells were unable to sustain the survival response and, in turn, underwent apoptosis.
Finally, in this work, we were interested in the identification of molecular actors during apoptosis, as induced by the lipid extracts. For the first approaches, we analysed caspase activity. In this assay the substrate (amino acid peptide DEVD) used to evaluate the caspase activation is recognised by both caspases-3 and -7. In view of this, our results suggest that the sub-extracts from N. glauca trigger apoptosis involving caspase pathways, but we are unable to identify the specific caspase involved using this method. Since apoptotic signalling pathways, which eventually cause DNA fragmentation, are largely mediated by the family of caspases, their activation in response to lipid extract treatments is in agreement with the chromatin destruction evidenced by DAPI and TUNEL experiments.
With the aim to identify the compounds responsible for the apoptotic effects on satellite cells we performed phytochemical screening, as a first approach, of the sub-extracts from N. glauca. We observed the presence of flavonoids, anthraquinones, triterpenes and steroids in the sub-extracts of hexane and chloroform. The presence of alkaloids in these sub-extracts was not observed. In the ethyl acetate sub-extract the presence of all the chemical structures examined was detected, but probably, are present in a low concentration respect the others sub-fractions. Among the different sub-fractions, we chose the one with greatest apoptotic power for further separation until obtaining the putative compound responsible for observed effects (Methods). Interestingly, our results indicate that the adverse effect of lipid extracts from N. glauca is mainly due to the presence of palmitic acid, as confirmed by NMR and GC-MS spectrometry. In agreement with the fact that palmitic acid is an apoptosis inducer in numerous systems and the relevance for us, in muscle tissue and in C2C12 cells (Review in refs. 31–34). Our results suggested that palmitic acid from N. glauca is able to modulate the genes related apoptosis. We observed an increase in PUMA mRNA levels, as well as the down-regulation of Bcl-2 mRNA levels induced by palmitic acid in C2C12 cells.
The data reported here, suggest that the effects of lipid extracts on satellite cells could affect muscle homeostasis, since satellite cells are critical for muscle regeneration and repair.35) Thus, N. glauca used in HRT to overcome menopausal symptoms like osteoporosis and loss of muscle mass, could worsen these symptoms. In addition, our results suggest that palmitic acid is responsible for the apoptosis observed. It is important to emphasise that this apoptotic effect triggered by palmitic acid, was observed in a specific cell type and that this response can vary or not exist if the experimental conditions are modified as a vegetable species of origin, concentrations or the target cell. We also provide knowledge about the molecular mechanisms that are activated by extracts of N. glauca to exert their negative effects on the muscle. Additional studies are necessary to further elucidate the signalling pathways, which mediate the apoptotic effect of palmitic acid in skeletal muscle cells. This knowledge may be of relevance to developing strategies to avoid undesirable side effects of this fatty acid, improving the medicinal capabilities of N. glauca.
Nicotiana glauca (GRAHAM) plant specimens were collected from their natural habitats in Buenos Aires Province, Argentina and were grown under green-house conditions. Plant names have been checked with the online website (www.theplantlist.org) of the Royal Botanic Gardens, Kew, accessed in August 2017. A herbarium voucher specimen has been kept at the Herbarium of the Department of Biology, Biochemistry and Pharmacy National University of the South. Local name in South America is palán-palán.
Extraction of Lipid ExtractsThe starting material plant was from leaves of N. glauca (126 g), which was extracted for two times with 96% ethanol at room temperature for 10 d. The ethanolic extract (crude extract) was concentrated under reduced pressure giving 26.3 g (21%); 4 g (non-lyophilized) of this residue was successively partitioned with hexane, chloroform and ethyl acetate. The extracted solutions were evaporated under reduced pressure and then lyophilized to yield 993 mg (24.8%) of hexane sub-extract, 61 mg (1.5%) of chloroform sub-extract and 164 mg (4.1%) ethyl acetate sub-extract. Of the three sub-extracts, the n-hexane sub-extract had the highest apoptotic effect in muscle cells (Results), thus it was selected for further separation by chromatography on silica gel, eluted with n-hexane/ethyl acetate (100 : 0→0 : 100, step-gradient system) to yield five fractions (F1–F5). Of these fractions, F3 had the greatest apoptotic effect (Results). This fraction (66.4 mg) was subjected to flash chromatography eluted with n-hexane/ethyl acetate (100 : 0 → 90 : 10, step-gradient system) yielding three sub-fractions (SF1–SF3) of which the sub-fraction SF1 was the most apoptotic. This sub-fraction led to obtain palmitic acid in pure form as a white solid (6.4 mg, 0.033% from the material plant), mp: 63°C. The structure of palmitic acid was confirmed by NMR and GC-MS spectrometry. 1H-NMR and 13C-NMR data were in agreement with previously reported findings (Zhao et al., 2017; Bordoloi et al., 2017); 1H-NMR (300 MHz, CDCl3): δ (ppm) 2.34 (t, J = 7.1 Hz, 2H), 1.63 (m, 2H), 1.25 (br s, 24H), 0.87 (t, J = 7.1 Hz, 3H); 13C-NMR (75 MHz, CDCl3): δ (ppm) 178.97, 33.97, 32.08, 29.85, 29.74, 29.58, 29.51, 29.39, 29.21, 24.84, 22.84, 14.26; MS (EI, 70 eV) m/z at 256.3 [M]+, 213.2, 157.1, 129.1, 97.1, 73.1.
The final residue of each phase was solubilized in isopropanol (60 µL) and stored at −20°C.
Cell Culture and TreatmentC2C12 murine skeletal myoblasts obtained from American Type Culture Collection (Manassas, VA, U.S.A.) were cultured in growth medium (Dulbecco’s modified Eagle’s medium (DMEM)) supplemented with 10% heat-inactivated fetal bovine serum, 1% nistatine, and 2% streptomycin. These cells have been widely used to study muscle functions.24,30,36) The C2C12 cells are murine myoblasts derived from satellite cells, whose behavior corresponds to that of progenitor lineage. This cell line is a subclone of C2 myoblasts37) which proliferate, differentiate and synthesize characteristic muscle proteins in culture.38,39) Since C2C12 cells are comparable to satellite cells,40) they represent an appropriate experimental model of them.
Cells were incubated at 37°C in a humid atmosphere of 5% CO2 in air. Cultures were passaged every 2 d with fresh medium. The treatments were performed with confluent cultures (120000 cells/cm2) in medium without serum for 20 min before treatments. The crude extract, sub-extracts, sub-fractions and palmitic acid were dissolved at 2.5 mg/5 mL isopropanol. Then, cells were exposed (1 : 1000 dilution in DMEM without serum) to the crude extract from N. glauca, each sub-extract: hexane, chloroform and ethyl acetate, palmitic acid or vehicle [0.001% isopropanol (control)] at the times indicated; or were treated with H2O2 0.5 mM (as positive control of apoptosis). After treatments, cells were lysed using a buffer (50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 0.2 mM Na2VO4, 2 mM ethylenediaminetetraacetic acid (EDTA), 25 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% NP40, 20 mg/mL leupeptin, and 20 mg/mL aprotinin). Protein concentration was estimated by Bradford (1976). For microscopical assays, cells were cultured in chamber slides.
TUNEL AssaysAfter the specific treatments, cells grown over coverslips, were processed for in situ localisation of nuclei exhibiting DNA fragmentation by the technique of TUNEL with the use of the apoptosis detection kit DeadEndTM Fluorometric TUNEL System (Promega, Madison, WI, U.S.A.). The protocols were followed according to the manufacturer´s instructions. Then cells were analysed by conventional fluorescence microscope (NIKON Eclipse Ti-S equipped with standard filter sets to capture fluorescent signals, and images were collected using a digital camera). At least 500 cells of each experimental condition were counted and apoptotic cells were identified by nuclei staining (TUNEL-positive cells). The results were expressed as percentage of apoptotic nuclei.
Quantitation of Apoptotic CellsAfter treatments, cells were fixed with cold methanol and then washed with phosphate buffered saline (PBS). Fixed cells were incubated for 30 min at room temperature (r.t.) in darkness with 1 : 500 of a solution of DAPI (5 mg/mL) and washed with PBS. Cells were examined using a fluorescence microscope equipped with standard filter sets to capture fluorescent signals. Images were collected using a digital camera. Apoptotic cells were identified by the condensation and/or fragmentation of their nuclei. The results were expressed as percentage of apoptotic cells. A minimum of 500 cells was counted for each treatment from at least three independent experiments.
MitoTracker Red StainingCoverslips with adherent cells were stained with MitoTracker red (Molecular Probes), prepared in dimethyl sulfoxide and then added to the cell culture medium at a final concentration of 1 mmol/L. After 15 min incubation at 37°C, cells were washed with PBS and fixed with cold methanol. Finally, coverslips were analysed by fluorescence microscopy as described.
Caspase-3/7 Activity AssayAfter specific treatments, cells were labeled with 6 µM CellEventTM caspase-3/7 reagent in PBS with calcium and magnesium for 30 min–37°C in the dark. The reagent consists of a four amino acid peptide (the caspase-3/7 cleavage sequence, DEVD) conjugated to a nucleic acid binding dye. This cell-permeant substrate is intrinsically non-fluorescent, because the DEVD peptide inhibits the ability of the dye to bind to DNA. After activation of caspase-3 or caspase-7 in apoptotic cells, the DEVD peptide is cleaved, enabling the dye to bind to DNA and produce a bright, fluorogenic response with an absorption/emission maxima of ca. 502/530 nm. Finally, the stained cells were analysed with a fluorescence microscope (NIKON Eclipse Ti-S equipped with standard filter sets). At least 500 cells of each experimental condition were counted and activation of caspases were identified by green fluorescence in nuclei (Caspase-3/7-positive cells). The results were expressed as percentage of Caspase-3/7-positive cells.
Western Blotting AnalysisCell cultures were scrapped and resuspended in lysis buffer (50 mM Tris–HCl, pH 7.4, 150 mM, NaCl, 0.2 mM Na2VO4, 2 mM EDTA, 25 mM NaF, 1 mM PMSF, 20 mg/mL leupeptin, and 20 mg/mL aprotinin). Lysates were collected by aspiration and centrifuged at 12000 × g for 15 min. The protein content of the supernatant was quantified by the Bradford procedure.41) Then, lysate proteins dissolved in Laemmli42) sample buffer were separated on 10–12% sodium dodecyl sulfate (SDS) polyacrylamide gels and electrotransferred to polyvinylidene difluoride membranes. Relative migration of unknown proteins was determined with molecular weight colored markers (Amersham, Piscataway, U.S.A.). Membranes were blocked 1 h at r.t. in PBS–T buffer (PBS 0.1% Tween-20) containing 5% dry milk. Membranes were incubated with primary antibodies overnight at 4°C, then washed three times in PBS–T and incubated in PBS–T containing 1% dry milk with peroxidase conjugated secondary antibodies for 1 h at r.t. Membranes were visualized using an enhanced chemiluminescent technique. For reprobing with other antibodies, membranes were incubated in stripping buffer (62.5 mM Tris–HCl, pH 6.8, 2% SDS, and 50 mM mercaptoethanol) for 30 min at 55°C, washed for 10 min in PBS–T, and then blocked/blotted as described above.
Quantitative Real Time RT-PCRAfter treatments, total RNA (10−6 C2C12 cells/condition) was extracted using the High Pure RNA Isolation kit and approximately 2 µg of total RNA was reverse transcribed with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc., U.S.A.) according to the manufacturer’s instructions. Quantitative measurement of RT-PCR was done using KAPA SYBR® FAST qPCR Master Mix (2X) under the standard conditions recommended by the manufacturer. Primer sets to amplify murine cDNAs used in the analysis were as follows: glyceraldehyde 3-phosphate dehydrogenase (GAPDH) set: forward 5′-CGT CCC GTA GAC AAA ATG GT-3′, reverse 5′-TTG ATG GCA ACA ATC TCC AC-3′; Bcl-2 set: forward 5′-CGT CAA CAG GGA GAT GTC A-3′, reverse 5′-TTC CAC AAA GGC ATC CCA GC-3′, PUMA set: forward 5′-TAC GAG CGG CGG AGA CAA G-3′, reverse 5′-GTG TAG GCA CCT AGT TGG GC-3′. The specificity of PCR products was confirmed by melting curve analysis. Relative quantification of gene expression was determined by the comparative CT method.43)
Statistical AnalysisData analysis was performed using standard statistical packages (InfoStat System, Córdoba, Argentina).44) Values are shown as the mean ± standard deviation (S.D.) of at least three independent experiments. The data were considered statistical significant when p < 0.05.
MaterialsAnti-phospho-Akt (Ser473) and anti-HSP27 antibodies were from Cell Signaling Technology Inc. (Danvers, U.S.A.). Anti-beta tubulin (1 : 10000) was from Thermo Fisher Scientific, Inc. (Rockford, U.S.A.). DAPI and MitoTracker Red (CMXRos) dyes were from Molecular Probes (Eugene, U.S.A.). The ECL blot detection kit was provided by Perkin-Elmer, Inc. (Waltham, U.S.A.). The protein marker was from Amersham (Buckinghamshire, England). TUNEL assay kit was from Promega (Promega Corp., Madison, U.S.A.), Cell Caspase-3/7 reagent was from Invitrogen (Carlsbad, CA, U.S.A.). High Pure RNA Isolation kit was from Roche Diagnostics (Mannheim, Germany). High Capacity cDNA Reverse Transcription Kit was purchased from Applied Biosystems, KAPA SYBR® qPCR Kit Master Mix Universal was from Biosystems, Inc. (Woburn, U.S.A.) and primer sets were from Invitrogen. All the other reagents used were of analytical grade. For column chromatography it was used silica gel 60, 70–230 mesh (Merck) and 200–425 mesh (Aldrich). The chromatographies were monitored by TLC on silica gel plates (60F-254) visualized under UV light and/or using p-anisaldehyde-acetic acid in ethanol spray reagent. NMR experiments were carried on a Bruker ARX 300 spectrometer. Palmitic acid was registered in deuterated chloroform (CDCl3, 99.8% D, Aldrich). Chemical shifts (δ) are reported in ppm from tetramethylsilane (TMS) using the residual solvent resonance (CDCl3: 7.26 ppm for 1H-NMR and 77.16 ppm for 13C-NMR. Multiplicities are abbreviated as follows: t = triplet, m = multiplet; br s = broad signal). Melting points were determined using a Büchi 510 apparatus and are not corrected. Mass spectra were obtained at 70 eV on an Agilent CG-78903 instrument with a MS-5977 A MSD selective mass detector.
This research was supported by grant from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET); Grant number: PIP11220110100544. LP, AV and LM are researcher members of CONICET; MBF is research member of CIC, DL and FM thank the CONICET for a doctoral fellowship.
The authors declare no conflict of interest.