2019 Volume 42 Issue 6 Pages 929-936
2019 Volume 42 Issue 6 Pages 929-936
Bisphosphonates (BPs) containing nitrogen (N-BPs) exhibit far stronger anti-bone-resorptive effects than non-N-BPs. However, repeated administration of N-BPs causes osteonecrosis selectively in jawbones. As BPs accumulate in large amounts within inflamed bones, any N-BP released from the pool accumulated within jawbones might directly act on cells in the surrounding soft-tissues and induce inflammation or necrosis. Here, we examined the local and systemic effects of zoledronate (the most potent N-BP with the highest incidence of jawbone-necrosis) on inflammatory cytokines in mice. Locally within ear-pinnas: (i) zoledronate induced long-lasting accumulation of interleuikin-1β (IL-1β) and IL-18, but not tumor necrosis factor-α (TNF-α), (ii) zoledronate and lipopolysaccharide (LPS, a cell-wall component of Gram-negative bacteria) mutually augmented the productions of IL-1β, IL-18, and TNF-α, and (iii) oxidronate (a toxic non-N-BP) by itself produced not only IL-1β and IL-18, but also TNF-α. In systemic experiments using intraperitoneal injection of zoledronate and/or LPS, (i) zoledronate by itself increased none of the above cytokines in serum, and (ii) in mice pretreated (3 d before) with zoledronate, the LPS-induced increases in serum IL-1β and IL-18 were greatly augmented with a delayed slight TNF-α augmentation. These results, together with previous ones, suggest that (a) pro-IL-1β and pro-IL-18 accumulate within cells in soft-tissues exposed to N-BPs, and infection may augment not only their production, but also the release of their mature forms, (b) IL-1β and IL-18 (possibly together with TNF-α) may play important roles in N-BP-induced inflammation and/or necrosis, and (c) mechanisms underlying the cytotoxic effects of BPs may differ between N-BPs and non-N-BPs.
Among the bisphosphonates (BPs), nitrogen-containing BPs (N-BPs) have much stronger anti-bone-resorptive effects than non-nitrogen-containing BPs (non-N-BPs)1) (Fig. 1). However, N-BPs have inflammatory/necrotic side effects, such as injuries to esophageal and gastric tissues upon oral administration and influenza-like acute inflammatory reactions following intravenous administration.2) In addition, BP-related osteonecrosis of jaws (BRONJ) with exposure of the necrotic jawbones has been a serious concern after repeated administration of N-BPs.3,4) Jawbones are unique among skeletal structures because they are frequently subject to bacterial infections via the teeth. Indeed, oral infection and/or tooth extraction have been thought to promote BRONJ.3,4)

Bisphosphonates (BPs), with a non-hydrolysable P–C–P structure, are analogs of pyrophosphate, which has a hydrolysable P–O–P structure. Alendronate and zoledronate are nitrogen-containing BPs (N-BPs). Etidronate, clodronate, and oxidronate are non-N-BPs. Relative potencies of the anti-bone-resorptive effects of BPs (with etidronate being tentatively indicated as 1.0) are shown within ( ).
BPs (irrespective of whether they are N-BPs or non-N-BPs) bind strongly to bone hydroxyapatite and thus accumulate within bones, especially inflamed bones.5,6) Hence, BPs are used for bone-scintigraphy to detect the sites of inflammation and/or metastases in bones. Oral infection causes inflammation around jawbones, and thus it might be expected to promote the accumulation of N-BPs within jawbones. In mice, topically injected N-BPs induce inflammation and/or necrosis at the injection site.7–10) Zoledronate, an N-BP, has the most potent anti-bone resorptive activity among the BPs (Fig. 1), and its incidence of BRONJ is the highest among N-BPs.3,4) Notably, zoledronate is detectable in the saliva of patients with BRONJ.11) In addition, bone-bound N-BPs inhibit the growth of adjacent non-bone cells in vitro.12) These findings suggest that jawbone-bound N-BPs may be released from the bone under certain conditions and injure the soft-tissues surrounding the jawbone, leading to necrosis.
In contrast to N-BPs, the clinically used non-N-BPs clodronate and etidronate (Fig. 1) have very scarce, if any, inflammatory/necrotic side effects.13) Indeed, in mice, no inflammatory/necrotic effects are observed even when they are injected topically9) or intraperitoneally14,15) at doses much higher than those at which N-BPs induce inflammation/necrosis. However, some non-N-BPs, such as oxidronate (Fig. 1), do induce inflammation and necrosis similar to those induced by N-BPs at the injection site9) when given at doses similar to those used for N-BPs.16) However, it has been reported that the mechanisms by which N-BPs and non-N-BPs exert cytotoxic effects are different.17) Interestingly, our recent pharmacological studies suggest that N-BPs may enter soft-tissue cells via phosphate transporters, and clodronate and that etidronate can inhibit this entry.9,18,19)
Interleukin-1α (IL-1α) and IL-1β are typical inflammatory and pyrogenic cytokines.20) We have reported that (i) although an N-BP by itself did not produce detectable levels of serum IL-1α or IL-1β in mice, their serum elevations induced by lipopolysaccharide (LPS, a cell-wall component of Gram-negative bacteria) are greatly augmented in mice previously injected with the N-BP,21,22) (ii) the inflammatory effects of N-BPs are weak or undetectable in mice deficient in both IL-1α and IL-1β,22) (iii) intraperitoneal injection of alendronate (an N-BP, Fig. 1) into mice increases IL-1β in soft tissues (such as liver, lung, and spleen),23) (iv) periodontal pathogenic bacteria cause in vitro activation of caspase-1 (the enzyme that converts pro-IL-1β to active or mature IL-1β),24) (v) alendronate directly stimulates macrophages to produce pro-IL-1β in vivo and in vitro, but the release of mature IL-1β is below detectable levels due to insufficient activation of caspase-1,25) and (vi) when it is injected into mice pretreated with alendronate, LPS activates caspase-1 and causes the release of mature IL-1β.25) These findings suggest that both IL-1α and IL-1β are involved in the inflammatory effects of N-BPs. It is known that in addition to its role in the production of mature IL-1β, activated caspase-1 acts on pro-IL-18 to produce and release the mature form. Release of IL-1β and IL-18 accompanies the cell death termed “pyroptosis.”26–29) In contrast, tumor necrosis factor-α (TNF-α) is thought to be involved in other types of cell death, namely “apoptosis” and/or “necroptosis.”26,28,29)
Informed by the results described above, we designed the present study, on mice, to examine (i) the effects of locally or systemically injected zoledronate and oxidronate on the productions of IL-1β, IL-18, and TNF-α, and (ii) the effects of LPS on the zoledronate-induced production of these cytokines.
BALB/c mice were purchased from SLC (Shizuoka, Japan). All experiments complied with the Guidelines for Care and Use of Laboratory Animals in Tohoku University and Tsurumi University. Zoledronate (Toronto Research Chemicals Inc., North York, ON, Canada) and oxidronate (synthesized to order) were dissolved in sterile saline, the pH of the solutions being adjusted to 7 with NaOH. A LPS from Escherichia coli O55:B5 prepared by Westphal’s method was obtained from Difco Laboratories (Detroit, MI, U.S.A.) and dissolved in sterile saline. RPMI 1640 solution, Triton X-100, 1 M N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) solution, bovine serum albumin, and gentamicin sulfate solution were obtained from Wako (Osaka, Japan). Proteinase inhibitor cocktail was from Sigma (St. Louis, MO, U.S.A.). Experimental protocols are described in the text or in the legend to the Figure relating to each experiment.
Injection of Test Reagents and SamplingTest reagents were injected subcutaneously into ear-pinnas (20 µL/ear-pinna) and whole ear-pinnas were removed after the indicated time. In other experiments, test reagents were intraperitoneally injected into mice (0.1 mL/10 g body weight of mouse) and blood was collected directly into test tubes following decapitation. Serum was recovered by centrifugation at 2000 × g at 4°C, then stored at −80°C until use.
Measurement of Cytokines in Ear-Pinnas and Blood SerumFrozen ear-pinnas were homogenized in RPMI 1640 solution containing Triton X-100 (5 µL/mL), HEPES (10 µmol/mL), bovine serum albumin (100 µg/mL), gentamicin sulfate (50 µg/mL), and proteinase inhibitor cocktail (10 µL/mL). The protease inhibitor cocktail contains 4-(2-aminoethyl)benzenesulfonyl fluoride, aprotinin, leupeptin, bestatin, pepstatin A, and E-64. The supernatant obtained by centrifugation (10000 × g for 10 min at 4°C) of the homogenate was subjected to the measurement of cytokines. IL-1β and IL-18 were measured using enzyme-linked immunosorbent assay (ELISA) kits from Thermo Scientific (Rockford, IL, U.S.A.) and MBL (Nagoya, Japan), respectively. The ELISA kit for TNF-α was from Life Technologies (Frederick, MD, U.S.A.). The procedures used were essentially the same as those described previously,23,25) although the ELISA kits and proteinase inhibitor cocktail came from different makers than those used previously.
Assessment of NecrosisAfter maximum inflammation (estimated as the red area) has been attained, the center of the inflammatory site can be seen to change in color from red to dark brown, or black, or as a tissue defect.6) This color change was evaluated as an indicator of the development of necrosis.
Statistical AnalysisExperimental values are given as the mean ± standard deviation (S.D.). The statistical significance of differences was evaluated using a Bonferroni post-hoc test after a one-way (Figs. 2–5) or a two-way (Fig. 6) ANOVA with the aid of InStat software (Instaq Scottsdale, AZ, U.S.A.). p Values less than 0.05 were considered to indicate significance.
We previously reported that subcutaneous injection of zoledronate (1, 2, or 4 mM) into mouse ear-pinnas induced dose-dependent swelling and necrosis in the ear-pinnas, with 4 mM zoledronate notably inducing necrosis in all the mice tested.6) In the present study, such injection of zoledronate into ear-pinnas induced dose-dependent production of IL-1β and IL-18 in the ear-pinnas at 3 d after its injection (Fig. 2). However, no significant effect of zoledronate was detected on the production of TNF-α, although the level of TNF-α tended to decrease. These results indicate that zoledronate stimulates the production of IL-1β and IL-18, but not of TNF-α.

Zoledronate (Zol) at the indicated concentrations was subcutaneously injected into both right and left ear-pinnas (20 µL/ear-pinna), and cytokines in the ear-pinnas (right and left ear-pinnas were combined) were measured 3 d after the injection. The values are means ± S.D. from 4 or 5 mice. ** p < 0.01, *** p < 0.001 vs. Zol 0 mM. n = 4 or 5.
Next, we examined the time-dependency of the changes in IL-1β, IL-18, and TNF-α induced after subcutaneous injection of 2 mM zoledronate into ear-pinnas. At this concentration, zoledronate reportedly caused necrosis in half of the mice tested at 6 d after its injection.6) In Fig. 3, the levels of cytokines are shown for all ear-pinnas (i.e., irrespective of whether they exhibited inflammation, necrosis, or both). Zoledronate produced gradual increases in IL-1β and IL-18. The number of necrotic ears per group increased with time (see the inserted table in Fig. 3). In this experiment, TNF-α had either decreased or tended to decrease by day 3. We then compared the cytokine levels on days 5 and 7 shown in Fig. 3 between inflammatory and necrotic ear-pinnas (Table 1). However, there were no significant differences between them. These results suggest that IL-1β and IL-18 (but not TNF-α) may accumulate within soft-tissue cells exposed to zoledronate.
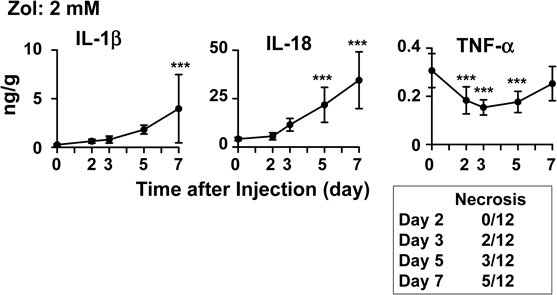
Zoledronate (Zol) (2 mM) was subcutaneously injected into ear-pinnas (20 µL/ear-pinna, both right and left ear-pinnas). At the indicated times after the injection, the ear-pinnas of the various mice were designated as either inflammatory or necrotic ear-pinnas. The cytokines in each ear-pinna were measured at the indicated times after the injection. Numbers of ear-pinnas designated as necrotic are shown in a table within the figure. Cytokine levels at days 5 and 7 are compared between inflammatory and necrotic ear-pinnas in a separate table (Table 1). The values are means ± S.D. from 12 ear-pinnas. *** p < 0.001 vs. day 0. n = 12.
| IL-1β (ng/g) | IL-18 (ng/g) | TNF-α (ng/g) | ||||
|---|---|---|---|---|---|---|
| Day 5 | Day 7 | Day 5 | Day 7 | Day 5 | Day 7 | |
| Inflammation | 1.8 ± 0.3 (9) | 4.1 ± 4.3 (7) | 23.8 ± 8.8 (9) | 33.3 ± 14.8 (7) | 0.17 ± 0.04 (9) | 0.26 ± 0.09 (7) |
| Necrosis | 1.9 ± 0.8 (3) | 3.8 ± 2.5 (5) | 15.7 ± 8.4 (3) | 36.2 ± 16.0 (5) | 0.19 ± 0.06 (3) | 0.25 ± 0.05 (5) |
The cytokine levels on days 5 and 7 shown in Fig. 3 were compared between inflammatory and necrotic ear-pinnas. The values are means ± S.D. from the indicated numbers of ear-pinnas. ( ): Number of tested ear-pinnas. Note: there were no significant differences between inflammatory and necrotic ear-pinnas.
As described in Introduction, infection is thought to be causally involved in BRONJ. Thus, we tested the effects of zoledronate (1 mM), LPS (0.1 mg/mL), and their combination on the levels of inflammatory cytokines at 3 d after their subcutaneous injection into ear-pinnas. As shown in Fig. 4, when given separately, zoledronate and LPS tended to increase IL-1β and IL-18. However, co-injection of zoledronate and LPS led to augmented production of these cytokines. Moreover, it is notable that this combination significantly increased TNF-α, too. These results suggest that zoledronate and LPS mutually augment the production or accumulation of IL-1β, IL-18, and TNF-α.

Saline (S), LPS (0.1 mg/mL), zoledronate (Zol) (1 mM), or a mixture containing LPS (0.1 mg/mL) and zoledronate (1 mM) was subcutaneously injected into ear-pinnas (20 µL/ear-pinna, both right and left ear-pinnas), and the cytokines in the ear-pinnas (right and left ear-pinnas were combined) were measured on day 3 after the injection. The values are means ± S.D. from 4 or 5 mice. * p < 0.05, *** p < 0.001 vs. S, ### p < 0.001 vs. LPS, $ p < 0.05, $$$ p < 0.001 vs. Zol, n = 4 or 5.
Although oxidronate is a non-N-BP (Fig. 1) it has inflammatory/necrotic effects on mouse ear-pinnas at 10 mM.9) Thus, we examined the effect of 10 mM oxidronate on the production of IL-1β, IL-18, and TNF-α in ear-pinnas. In Fig. 5, results are shown separately for inflammatory ear-pinnas and necrotic ear-pinnas. Like zoledronate, 10 mM oxidronate increased IL-1β and IL-18. However, unlike zoledronate, oxidronate increased TNF-α, too. The levels of these cytokines were greater (IL-18) or tended to be greater (IL-1β and TNF-α) in the necrotic ear-pinnas than in the inflammatory ear-pinnas.

Saline (S) or oxidronate (Oxi) (10 mM) was subcutaneously injected into ear-pinnas (20 µL/ear-pinna, both right and left ear-pinnas). On day 3 after the injection, ear-pinnas with inflammation alone and necrosis were separated, and cytokine levels in the ear-pinnas were measured. The values are means ± S.D. from 3 or 4 ear-pinnas. ** p < 0.01, *** p < 0.001 vs. S, # p < 0.05 vs. Oxi (inflammation), n = 3 or 4.
It is known that IL-1β and IL-18 are formed within cells as pro-types and then converted to their mature types by caspase-1, and that the mature types are then released from the cells.30–32) In the experiments employing local injections described above, we could not distinguish pro-types from mature types. We previously reported that intraperitoneal (i.e., systemic) injection of alendronate (an N-BP) into mice increased pro-IL-1β in tissues, and that LPS converted this to mature IL-1β via activation of caspase-1.25) Indeed, intraperitoneal injection of alendronate into mice increases IL-1β in various tissues, but not in the serum, and intraperitoneal or intravenous injection of LPS into mice 3 d after an injection of alendronate markedly augments the serum level of IL-1β.22,23) Here, we examined whether pretreatment with intraperitoneal zoledronate might lead to augmented effects of intraperitoneal LPS injection on the serum levels of IL-1β, IL-18, and TNF-α in mice. In mice injected with zoledronate alone (10 µmol/kg, ca. 0.25 µmol/mouse), we could detect no IL-1β, IL-18, or TNF-α in the serum (data not shown). As shown in Fig. 6, LPS alone (0.1 mg/kg, ca. 2.5 µg/mouse) only tended to increase them. However, LPS markedly elevated the serum levels not only of IL-1β, but also of IL-18 in mice pre-treated with zoledronate 3 d before the LPS injection. The only significant difference in TNF-α between the saline→LPS and zoledronate→LPS groups was that there were small augmentations at 4 and 6 h after the LPS injection in the latter group.

Saline (S) or zoledronate (Zol) (10 µmol/kg, ca. 0.25 µmol/mouse) was intraperitoneally injected into mice. Three days later, LPS (0.1 mg/kg, ca. 2.5 µg/mouse) was intraperitoneally injected into the same mice, and serum levels of IL-1β, IL-18, and TNF-α were measured at the indicated numbers of hours after the injection of LPS. The values are means ± S.D. from 3 or 4 mice. ** p < 0.01, *** p < 0.001 vs. respective time of S→LPS, ## p < 0.01 vs. time 0 of S→LPS, $$ p < 0.01, $$$ p < 0.001 vs. time 0 of Zol→LPS. n = 3 or 4.
In the present study, we focused on the local and systemic effects of zoledronate on typical inflammatory cytokines (IL-1β, IL-18, and TNF-α) in mice. The results may be summarized as follows. In local experiments using ear-pinnas, (i) zoledronate induces long-lasting accumulation of IL-1β and IL-18, (ii) zoledronate and LPS (a cell-wall component of Gram-negative bacteria) mutually augment the productions of IL-1β, IL-18, and TNF-α, and (iii) oxidronate (a toxic non-N-BP) by itself stimulates production not only of IL-1β and IL-18, but also of TNF-α. In systemic experiments using intraperitoneal injection of zoledronate and/or LPS, (a) zoledronate by itself increased none of the above cytokines in serum, and (b) in mice pretreated (3 d before) with zoledronate, the LPS-induced increases in serum IL-1β and IL-18 were greatly augmented with a delayed slight TNF-α augmentation. These findings are discussed below. We believe that the above results make this the first report to demonstrate the ability of inflammatory or toxic BPs to stimulate the production of IL-18.
Accumulation of IL-1β and IL-18 within CellsAs described in Introduction, our findings from previous murine experiments suggest that both IL-1α and IL-1β are causally involved in the inflammatory effects of N-BPs. Experiments by other investigators support this idea.33–35) Indeed, IL-1β has been detected in the saliva or gingival crevicular fluid from BRONJ patients.36,37) In addition to IL-1, the present study suggested (possibly for the first time) that IL-18, too, may be causally involved in the inflammatory and/or necrotic effects of N-BPs.
Caspase-1 is known to be an enzyme producing mature IL-1β and IL-18 from their precursors.31,32,38) N-BPs inhibit farnesyl pyrophosphate (FPP) synthase in the mevalonate pathway of cholesterol biosynthesis in various cells, leading to a reduction in protein prenylation.1) Reduced prenylation has been suggested to activate caspase-1 and lead to a sustained secretion of IL-1β, resulting in severe chronic inflammation in children with genetically reduced mevalonate kinase in the cholesterol biosynthesis pathway.39,40) Moreover, reduced prenylation also reportedly increases IL-1β mRNA in vitro.40) Indeed, various statins, inhibitors of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase in the mevalonate pathway, have also been shown to induce in vitro an activation of caspase-1 and productions of not only IL-1β but also IL-18 in peripheral-blood mononuclear cells.41–44) Interestingly it has also been shown that in the presence of bacteria, the production of these cytokines is greatly increased by fluvastatin in a synergistic way.41) Thus, it seems likely that N-BPs are capable by themselves of producing pro-IL-1β and pro-IL-18 and also causing release of their mature forms.
In the present study, we showed that (i) in local experiments, zoledronate induces accumulation of IL-1β and IL-18 within tissues, and (ii) in systemic experiments, while zoledronate by itself increases neither of these cytokines in serum, pretreatment with zoledronate leads to marked augmentation of the effects of intraperitoneal LPS on the serum levels of IL-1β and IL-18. Concerning the alendronate-induced production of IL-1β in the spleen of mice, our previous results obtained from Western blotting demonstrated that (i) alendronate directly stimulates macrophages to produce pro-IL-1β in vivo and in vitro, but that the release of mature IL-1β is below detectable levels due to insufficient activation of caspase-1, and (ii) LPS activates caspase-1 and causes a release of mature IL-1β when it is injected into mice pretreated with alendronate.25) These results, together with the ones in reports described above, suggest that (i) N-BPs induce a long-lasting co-existence of the pro-forms of IL-1 and IL-18 in tissues, (ii) although N-BPs by themselves may activate caspase-1, the magnitude of this effect may be not sufficient to produce detectable serum levels, and (iii) in N-BP-treated mice, LPS augments the release of the mature forms of IL-1β and IL-18 via activation of caspase-1, resulting in them increasing in the serum.
In the present study, we again tried to detect pro-IL-1β, mature IL-1β, and conversion of inactive caspase-1 to active caspase-1 by Western blotting. However, we could not detect them, possibly because there may be much fewer macrophages in the ear-pinna than in the spleen. Indeed, the increase in IL-1β was several tens ng/g in the spleen,25) against only several ng/g in the ear-pinna.
Roles of IL-1β, IL-18, TNF-α, and LPS (or Infection) in the Pathogenesis of BRONJNon-N-BPs are converted into cytotoxic ATP analogs within cells.17) Thus, the mechanisms by which non-N-BPs exert cytotoxic effects are different from those that mediate the cytotoxic effects of N-BPs (see above). In the present study, zoledronate increased IL-1β and IL-18, but not TNF-α, while the toxic non-N-BP oxidronate increased not only IL-1β and IL-18, but also TNF-α (Fig. 5). This supports the idea that the mechanisms underlying the cytotoxic effects of zoledronate and oxidronate are different. In addition, the effect of oxidronate supports IL-1β, IL-18, and TNF-α being important during the pathogenesis of inflammation and/or necrosis.
IL-1 and TNF-α act synergistically to induce inflammatory as well as toxic reactions.45–47) These cytokines and LPS also act synergistically to induce inflammation and tissue injury, the effects being observable as local Shwartzman reactions in animal skins.48–50) The present findings and the reported properties of inflammatory cytokines described above suggest that the long-lasting co-existence of IL-1β, IL-18, and TNF-α may be induced by N-BPs and LPS around infected jawbones and that their synergistic or cooperative effects may play important roles in the pathology of BRONJ. As described in Introduction, it is thought that IL-1β and IL-18 are involved in pyroptosis, and that TNF-α is involved in apoptosis and/or necroptosis. TNF-α is produced in various cells other than macrophages in response to LPS.51) It should also be noted that although IL-1β is expressed upon activation only in cells of hematopoietic origin, pro-IL-1α is present in a wide variety of cells and is expressed in hematopoietic and non-hematopoietic cells in response to appropriate stimuli.52) Thus, in a scenario in which there is (a) a prolonged release of N-BPs from jawbones that have previously accumulated them and (b) a co-existence of LPS (or infection), it is possible that IL-1β, IL-18, TNF-α, and IL-1α, in soft-tissues surrounding the jawbones might contribute to the induction of inflammation and necrosis (i.e., BRONJ), and to the subsequent repair of the injury.
PerspectiveLPS is known to stimulate Toll-like receptor (TLR)-4. It is notable that IL-1 (IL-1α and IL-1β) and IL-18 belong to the IL-1 family, and that the IL-1 and TLR families share similar functions.53) During the course of cholesterol biosynthesis, farnesyl pyrophosphate (FPP) is formed from isopentenyl pyrophosphate. As we noted in our recent review,19) FPP is a precursor of various substances that are important for the functions and structure of eukaryotic cells, and N-BPs inhibit the synthesis of FPP, resulting in a variety of cellular dysfunctions (including injuries and death among cells) not only in osteoclasts but in other cells, too. Dinarello pointed out that more than any other cytokine family, the IL-1 family is primarily associated with innate immunity, and the IL-1 family includes various other inflammatory, as well as anti-inflammatory, cytokines.53) Our findings suggest that the N-BP-induced cellular dysfunctions that occur via reduced production of FPP may trigger or augment self-defensive, inflammatory, or innate immune responses. Hence, it might be very interesting to examine the effects of N-BPs on members of the IL-1 family other than IL-1α, IL-1β, and IL-18.
The results obtained in the present study, together with previous ones, suggest that (a) pro-IL-1β and pro-IL-18 accumulate within cells in soft-tissues exposed to N-BPs, and that infection may augment not only their production, but also the release of their mature forms, (b) IL-1β and IL-18 (possibly together with TNF-α) may play important roles in N-BP-induced inflammation and/or necrosis, and (c) the mechanisms underlying the cytotoxic effects of BPs may differ between N-BPs and non-N-BPs. The main points of the above discussion are summarized in Fig. 7.

This work was supported by Grants from the Japan Society for the Promotion of Science [21792101 (Funayama), 16K11672 (Endo)]. We are grateful to Dr. Robert Timms for editing the manuscript.
The authors declare no conflict of interest.