2019 Volume 42 Issue 6 Pages 954-959
2019 Volume 42 Issue 6 Pages 954-959
Vitamin C is a natural nutrient with antioxidant properties and is used as a health supplement. In this study, we examined the effects of intraperitoneal administration of high-dose vitamin C (4 g/kg) on dextran sodium sulfate (DSS)-induced ulcerative colitis. We prepared a mouse ulcerative colitis model by administering DSS for 7 d along with high-dose vitamin C each day during DSS treatment. Ulcerative colitis induced by DSS was ameliorated by high-dose vitamin C administration. Blood levels of interleukin-6, tumor necrosis factor-α, hydrogen peroxide (H2O2), and iron were elevated in DSS-treated mice but lowered by high-dose vitamin C administration. Contrarily, the levels of H2O2 and iron and the numbers of terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL)-positive cells in the colon were further increased by high-dose vitamin C administration. The expression levels of fibroblasts, collagen type I, and collagen type III decreased in the DSS-treated mice but increased in mice administered high-dose vitamin C. These results suggest that high-dose vitamin C administration can improve ulcerative colitis.
In ulcerative colitis, the tunica mucosa of the colon is inflamed. The colon may degenerate and become ulcerated in response to chronic inflammation. The incidence of ulcerative colitis is rapidly increasing.1) Recurring inflammation of the colonic tunica mucosa involves symptoms like diarrhea and blood in the feces, with fecal abnormality being the most common symptom. There are several causes of ulcerative colitis. Environmental and genetic factors may be responsible for the breakdown of the intestinal tunica-mucosa barrier.2,3) When the colonic epithelium deteriorates, it may be invaded by pathogenic bacteria.4) Mucosal immunity may result in the irruption of intestinal bacteria into the colon, which can induce cytokine production and chronic inflammation. Tumor necrosis factor (TNF)-α and interleukin (IL)-6 may be important causative factors.5) Additionally, fibrosis is a major complication in inflammatory bowel disease, which may be mediated by the intestinal fibroblasts.6) Collagen I and III secreted from fibroblasts play important roles in the recovery from inflammation.7,8)
Recently investigated drug therapies for ulcerative colitis include the anti-inflammatory agents 5-aminosalicylic acid,9) and adrenocorticosol steroid,10) immunomodulators cyclosporine11) and tacrolimus,12) and anti-TNF-α antibody infliximab.13) However, the causes and mechanisms of the onset of ulcerative colitis remain poorly understood. Other treatment options for this disorder are needed.
Vitamin C is a water-soluble molecule naturally present in certain foods and is used as a preservative and food supplement. Vitamin C is an important antioxidant14) and plays a vital role in immune function.15) Vitamin C is essential for collagen biosynthesis.16) Thus, vitamin C may effectively prevent or lower the risk of contracting several diseases like chronic obstructive pulmonary disease,17) hypertension,18) and the common cold.19) Additionally, it was reported that high-dose vitamin C administration has anticancer effects.20–22) Our previous analyses indicated that high doses of vitamin C was significantly effective against colorectal cancer (unpublished). However, the effects of high-dose vitamin C therapy on ulcerative colitis are unknown.
In this study, we examined the effects of high-dose vitamin C administration on a dextran sodium sulfate (DSS)-induced mouse ulcerative colitis model. We also investigated the mode of action of high-dose vitamin C administration against ulcerative colitis.
Hairless male mice (HR-1; 7-week-old) were purchased from SLC (Hamamatsu, Shizuoka, Japan) and housed under a 12-h light/12-h dark cycle at a constant temperature of 23 ± 1°C and 50 ± 10% relative humidity. This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of Suzuka University of Medical Science (approval number: 34). All surgeries were performed under pentobarbital anesthesia and every effort was made to minimize animal suffering.
Induction of Experimental Colitis in MiceAt 8 weeks of age, the mice were randomly assigned to four groups (n = 6 per group): control, vitamin C, DSS, and DSS + vitamin C. Mice in the DSS and the DSS + vitamin C groups were fed 5.0% (w/v) DSS (molecular weight (MW): 36000–50000 Da; MP Biomedicals, Solon, OH, U.S.A.) in drinking water for 7 d to induce colitis.23–25) Disease development was monitored by observing and weighing the feces of each mouse. Colitis severity was determined by measuring the body weight, fecal condition, and colon length. The fecal condition score was determined using two parameters: stool consistency (0 = normal; 1 = soft; 2 = very soft but formed; 3 = liquid) and fecal bleeding according to the guaiac paper test (0 = negative, 1 = faintly blue, 2 = moderately blue, 3 = dark blue, 4 = visible blood). The sums of both factors were used as the individual disease activity scores.26)
In the vitamin C and DSS/vitamin C groups, vitamin C (4g/kg body weight) was administered intraperitoneally for 7 d as previously described.21) Control animals were given plain water. Furthermore, the dose of vitamin C used in the experiment is the volume that showed an effect in a previous study.27) Additionally, we examined the concentration dependence of the effects of vitamin C addition. A concentration of (4 g/kg body weight showed the least effects.
Colon Preparation and StainingFor the histological analyses, the mice were sacrificed at 7 d after the start of the experiment. Colon specimens were fixed in phosphate-buffered paraformaldehyde (4%), embedded in frozen Tissue Tek, OCT compound, and sliced into 5-µm-thick sections. Apoptosis was identified in situ in the colon sections with an apoptosis detection kit (TaKaRa Biomedicals, Tokyo, Japan).
Western BlottingThe colon samples were homogenized in lysis buffer (Kurabo, Osaka, Japan) and centrifuged at 8000 × g for 10 min. The supernatants from each sample were then isolated and stored at −80°C until further analysis. Western blotting was performed as previously described.28) Briefly, the samples were separated by electrophoresis and the membranes were incubated at 25°C for 1 h with primary antibodies against S100A4, a fibroblast marker (1 : 1000; RB-1804-A0, Thermo Fisher Scientific, Waltham, MA, U.S.A.), collagen type I (1 : 1000; 234167, Millipore, Billerica, MA, U.S.A.), collagen type III (1 : 1000; ab7778, Abcam, Cambridge, U.K.), or β-actin (1 : 5000; A5441-100UL, Sigma-Aldrich Corp., St. Louis, MO, U.S.A.). Immune complexes on the membranes were visualized with horseradish peroxidase-conjugated secondary antibody (1 : 1000; Novex, Frederick, MD, U.S.A.) and detected with ImmunoStar Zeta regent (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The images were acquired with the Multi-Gauge Software Program (FUJIFILM, Greenwood, SC, U.S.A.).
Measurement of Plasma IL-6, TNF-α, H2O2, and IronThe plasma levels of IL-6 and TNF-α were determined using commercial enzyme-linked immunosorbent assay kits (IL-6: BioLegend, San Diego, CA, USA; TNF-α: R&D Systems, Minneapolis, MN, U.S.A.) in accordance with the manufacturer’s instructions. Plasma and colonic H2O2 and iron levels were determined with an OxiSelect™ STA-347 in vivo ROS/RNS assay kit (Cell Biolabs, Inc., San Diego, CA, U.S.A.) and a metalloassay kit (ferrozing method) (Metallogenics, Chiba, Japan) in accordance with the manufacturer’s instructions.
Statistical AnalysesAll data are presented as the mean ± standard deviation (S.D.). The results were analyzed with Microsoft Excel 2010 software (Microsoft Corp., Redmond, WA, U.S.A.). Differences between groups were evaluated by one-way ANOVA, followed by Tukey’s post-hoc test in SPSS version 20th software (SPSS, Inc., Chicago, IL, U.S.A.). The results were considered significant at p < 0.05.
Diarrhea and fecal bleeding were observed in DSS-treated mice (Figs. 1A, B). The colitis scores of vitamin C/DSS-treated mice were lower than those of DSS-treated mice (Fig. 1C). There were decreases in the body weight (Fig. 1D) and colon length (Figs. 1E, F) in DSS-treated mice relative to the controls. The average colon length of vitamin C/DSS-treated mice was greater than that of DSS-treated mice.
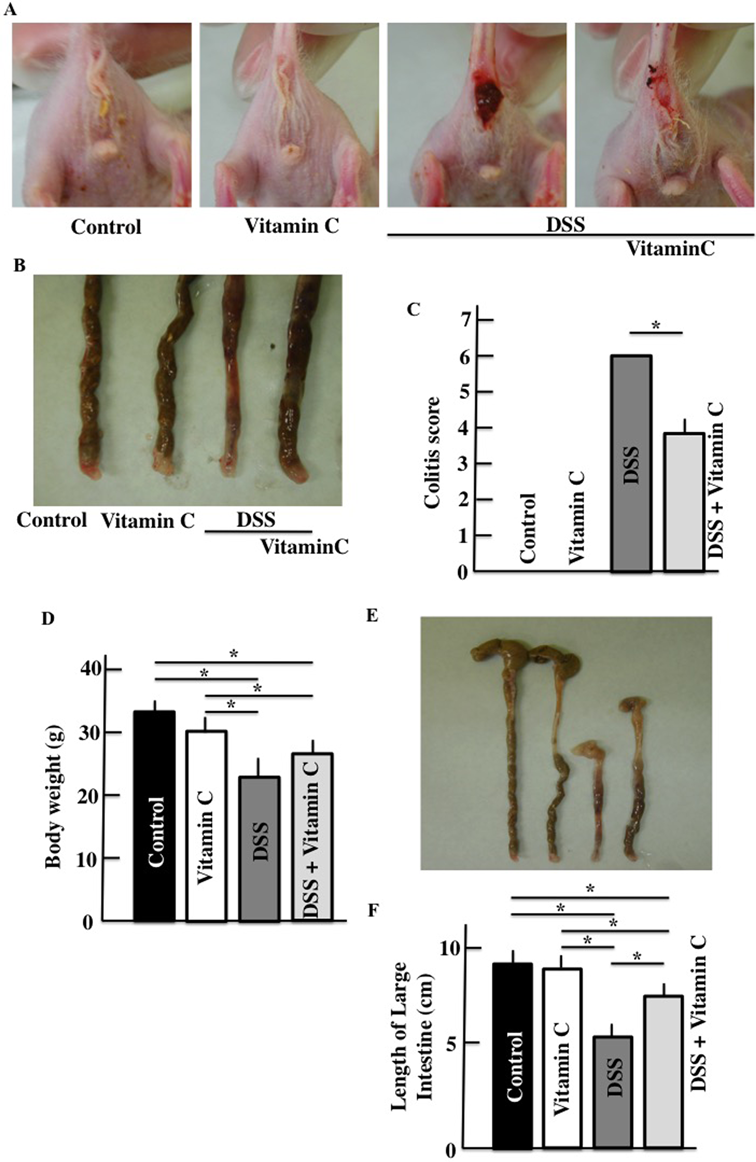
Colitis scores (A–C), body weights (D), and colon length (E, F). Values are presented as the mean ± S.D. of six animals. * p < 0.05. (Color figure can be accessed in the online version.)
Plasma IL-6 and TNF-α levels were higher in DSS-treated mice than those in untreated control mice. However, plasma IL-6 and TNF-α were both lower in the vitamin C/DSS-treated mice than in DSS-treated mice (Fig. 2).
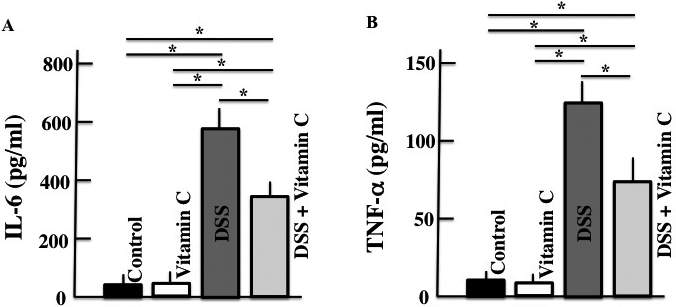
Values are presented as the mean ± S.D. of six animals. * p < 0.05.
Active oxygen was also measured because its levels are closely associated with vitamin C levels. In DSS-treated mice, the plasma and colonic H2O2 and iron and expression of apoptosis-positive cells in the colon were higher than those in untreated control mice. In vitamin C/DSS-treated mice, only colonic H2O2 and iron levels and apoptosis expression in the colon were higher than in DSS-treated mice (Fig. 3).
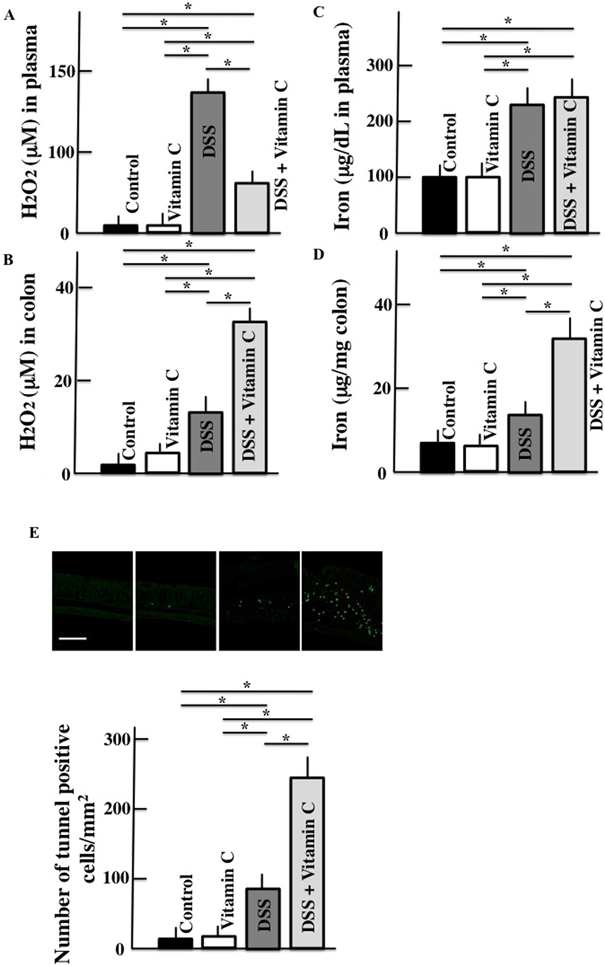
Scale bar = 100 µm. Values are presented as the mean ± S.D. of six animals. * p < 0.05. (Color figure can be accessed in the online version.)
Collagen generation was evaluated because it is regulated by vitamin C. Fibroblast, collagen type I, and collagen type III expression levels in the colon of DSS-treated mice were lower than those in untreated control mice. However, the levels of all these parameters were greater in mice administered high-dose vitamin C than in DSS-treated mice (Fig. 4).
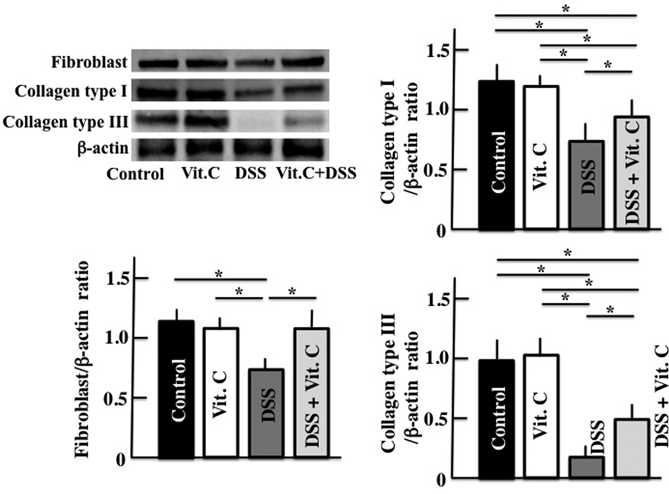
Equal protein loading was assessed using mouse β-actin. Vit. C: Vitamin C. Values are presented as mean ± S.D. of six animals. * p < 0.05.
In this study, the symptoms of DSS-induced colitis were ameliorated by high-dose vitamin C administration. Plasma IL-6 and TNF-α levels were increased in DSS-treated mice but decreased in high-dose vitamin C-treated mice relative to the controls. High-dose vitamin C lowered plasma H2O2 when compared with the untreated controls, but increased H2O2 in the inflamed colon relative to the control. High-dose vitamin C administration also increased free iron and apoptosis expression levels in the inflamed colon when compared with the untreated control. Additionally, the expression levels of colonic fibroblasts, collagen type I, and collagen type III decreased in response to DSS-treatment but increased in response to high-dose vitamin C administration.
Vitamin C is an antioxidant and removes water-soluble reactive oxygen species (O2−, H2O2, and 1O2).29) Therefore, vitamin C lowered plasma H2O2 levels relative to DSS-treated mice in this study. However, in vitamin C/DSS-treated mice, H2O2 levels in the inflamed colon were higher than those in DSS-treated mice. This is because the risk of apoplexy increases with DSS treatment, which increases hemoferrum levels. Additionally, iron in the colon of vitamin C/DSS-treated mice showed a greater increase. As reported by Agget, because vitamin C is a reducing agent, Fe3+ is converted to Fe2+ and iron absorption increases through divalent metal transporter 1.30) Additonally, according to Wienk et al., vitamin C increases iron uptake, particularly in the intestinal mucosa.31) The increase in iron in the colon caused by vitamin C is depicted in Fig. 3D. As reported by Chen et al.,32) ascorbic acid tends to be converted to ascorbate radical in the extracellular field (or tissue) rather than in the blood, easily leading to the production of H2O2. Thus, we considered that H2O2 was increased by vitamin C in the colon tissue, as shown in Fig. 3B. Additionally, the damaged cells of the colon may have been eliminated by apoptosis and the colon length did not change following colon remodeling. The anticancer efficacy of high-dose vitamin C administration may be explained by its ability to induce apoptosis in tumor cells. In this study, both colonic H2O2 and free iron levels increased in response to vitamin C administration. High-dose vitamin C administration induced colonic apoptosis and reduced ulceration. However, the actual vitamin C concentration present in the colon is unknown and requires further investigation.
In inflamed or otherwise-damaged tissues, fibroblast levels increase, resulting in the production of collagen type III. In the presence of vitamin C, lesions may heal because the vitamin suppresses collagen type III and induces the formation of collagen type I.7) These enable tissues to recover from the injury.7,8) In this study, fibroblast proliferation and increases in the expression levels of collagen type I and III were observed in response to high-dose vitamin C administration. This may have accelerated the healing of ulcers induced by DSS. However, recovery from these lesions generally requires several weeks. High-dose vitamin C administration may induce fibroblast proliferation and collagen production at an early treatment stage. Further studies are required to determine the exact modes of action and optimal dosages of vitamin C needed to treat ulcerative colitis.
High-dose vitamin C administration ameliorates DSS-induced ulcerative colitis. Its therapeutic mode of action may include the induction of apoptosis by H2O2 and enhancement of collagen production. Therefore, vitamin C may be useful for treating ulcerative colitis both safely and effectively. However, the exact mechanisms and optimal doses of high-dose vitamin C administration remain unknown and require further investigation.
This study was supported by Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (JSPS; Grant Number 18K06802).
The authors declare no conflict of interest.