2019 Volume 42 Issue 5 Pages 840-844
2019 Volume 42 Issue 5 Pages 840-844
In drug absorption and permeability experiments, an unstirred water layer (UWL) is known to cause differences in the estimated permeability of drugs between in vitro and in vivo experiments. Therefore, it is necessary to develop a new method to allow for accurate measurements of in vitro drug absorption through the reduction of the UWL effect. Previously, we have developed an artificial intestinal tract that mimics the tubular structure of the human intestine and enables study of drug absorption under flow conditions. In order to determine whether our artificial intestinal tract has the potential to reduce the effect of a UWL on drug absorption, the present study evaluated drug absorption in Caco-2 cells using this artificial system. The viability and tight junction structure of Caco-2 cells on the artificial intestinal tract were intact during perfusion. The cumulative amount of the highly lipophilic drugs imipramine and chlorpromazine accumulated in Caco-2 cells cultured on the cell culture plate was 1.5 times higher under mechanical agitation, whereas that of cells on the artificial intestinal tract was 6.5 times higher when internal flow was applied. In addition, the cumulative amounts of 5-aminosalicylic acid and clonidine, drugs with low lipophilicity, accumulated in Caco-2 cells on the artificial intestinal tract were unchanged by internal flow. These results indicate that the artificial intestinal tract enables effective reduction of the UWL thickness at the Caco-2 cell-surface, and allows evaluation of in vitro drug absorption under conditions similar to those found in vivo.
In vitro absorption and permeability experiments are widely used in the discovery and development of orally administered drugs.1,2) The standard method is to use human intestinal epithelial Caco-2 cells cultured on cell culture plates or Transwell® plates.2,3) Many reports have demonstrated that the transport efficiency of drugs obtained from in vitro studies are well correlated with in vivo bioavailability.4,5) However, contrasting reports have also indicated that the in vitro permeabilities of some drugs were not in accordance with those obtained from in vivo experiments. These inconsistencies in values arise from the functional differences between Caco-2 cells and human or animal small intestines.4,6,7)
An unstirred water layer (UWL) is one of the critical causes of difference in estimated drug permeability, not only between in vitro and in vivo experiments but also among in vitro studies.8–10) A UWL occurs as a result of a complex hydrogel, which is composed of glycoproteins, lipids, and salts, covering the surface of intestinal epithelial cells. This water layer restricts the diffusion of various kinds of drugs, acting as a considerable barrier against drug absorption.11,12) It has been reported that the UWL on the surface of intestinal epithelium under in vitro static conditions is much thicker than that under in vivo peristaltic conditions.10) Therefore, it is necessary to take the effect of UWL into consideration when in vitro absorption and permeability experiments are carried out. So far, many efforts have been made to control the thickness of a UWL in in vitro studies by using gas lift or magnetic stirring, in order to precisely predict the bioavailability of drugs.13,14) However, even in these approaches, agitation at the cell-surface was still found to be insufficient. Therefore, the construction of an effective method for stirring at the cell-surface is necessary to further reduce the thickness of the UWL and to allow accurate evaluation of drug absorption in vitro.
We have previously developed an artificial intestinal tract system which mimics the human intestinal tubular structure.15) This device is composed of a flat polydimethyl siloxane membrane with collagen coating that can easily form the desired tubular structure through the application of air pressure using pneumatic balloon actuators. Subsequently, cells can be seeded and cultivated on the membrane while it is in its flat state, and the membrane can then be actuated to form the tubular structure necessary for conducting drug absorption studies under in vivo small intestine-mimetic conditions. Moreover, this artificial intestinal tract also enables the efficient agitation of the cell-surface through internal flow, and therefore, this system has the potential to effectively evaluate in vitro drug absorption without the influence of a UWL.
In this study, we evaluated the in vitro absorption of several drugs with varying lipophilicity in Caco-2 cells using our artificial intestinal tract system and determined its utility for drug absorption experiments.
Caco-2 human epithelial colorectal adenocarcinoma cells were purchased from DS Pharm Biomedical Co., Ltd. (Osaka, Japan). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, nonessential amino acids, penicillin G (100 U/mL), and streptomycin (100 µg/mL) at 37°C with 5% CO2.
Preparation of Caco-2 Cell Monolayer on an Artificial Intestinal TractAn artificial intestinal tract was constructed according to our previous report.15) A photograph of the system is shown in Fig. 1A. The collagen-coated inner membrane of the artificial intestinal tract was seeded with 1 × 104 Caco-2 cells. The cells were cultured for 7 d to reach confluency and were then cultured for a further 7 d. The culture medium was replaced every 24 h.
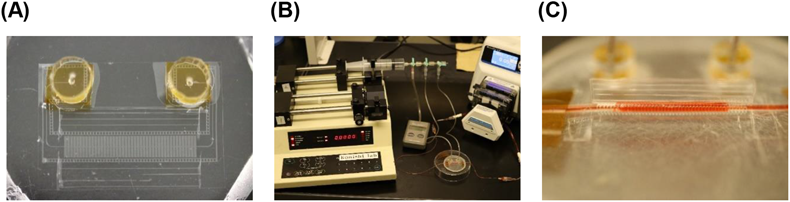
For drug absorption studies, the artificial intestinal tract (A) was connected with an infusion pump (B), and drug solution was perfused into the artificial intestine (C). (Color figure can be accessed in the online version.)
After the Caco-2 cell monolayer was prepared, the artificial intestinal tract was actuated from a flat membrane to form a circular tube by constant air infusion into pneumatic balloon actuators. Images of the artificial intestinal tract during actuation are shown in Figs. 1B and C. Hanks’ balanced salt solution (HBSS) (Sigma-Aldrich, St. Louis, MO, U.S.A.) (pH 7.4) with 5% glucose and 0.1% dimethyl sulfoxide was perfused in the artificial intestinal tract for 1 h at flow rates of 0.05 and 0.5 mL/min, in separate experiments. Later, the artificial intestinal tract was opened, and the cells were washed three times with ice-cold HBSS. Cell viability was measured using Cell Count Reagent SF (Nacalai Tesque, Kyoto, Japan). The results were expressed as % viability.
Observation of Tight Junction Structure of Caco-2 Cell MonolayerAfter the cell viability assay, the cells were fixed with 4% paraformaldehyde phosphate buffer solution for 15 min, and the resultant cells were incubated with ZO-1 antibody, Alexa Fluor® 488 conjugate (Thermo Fisher Scientific K.K., Kanagawa, Japan) for 1 h. After washing three times with ice-cold HBSS, cover slips were mounted on the cells with Fluoro-KEEPER antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (Nacalai Tesque). The cells were observed using fluorescence microscope BZ-X710 (KEYENCE Corporation, Tokyo, Japan).
Drug Absorption Experiments in Caco-2 Cells Using a Cell Culture PlateCaco-2 cells were seeded on a collagen-coated 24-well culture plate at a density of 1 × 105 cells and cultured for 14 d. The culture medium was replaced with HBSS (Sigma-Aldrich) (pH 7.4) with 5% glucose, 0.1% dimethyl sulfoxide containing 50 µM of imipramine, 50 µM of chlorpromazine, 1 mM of 5-aminosalicylic acid (5-ASA), or 100 µM of clonidine, and incubated for 1 h with or without mechanical agitation using a water bath shaker (TAITEC, Saitama, Japan). Later, the cells were washed three times with ice-cold HBSS. The cells were collected in acetonitrile and centrifuged at 10000 × g for 10 min at 4°C. The drug concentration of the resultant supernatant was measured by HPLC. The resultant cell-pellet was lysed using lysis buffer (0.05% Triton X-100, 2 mM ethylenediaminetetraacetic acid, 0.1 M Tris, pH 7.8), and its protein concentration was measured using BCA protein assay kit (TaKaRa Bio Inc., Shiga, Japan).
Drug Absorption Experiments in Caco-2 Cells Using an Artificial Intestinal TractAfter preparing Caco-2 cell monolayer, the artificial intestinal tract was actuated from a flat membrane to a circular tube by constant air infusion into pneumatic balloon actuators. HBSS (Sigma-Aldrich) (pH 7.4) with 5% glucose, 0.1% dimethyl sulfoxide containing 50 µM of imipramine, 50 µM of chlorpromazine, 1 mM of 5-ASA, or 100 µM of clonidine was perfused in the artificial intestinal tract for 1 h at flow rates of 0.05 and 0.5 mL/min, in separate experiments. Then, the artificial intestinal tract was opened, and the drug concentration and protein concentration in the cells were measured as per the procedure described in the “Drug absorption experiments in Caco-2 cells using a cell culture plate” section.
Analytical MethodsAnalyses were performed on a C18 column (4.6 mm i.d. × 150 mm; pore size, 12 nm; particle size, 5 µm, Nacalai Tesque) by an HPLC system, comprising a model LC-20AD HPLC pump (Shimadzu, Kyoto, Japan), a model SIL-10AC auto sampler (Shimadzu), a model SPD-20 A ultraviolet detector (Shimadzu), and a model RF-10XL fluorescence detector. The compositions of mobile phases were as follows: imipramine, chlorpromazine, and clonidine, 30 mM ammonium acetate with 0.05% triethylamine (pH 5.86)-acetonitrile (60 : 40, v/v); 5-ASA, 20 mM sodium dihydrogenphosphate dehydrate (pH 2.2)-acetonitrile (95 : 5, v/v). The mobile phase was pumped at a flow rate of 1.0 mL/min, and imipramine, chlorpromazine, and clonidine were detected by absorbance at 245 nm. The fluorescence of 5-ASA was detected (Excitation: 311 nm, Emission: 449 nm). The injection volume was 20 µL. The drug concentration in samples was calculated by an absolute standard curve method.
Statistical AnalysisResults are presented as the mean ± standard deviation (S.D.) of three experiments. ANOVA was used to test the statistical significance of differences between groups. Multiple comparisons between control groups and other groups were performed with the Dunnett’s test.
Our artificial intestinal tract is a freely openable system, enabling the cultivation of cells under static and flat conditions before the absorption study is conducted (Fig. 1). Although this system mimics conditions of human small intestine, such as tension from the tubular structure and shear stress from the perfusion, these forces which are applied to Caco-2 cells during the actuation of the artificial intestinal tract may affect cell function. Therefore, we evaluated the viability and function of Caco-2 cells following actuation of the artificial intestinal tract. As shown in Fig. 2A, the cell viability was unchanged after the perfusion at flow rates of 0.05 and 0.5 mL/min. In addition, we observed that the structure of the tight junction was maintained intact following perfusion (Fig. 2B). When the perfusion was carried out at a flow rate of 0.1 mL/min, the detachment of Caco-2 cell monolayer was observed. These results indicate that the actuation of the intestinal tract and the perfusion do not affect the viability of Caco-2 cells as long as the flow rate of the perfusion is less than or equal to 0.5 mL/min.
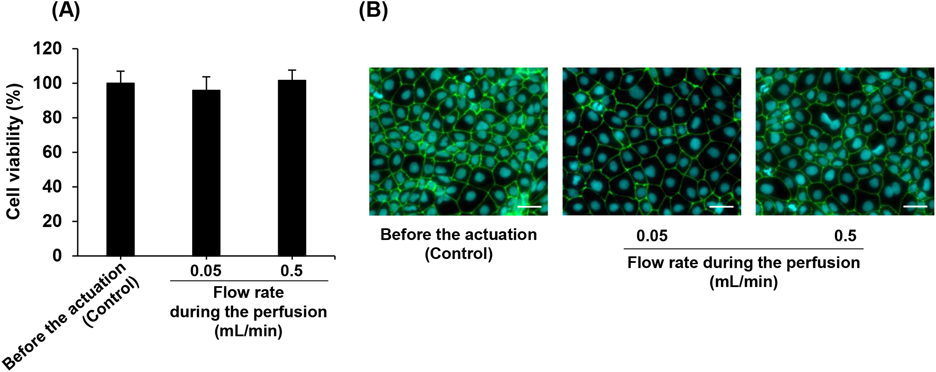
HBSS with 5% glucose and 0.1% dimethyl sulfoxide was perfused in the artificial intestinal tract for 1 h at flow rates of 0.05 and 0.5 mL/min. (A) Each value represents the mean ± S.D. (n = 3). (B) Scale bar: 50 µm. (Color figure can be accessed in the online version.)
In order to determine whether an artificial intestinal tract can decrease the effects of a UWL on in vitro drug absorption studies, we evaluated the absorption of two lipophilic drugs imipramine and chlorpromazine by Caco-2 cells on the artificial intestinal tract. As a control, we performed the absorption experiments in Caco-2 cells seeded on cell culture plates under mechanical agitation, following the method we previously reported.16) The cumulative amounts of imipramine and chlorpromazine absorbed by Caco-2 cells cultured on the cell culture plate were improved by only approximately 1.5-fold with mechanical agitation (Figs. 3A, B). In contrast, a remarkable flow rate-dependent increase of imipramine and chlorpromazine accumulation in Caco-2 cells was observed when the experiments were carried out using the artificial intestinal tract (Figs. 3C, D). Cumulative accumulation of imipramine and chlorpromazine in Caco-2 cells on the artificial intestinal tract were approximately 6.5 times higher with internal flow than those without the internal flow. These results suggest that the internal flow of the artificial intestinal tract effectively agitates the surface of Caco-2 cells, resulting in reduction of the UWL thickness. Hidalgo et al. reported that the permeability of testosterone across the Caco-2 cell monolayer increased approximately 3.5 times increased by gas (O2/CO2) lift at a rate of 40 mL/min, using side-by-side diffusion cells which can circulate transport buffer by gas (O2/CO2) lift.13) In addition, Yano et al. showed that the permeability of highly lipophilic heptyl benzene across Caco-2 cell monolayer was approximately doubled by magnetic stirring at a rate of 900 rpm, using a dissolution/permeation system which consisted of side-by-side chambers and magnetic stirrers.14) The artificial intestinal tract system used in the present study is able to remove a UWL more efficiently than the above-mentioned methods reported in the literature. This may be due to the tubular structure of the system which can provide Caco-2 cells with uniform shear stress. It has been reported that transport across a UWL is a rate-limiting process in the intestinal absorption of highly lipophilic drugs, and therefore the absorption rate of these drugs in the epithelium is known to vary inversely with the thickness of a UWL.9,10) Based on this information and our results, a 6-fold reduction in the UWL thickness on the surface of Caco-2 cells can be achieved using the artificial intestinal tract. As the UWL in the intestine under static conditions has been reported to be over 6 times thicker than that under physiological dynamic conditions,10) the artificial intestinal tract system would enable the study of in vitro absorption by simulating conditions similar to those of in vivo physiological conditions, particularly with regard to the presence of a UWL, although the rates of perfusion (0.05 and 0.5 mL/min) are not relevant to the physiological flow rate of fluid through the small intestine (2.5–20 mL/min).17)
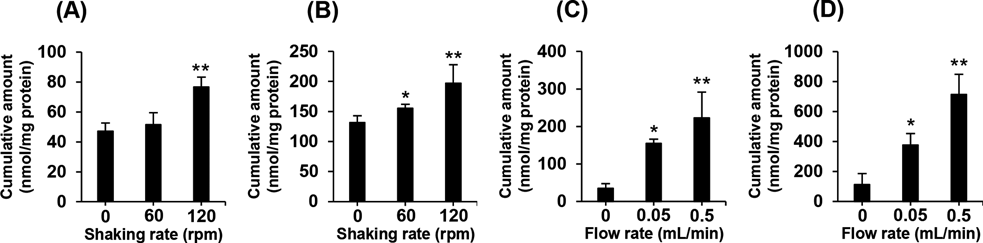
The cumulative amount of imipramine (A) and chlorpromazine (B) in Caco-2 cells on a cell culture plate. The cells were incubated with 50 µM of imipramine, or 50 µM of chlorpromazine for 1 h. Culture plates were agitated at 0, 60, or 120 rpm using a water bath shaker. The cumulative amount of imipramine (C) and chlorpromazine (D) accumulated in Caco-2 cells on an artificial intestinal tract. 50 µM of imipramine, or 50 µM of chlorpromazine were perfused for 1 h at a flow rate of 0.05, or 0.5 mL/min. Each value represents the mean ± S.D. (n = 3). * p < 0.05; ** p < 0.01, compared with no agitation (0 rpm) or no flow (0 mL/min).
We also investigated the accumulation of 5-ASA and clonidine, drugs with poor lipophilicity, in Caco-2 cells. The cumulative amounts of both 5-ASA and clonidine accumulated in Caco-2 cells cultured on cell culture plates was unchanged between cells that were subjected to mechanical agitation and those that were not (Figs. 4A, B). Similarly, no increase in accumulation of these drugs was observed in the cells on the artificial intestinal tract with internal flow (Figs. 4C, D). It has been reported that although a UWL greatly restricts the uptake of lipophilic drugs, this effect is not observed in hydrophilic drugs because they can freely diffuse through a UWL.12,18) Therefore, it is to be expected that absorption of 5-ASA and clonidine in Caco-2 cells would be unaffected by mechanical agitation or internal flow. These results further indicate that the internal flow of our artificial intestinal tract reduces the effect of a UWL on drug absorption by the Caco-2 cell monolayer.
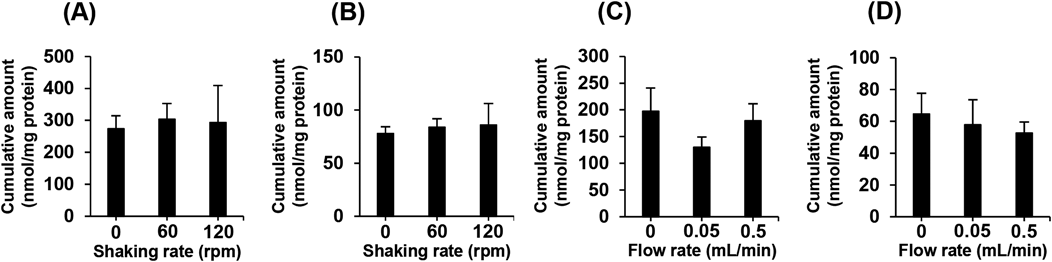
The cumulative amount of 5-ASA (A) and clonidine (B) in Caco-2 cells on a cell culture plate. The cells were incubated with 1 mM of 5-ASA, or 100 µM of clonidine for 1 h. Culture plates were agitated at 0, 60, or 120 rpm using a water bath shaker. The cumulative amount of 5-ASA (C) and clonidine (D) in Caco-2 cells on an artificial intestinal tract. 1 mM of 5-ASA, or 100 µM of clonidine were perfused for 1 h at a flow rate of 0.05, or 0.5 mL/min. Each value represents the mean ± S.D. (n = 3).
In conclusion, we successfully demonstrated increased drug absorption by Caco-2 cells through reduction of the UWL effect using our artificial intestinal tract. Further studies are needed because the evaluation of the permeability of drugs across Caco-2 cell monolayer, rather than the uptake of drug in Caco-2 cell, is important for more accurate estimation of human oral absorption of drugs. We are now developing a new artificial intestinal tract system of which its inner membrane is partly consists of a microporous membrane, and evaluating the time-dependent transport of drug across Caco-2 cell monolayer using this system. Nevertheless, this system could contribute to the development of a precise in vitro method for estimating drug permeability in the human small intestine.
This study was supported in part by a Grant from the Strategic Research Foundation Grant-aided Project for Private Universities and by a Grant-in-Aid for Challenging Exploratory Research (Grant Number 15K12526) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Ritsumeikan Global Innovation Research Organization (R-GIRO) project at Ritsumeikan University. We are grateful to Dr. Koji Hattori and Mr. Satoshi Sugama (Ritsumeikan University) for supporting the preparation and actuation of an artificial intestinal tract system.
The authors declare no conflict of interest.