2019 Volume 42 Issue 6 Pages 968-976
2019 Volume 42 Issue 6 Pages 968-976
Previously, we reported that adenosine N1-oxide (ANO), which is found in royal jelly, inhibited the secretion of inflammatory mediators by activated macrophages and reduced lethality in lipopolysaccharide (LPS)-induced endotoxin shock. Here, we examined the regulatory mechanisms of ANO on the release of pro-inflammatory cytokines, with a focus on the signaling pathways activated by toll-like receptor (TLR)4 in response to LPS. ANO inhibited both tumor necrosis factor (TNF)-α and interleukin (IL)-6 secretion from LPS-stimulated RAW264.7 cells without affecting cell proliferation. In this response, phosphorylation of mitogen-activated protein kinase (MAPK) family members (extracellular signal-regulated kinase (ERK)1/2, p38 and SAPK/c-Jun N-terminal kinase (JNK)) and nuclear factor-κB (NF-κB) p65 was not affected by treatment with ANO. In contrast, phosphorylation of Akt (Ser473) and its downstream molecule glycogen synthase kinase-3β (GSK-3β) (Ser9) was up-regulated by ANO, suggesting that ANO stimulated GSK-3β phosphorylation via phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway. The phosphorylation of GSK-3β on Ser9 has been shown to negatively regulate the LPS-induced inflammatory response. Activation of PI3K/Akt signaling pathway has also been implicated in differentiation of mesenchymal stem cells into osteoblasts and adipocytes. As expected, ANO induced alkaline phosphatase activity and promoted calcium deposition in a mouse pre-osteoblastic MC3T3-E1 cell line. The ANO-induced differentiation into osteoblasts was abrogated by coincubation with Wortmannin. Furthermore, ANO promoted insulin/dexamethasone-induced differentiation of mouse 3T3-L1 preadipocytes into adipocytes at much lower concentrations than adenosine. The protective roles of PI3K/Akt/GSK-3β signaling pathway in inflammatory disorders have been well documented. Our data suggest that ANO may serve as a potential candidate for the treatment of inflammatory disorders. Promotion of osteogenic and adipocyte differentiation further suggests its application for regenerative medicine.
Adenosine is a key molecule that regulates numerous physiological processes by activating four G protein-coupled adenosine receptors (ARs), A1, A2A, A2B and A3 ARs.1) Regarding the adenosine-mediated regulation of inflammatory and immune responses, adenine nucleotides such as ATP and ADP are released extracellularly from damaged cells at sites of injury and inflammation. After release, ATP and ADP are catabolized sequentially by ectoenzymes CD39 and CD73, resulting in an increase of extracellular adenosine. Accumulated adenosine exhibits potent immunosuppressive activities that dampen inflammatory responses. Adenosine down-regulates the release of pro-inflammatory mediators primarily through the A2A AR.1,2) In addition to its anti-inflammatory effects, adenosine plays an important role in promoting wound healing and tissue repair.3) Thus, adenosine is a potent endogenous molecule that is critical for resolution of inflammation and tissue remodeling. However, the bioavailability of adenosine is limited by its extremely short half-life. That is, adenosine is rapidly metabolized in blood by its conversion to adenosine monophosphate by adenosine kinase or its change to inosine by adenosine deaminase.4)
Adenosine N1-oxide (ANO), which is found in royal jelly, is an oxidized product of adenosine at the N1 position of the adenine base moiety. We recently reported that ANO is refractory to adenosine deaminase-mediated conversion to inosine and possesses anti-inflammatory activities both in vitro and in vivo.5) ANO inhibited the secretion of inflammatory mediators at much lower concentrations than adenosine and dipotassium glycyrrhizinate when used with mouse peritoneal macrophages or the human monocytic cell line (THP-1) after stimulation with lipopolysaccharide (LPS) and interferon-γ (IFN-γ). From a mechanistic analysis, we inferred that ANO would suppress both tumor necrosis factor (TNF)-α and interleukin (IL)-6 secretion from LPS-stimulated RAW264.7 cells, a mouse macrophage-like cell line, through the up-regulation of the anti-inflammatory transcription factor c-Fos.5) However, we observed that forskolin, which up-regulates c-Fos via a cAMP-dependent pathway, inhibited TNF-α secretion from LPS-stimulated RAW264.7 cells but failed to inhibit IL-6 secretion. These results suggest the existence of a mechanism independent of c-Fos by which ANO can suppress secretion of pro-inflammatory cytokines. In this study, the mechanism(s) of the anti-inflammatory actions of ANO was thoroughly investigated.
BALB/c female mice, aged 8–12 weeks, were purchased from Charles River Japan (Kanagawa, Japan). All animal experiments described in this article were approved by the Laboratory Animal Care Committee of the Hayashibara Co., Ltd.
ReagentsANO was prepared according to the procedure described previously.5) LPS (Escherichia coli O55:B5), adenosine and forskolin were purchased from Sigma-Aldrich Japan (Tokyo, Japan). Wortmannin and H-89 were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Mouse cytokine standards for enzyme-linked immunosorbent assay (ELISA) (TNF-α, IL-6) were obtained from BD Biosciences (San Diego, CA, U.S.A.). The following monoclonal antibody (mAb) pairs for ELISA capture and biotinylated detection were purchased from BD Pharmingen for TNF-α, G281-2626 and MP6-XT3; IL-6, MP5-20F3 and MP5-32C11.
Anti-inflammatory Effects of ANOMurine peritoneal macrophages were elicited by intraperitoneal injection of 2 mL 4% Brewer thioglycollate medium (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan). Peritoneal exudate cells were collected by lavage 3 to 4 d after injection. Cells were washed twice with a medium and plated in 10-cm diameter plastic dishes (Nippon Becton Dickinson, Tokyo, Japan) at a density of 1 × 108 cells/dish in 10 mL of RPMI1640 medium (Sigma-Aldrich Japan) containing 10% (v/v) fetal bovine serum (FBS, Life Technologies, Grand Island, NY, U.S.A.), 100 units/mL penicillin G and 100 µg/mL streptomycin sulfate. After 2 h incubation (37°C, 5% CO2), non-adherent cells were removed by rinsing. RPMI1640 medium containing 10% FBS and antibiotics was then added to the adherent cells that were recovered by scraping. The recovered cells were used as peritoneal macrophages.
RAW264.7 cells were maintained in RPMI1640 medium containing 10% FBS and antibiotics. For pro-inflammatory cytokine production, RAW264.7 cells or peritoneal macrophages were seeded at 5 × 104 cells per well in flat bottom 96-well microplate and stimulated with LPS (2 µg/mL) in the presence or absence of varying concentrations of ANO or adenosine at 37°C for 24 h. In some experiments, peritoneal macrophages were incubated with various concentrations of H-89 or Wortmannin for 30 min before stimulation with LPS in the presence of ANO. In other experiments, RAW264.7 cells were stimulated with LPS (2 µg/mL) in the presence or absence of varying concentrations of forskolin, an activator of adenylyl cyclase at 37°C for 24 h. After a 24 h incubation period, the culture supernatants were collected for the measurement of cytokines.
For the measurement of cell proliferation, 20 µL alamarBlue dye (Trek Diagnostic Systems, OH, U.S.A.), a redox indicator, was added to each microplate well for the last 2 to 3 h of the incubation period. Fluorescence intensity (FI) was measured at 544 nm excitation wavelength and 590 nm emission wavelength.
For the measurement of intracellular cAMP, peritoneal macrophages and RAW264.7 cells (1.5–2 × 106 cells/well of 12 well plate) were incubated for 30 min with the indicated concentrations of ANO or forskolin. After the incubation, cells were incubated for 20 min with 0.1 N HCl, and disrupted by pipetting. Intracellular cAMP levels were determined by ELISA according to the manufacturer (Cayman Chemical, Ann Arbor, MI, U.S.A.).
Western Blotting AnalysisWestern blotting was performed using cell extracts from RAW264.7 cells stimulated with LPS (2 µg/mL) in the presence or absence of varying concentrations of ANO, as described previously.5) The membranes were probed with anti-phospho-p42/p44 (extracellular signal-regulated kinase (Erk)1/2) (Thr202/Tyr204) rabbit pAb, anti-phospho-SAPK/c-Jun N-terminal kinase (JNK) (Thr183/Tyr185) rabbit pAb, anti-phospho-p38 mitogen-activated protein kinase (MAPK) (Thr180/Tyr182) rabbit mAb (D3F9), anti-phospho-Akt (Ser473) rabbit mAb (D9E), anti-phospho-glycogen synthase kinase-3β (GSK-3β) (Ser9) rabbit mAb (D85E12) or anti-phospho-nuclear factor-κB (NF-κB) p65 (Ser536) rabbit mAb (93H1) (Cell Signaling Technology, Danvers, MA, U.S.A.) and then specific bands were detected by a chemiluminescent system. After treatment with reprobing solution (Restore Western blot Stripping Buffer; Pierce Biotechnology, Rockford, IL, U.S.A.) for 15 min at room temperature, the membrane was used for second detection with anti-p42/p44 (Erk1/2) rabbit mAb (137F5), anti-SAPK/JNK rabbit mAb (56G8), anti-p38 MAPK rabbit pAb, anti-Akt (pan) rabbit mAb (C67E7), or anti-β-Actin (ACTBD11B7; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.).
Osteogenic Differentiation of MC3T3-E1 CellsMouse pre-osteoblastic cell line, MC3T3-E1 cells were maintained in α-modified minimal essential medium (α-MEM) (Sigma-Aldrich Japan) containing 10% FBS and antibiotics. In differentiation cultures, MC3T3-E1 cells were seeded at 5 × 104 cells per well in flat bottom 24-well plates and cultured for 3 d at 37°C in 5% CO2. After rinsing with phosphate buffered saline (PBS), the semi-confluent cells were treated with various concentrations of ANO in fresh α-MEM containing 10% FBS, 10 mM β-glycerophosphate, and 10 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES), and the culture medium was replaced every 3 or 4 d. After 6 d of culture, alkaline phosphatase (ALP) activity was measured by using a LabAssay ALP Kit (FUJIFILM Wako Pure Chemical Corporation).
For the measurement of calcium deposition, the cells were washed with PBS and treated with 0.5 mL of 2 N HCl to solubilize extracellular calcium deposits after 7 d of culture. The calcium concentrations were measured with a Calcium Assay Kit (FUJIFILM Wako Pure Chemical Corporation).
Adipocyte Differentiation of 3T3-L1 Cells3T3-L1 cells, a preadipocyte cell line, were maintained in growth medium (Dulbecco’s modified Eagle’s medium) (Nissui Pharmaceutical Co., Tokyo, Japan) containing 10% FBS and antibiotics. Differentiation was initiated by adding medium containing 0.5 µM dexamethasone and 10 µg/mL insulin in the presence or absence of varying concentrations of adenosine or ANO. The medium was changed every 2 d. After 10 d of culture, differentiation of 3T3-L1 cells into mature adipocytes was confirmed by Oil Red O staining.6) Dye was eluted with 2-propanol, and absorption of the eluate was measured at 490 nm.
Statistical AnalysisData were analyzed by one-way ANOVA followed by Dunnett’s multiple-comparison test. In some experiments, data were analyzed by one-way ANOVA followed by Tukey multiple-comparison test. p-Values (<0.05) were considered statistically significant.
ANO exerts anti-inflammatory effects by inhibiting the secretion of pro-inflammatory mediators from activated macrophages.5) To explore the mechanisms for the anti-inflammatory effects of ANO, we first compared it with adenosine for their inhibitory effects on pro-inflammatory cytokine production. For this purpose, RAW264.7 cells were stimulated with 2 µg/mL LPS. Figures 1A and B showed the dose–response curve of their inhibitory effects on TNF-α and IL-6 production, respectively. As observed with murine peritoneal macrophages,5) ANO effectively inhibited both TNF-α and IL-6 production at low concentrations in a dose-dependent manner. These reductions were not due to the inhibition of cell growth (Fig. 1C). Adenosine also inhibited the secretion of TNF-α and IL-6 in a dose-dependent fashion, but much higher concentrations were required compared to ANO (Figs. 1A, B).
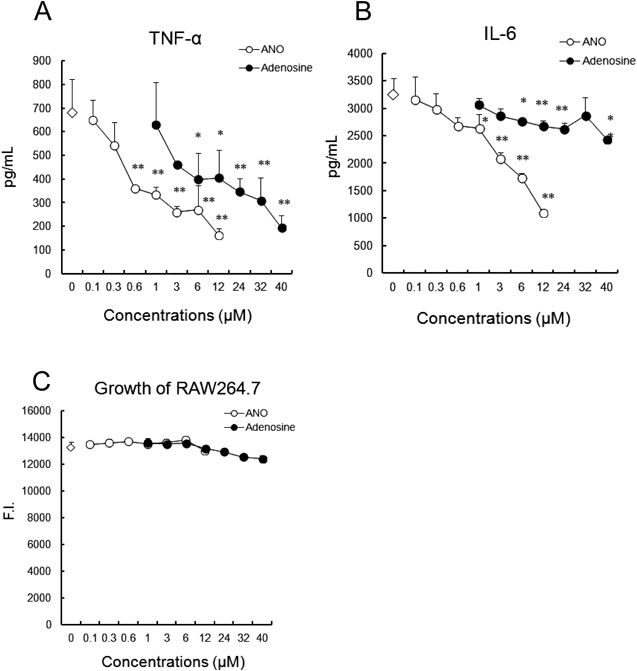
RAW264.7 cells (5 × 104 cells/well) were stimulated with LPS (2 µg/mL) in the presence or absence of varying concentrations of ANO or adenosine at 37°C for 24 h. Then, the levels of secreted TNF-α (A) and IL-6 (B) were measured by ELISA. Cell growth was assessed by adding 20 µL/well alamarBlue dye for the last 2–3 h of the incubation period. Data are expressed as fluorescence intensity (FI) values (C). Each value represents the means ± S.D. of triplicate cultures. * p < 0.05; ** p < 0.01, significantly different when compared with control culture.
Recently, it was reported that cAMP suppressed pro-inflammatory cytokine production by up-regulating the transcription factor, c-Fos.7) We observed that ANO significantly increased intracellular cAMP production in both peritoneal macrophages and RAW264.7 cells in a dose-dependent manner (Fig. 2). Furthermore, the increase in both mRNA and protein expression of c-Fos was observed when RAW264.7 cells were stimulated with LPS in the presence of ANO.5) We then examined the role of cAMP in ANO-mediated suppression of pro-inflammatory cytokines. Towards that end, we investigated the influence of forskolin on the secretion of TNF-α and IL-6 from LPS-stimulated RAW264.7 cells, since forskolin reportedly increased intracellular cAMP levels, probably by directly activating adenylyl cyclase (Fig. 2). As shown in Fig. 3A, forskolin significantly inhibited TNF-α production in a dose-dependent manner compared with vehicle control. The inhibitory effects of forskolin were not due to the inhibition of cell growth (Fig. 3C). In contrast, the same doses of forskolin failed to inhibit the secretion of IL-6 (Fig. 3B). These results suggest the existence of a mechanism different from that of the up-regulation of cAMP levels in the ANO-induced suppression of IL-6 secretion.

Peritoneal macrophages (A) and RAW264.7 cells (B) (1.5–2 × 106 cells/well of 12 well plate) were incubated for 30 min with indicated concentrations of ANO or forskolin (FSK). Culture supernatants were aspirated and the cells were lysed by incubation for 20 min with 0.1 N HCl, followed by disruption using a cell scraper. Intracellular cAMP levels were determined by ELISA. Each value represents the means ± S.D. of triplicate cultures. * p < 0.05; ** p < 0.01, significantly different when compared with control culture.

RAW264.7 cells were stimulated with LPS (2 µg/mL) in the presence or absence of varying concentrations of forskolin at 37°C for 24 h. Then, levels of secreted TNF-α (A) and IL-6 (B) were measured by ELISA. Cell growth was assessed by adding 20 µL/well alamarBlue dye for the last 2–3 h of the incubation period. Data are expressed as FI values (C). Each value represents the means ± S.D. of triplicate cultures. * p < 0.05, significantly different when compared with vehicle control culture.
LPS stimulation of macrophages activates MAPKs and NF-κB pathways, resulting in the release of pro-inflammatory cytokines.8) To identify the signaling pathway related to the anti-inflammatory mechanism of ANO, we first examined whether ANO regulated the phosphorylation of MAPKs, ERK1/2, SAPK/JNK, and p38 MAPK in LPS-stimulated RAW264.7 cells. Western blotting analysis was carried out by using antibodies against phosphorylated- or total forms of the above-mentioned proteins. Treatment of cells with LPS (2 µg/mL) resulted in phosphorylation of MAPKs compared to unstimulated control cells. The expression levels of phosphorylated and total forms of MAPKs in RAW264.7 cells treated with LPS were comparable with and without 5 µM ANO (Fig. 4A). Because ANO at doses above 1 µM significantly inhibited the secretion of both TNF-α and IL-6 by LPS-stimulated RAW264.7 cells (Figs. 1A, B), the results suggested that ANO did not modulate the signaling pathways of MAPKs. Next, we examined the effect of ANO on the NF-κB signaling pathway. Treatment with 5 µM ANO did not affect the phosphorylation of p65 (Fig. 4B).

RAW264.7 cells were treated with LPS (2 µg/mL) in the presence or absence of ANO (5 µM) for the indicated periods. The phosphorylations of ERK1/2 (Thr202/Tyr204), SAPK/JNK (Thr183/Tyr185), p38MAPK (Thr180/Tyr182) (A) and p65 (Ser536) and Akt (Ser473) (B) were detected by Western blotting analysis. To verify total protein, blots were stripped and reprobed with the indicated antibodies as described in the Materials and Methods section. β-Actin was used to calibrate sample loading against p65. NS; non-stimulated cells.
It has been shown that PI3K/Akt signaling pathway regulates the secretion of pro-inflammatory cytokines.9,10) We therefore examined whether ANO could activate the PI3K/Akt signaling pathway in LPS-stimulated RAW264.7 cells. As shown in Fig. 4B, LPS treatment resulted in phosphorylation of Akt. Addition of ANO (5 µM) together with LPS further increased the phosphorylation levels of Akt. In our preliminary study, we observed that ANO alone stimulated phosphorylation of Akt in RAW264.7 cells (data not shown).
Because phosphorylated Akt leads to phosphorylation of GSK-3β protein, we examined whether ANO induced the phosphorylation of GSK-3β. For this purpose, RAW264.7 cells were incubated with varying concentrations of ANO in the presence of LPS for 30 min. Using a mAb that recognized phosphorylated GSK-3β at Ser9, Western blotting analysis showed that ANO treatment caused an increase in phosphorylation of both Akt and GSK-3β proteins. The ratios of phosphorylated Akt/total Akt protein and phosphorylated GSK-3β/β-actin protein were significantly and dose-dependently increased by the addition of ANO (Figs. 5A, B).

RAW264.7 cells were treated for 30 min with LPS (2 µg/mL) in the presence or absence of varying concentrations of ANO. The phosphorylations of Akt (Ser473) and GSK-3β (Ser9) were detected by Western blotting analysis (A and B). To verify total Akt and β-actin, blots were stripped and reprobed with the indicated antibodies as described in the Materials and Methods section. β-actin was used to calibrate sample loading against GSK-3β. The optical density ratio of phospho-Akt to total Akt and of phospho-GSK-3β to β-actin was shown (A and B). Each value represents the means ± S.D. of three independent experiments. * p < 0.05; ** p < 0.01, significantly different when compared with control cells. # p < 0.05, significantly different when compared with LPS alone. NS; non-stimulated cells.
To evaluate the involvement of the PI3K/Akt signaling pathway in ANO-induced suppression of TNF-α and IL-6 release, murine peritoneal macrophages were pretreated with a PI3K/Akt inhibitor Wortmannin before stimulation with LPS in the presence of 1 µM ANO. Peritoneal macrophages were also pretreated with a protein kinase A (PKA) inhibitor H-89 to confirm the involvement of the cAMP/PKA signaling pathway. In this experimental system, we previously showed that the adenosine-induced suppression of TNF-α release was completely abrogated by H-89 pretreatment.5) However, as shown in Fig. 6, pretreatment with H-89 did not abrogate the ANO-induced suppression of both TNF-α and IL-6, but rather enhanced suppression. On the other hand, as shown previously, the ANO-induced suppression of TNF-α release was partially but significantly restored by the pretreatment with Wortmannin (Fig. 6A). Interestingly, Wortmannin pretreatment resulted in complete abrogation of the ANO-induced suppression of IL-6 release (Fig. 6B).

Peritoneal macrophages were incubated with vehicle or various concentrations of H-89 or Wortmannin for 30 min before stimulation with LPS (2 µg/mL) in the presence of ANO (1 µM). After 24 h, levels of TNF-α (A) and IL-6 (B) in the culture supernatants were determined by ELISA. Values represent the means ± S.D. of quadruplicate cultures. ## p < 0.01, significantly different when compared with LPS stimulation in the absence of ANO. * p < 0.05; ** p < 0.01, significantly different when compared with vehicle control culture in the presence of 1 µM ANO.
PI3K/Akt signaling pathway plays essential roles in the proliferation and differentiation of mesenchymal stem cells (MSCs), which are capable of differentiating into several lineages including bone, cartilage and fat.11) It has been shown that the PI3K/Akt signaling pathway is involved in regulation of osteoporosis.12,13) Therefore, we determined the effect of ANO on ALP activity and calcium deposition in the murine pre-osteoblastic cell line, MC3T3-E1. MC3T3-E1 cells were cultured with varying concentrations of ANO in the presence of 10 mM β-glycerophosphate for 6 to 7 d. After 6 d incubation period, ANO induced ALP activity in a dose-dependent fashion. At a dose of 10 µM ANO, ALP activity was increased more than 2.5-fold that of control culture (Fig. 7A). The up-regulation of ALP activity by ANO was not due to promotion of the cell growth (Fig. 7C).

MC3T3-E1 cells were cultured with varying concentrations of ANO in the presence of 10 mM β-glycerophosphate. After 6 d of culture, ALP activity (A) and growth of MC3T3-E1 cells (C) were determined. Calcium deposition (B) was measured after 7 d of exposure. Wortmannin was added to the differentiation cultures containing 10 µM ANO, and ALP activity was determined (D). Each value represents the means ± S.D. of triplicate cultures. ALP activities were expressed as the percentage of control culture. ** p < 0.01, significantly different when compared with control culture (A and B), one-way ANOVA with Tukey multiple-comparison (D).
Calcium deposition in cell culture is one of the most important markers of bone formation and osteoblast differentiation. The levels of calcium deposition were measured after 7 d of incubation. MC3T3-E1 cells treated with ANO exhibited higher deposition of extracellular calcium when compared with those of control cells (Fig. 7B). When Wortmannin, PI3K inhibitor, was added to the differentiation cultures containing 10 µM ANO, the increase in ANO-induced ALP activity was significantly abrogated (Fig. 7D). These results suggest that ANO promotes osteogenic differentiation of MC3T3-E1 cells through the activation of the PI3K/Akt signaling pathway.
We then examined whether ANO promotes adipocyte differentiation using 3T3-L1 cells. When 3T3-L1 cells were cultured with varying concentrations of ANO in the presence of dexamethasone and insulin, lipid content in adipocytes as quantified with Oil Red O staining increased dose-dependently with ANO (Fig. 8). At a dose of 4 µM ANO, a 2-fold significant increase in lipid content was observed as compared with that of control culture. Addition of 4–100 µM adenosine to the differentiation culture appeared to increase the lipid content dose-dependently, however, it did not show any statistical significances.

3T3-L1 cells were cultured with varying concentrations of adenosine or ANO in the presence of 0.5 µM dexamethasone and 10 µg/mL insulin. After 10 d of culture, adipocyte differentiation of 3T3-L1 cells was determined by Oil Red O staining. Each value represents the means ± S.D. of triplicate cultures. ** p < 0.01, significantly different when compared with control culture.
In our previous study, we inferred that ANO suppressed LPS-induced pro-inflammatory cytokine production, at least in part, through the up-regulation of the anti-inflammatory transcription factor c-Fos.5) ANO increased both mRNA and protein expression of c-Fos in LPS-stimulated RAW264.7 cells. c-Fos protein, which is up-regulated by cAMP and stabilized following phosphorylation by LPS-activated IκB kinase β (Ikkβ), physically binds to the p65 subunit of NF-κB. Through this binding, the recruitment of p65: p65 homodimer to the TNF promoter region is reduced, resulting in the suppressed production of TNF-α protein.7) However, the results obtained in our present study suggest the involvement of at least one additional pathway in ANO’s down-regulation of IL-6 secretion.
We analyzed two LPS-activated signaling pathways, the NF-κB pathway and the MAPK pathway, both of which have been shown to stimulate the release of inflammatory mediators.8) Our results indicated that the phosphorylation of MAPK family members (ERK1/2, p38 and SAPK/JNK) and the NF-κB p65 molecule was not affected by treatment with ANO in the presence of LPS. In contrast, the phosphorylation of Akt and its downstream molecule GSK-3β was up-regulated by ANO. Furthermore, while the ANO-induced suppression of TNF-α release was partially but significantly restored by the pretreatment with Wortmannin, the same treatment completely abrogated the ANO-induced suppression of IL-6 release. These results suggest that ANO stimulated phosphorylation of GSK-3β at Ser9 via the PI3K/Akt signaling pathway, resulting in the down-regulation of pro-inflammatory cytokines secretion. The difference in relative contribution of ANO to the regulation of TNF-α versus IL-6 secretion by LPS-stimulated peritoneal macrophages through activation of the PI3K/Akt signaling pathway may be related with the difference in the effects of forskolin on the secretion of TNF-α and IL-6 by LPS-stimulated RAW264.7 cells. It seems likely that cAMP/c-Fos-mediated inhibitory pathway is not involved in the ANO-induced suppression of IL-6 release by LPS-stimulated peritoneal macrophages or RAW264.7 cells.
It is shown that the PI3K/Akt signaling pathway negatively regulates the LPS-induced inflammatory response by inhibiting the function of GSK-3 through phosphorylation on Ser9.10) A specific GSK-3 inhibitor, SB216763, reduced the production of pro-inflammatory cytokines IL-1β, IFN-γ, IL-12 p40 and IL-6 by human peripheral blood monocytes stimulated with TLR2, TLR4, TLR5 or TLR9 agonists. It also up-regulated the anti-inflammatory cytokine IL-10.14) In accordance with our results, neither the amount nor the duration of NF-κB p65 phosphorylation in LPS-stimulated human monocytes was affected by the addition of SB216763.
In our preliminary study, we observed that lithium chloride, a well-known inhibitor of GSK-3β, suppressed the release of TNF-α by peritoneal macrophages stimulated with LPS (1 µg/mL) and IFN-γ (10 IU/mL). This indicates that the inhibition of GSK-3β function reportedly down-regulates pro-inflammatory cytokine secretion in our experimental system. However, we have not examined whether Akt inhibitor suppresses ANO-induced GSK-3β phosphorylation. We consider that this issue needs to be addressed in future studies.
The mechanism by which GSK-3β inhibition down-regulates the production of pro-inflammatory cytokines was ascribed to the augmentation of the cAMP response element binding protein (CREB) binding to the nuclear coactivator CREB-binding protein (CBP) following phosphorylation of GSK-3β on Ser9, which in turn suppresses the binding of NF-κB p65 to CBP.14,15) These results suggest that phosphorylation of GSK-3β by activating the PI3K/Akt signaling pathway provides an alternative mechanism by which ANO suppresses pro-inflammatory cytokine secretion.
The relationship between up-regulation of c-Fos expression and phosphorylation of GSK-3β, both of which were observed in ANO-treated RAW264.7 cells, remains to be determined. Reportedly, inactivation of the enzymatic activity of GSK-3β by phosphorylation on Ser9 upregulates CREB phosphorylation.16) Furthermore, the phosphorylation of CREB plays a crucial role in Fos transcription.17) It seems likely that ANO increased Fos expression through phosphorylation of GSK-3β followed by CREB phosphorylation in addition to cAMP-mediated up-regulation.
With respect to the differences in the efficacy between ANO and adenosine on the inhibition of LPS-induced TNF-α and IL-6 production, we consider the following two possibilities: differences in their metabolic stability and the intracellular inhibitory signaling pathways.
Adenosine is irreversibly converted to inosine by adenosine deaminase, which is present in serum. We have shown previously that ANO is refractory to deamination by adenosine deaminase, whereas adenosine is rapidly degraded and is not found in the culture medium supplemented with 10% FBS after 6 h of incubation.5) The inhibitory activity of adenosine on both TNF-α and IL-6 secretion by LPS-activated murine peritoneal macrophages was increased by the addition of adenosine deaminase inhibitor EHNA, but was still inferior to that of ANO. These results suggest that the potent anti-inflammatory activity of ANO could not be solely accounted for by its refractoriness to adenosine deaminase.
In LPS-stimulated peritoneal macrophages, ANO-mediated inhibition of TNF-α and IL-6 production was not reversed by H-89, whereas adenosine-mediated inhibition of TNF-α was completely recovered by H-89 (Fig. 6). Thus, the intracellular signaling pathways induced by ANO and adenosine seem to differ.
Regarding the activation of the PI3K/Akt signaling pathway by adenosine or its derivatives, the non-selective adenosine receptor agonist NECA induces human microvascular endothelial cells to produce endothelial nitric oxide synthase (eNOS) via A2B AR by activating the PI3K/Akt signaling pathway.18) Furthermore, Tian et al. reported that a highly selective A2B AR agonist, BAY60-6583, reduced myocardial ischemia/reperfusion injury by modulating macrophage phenotype, switching it from M1 to M2 via the PI3K/Akt pathway.19) We previously showed that ANO-induced inhibition of TNF-α secretion is significantly abrogated by pretreatment with MRS1754, an A2B AR antagonist.5) These results suggest that ANO may inhibit pro-inflammatory cytokine production by activating the PI3K/Akt signaling pathway via A2B AR. Further studies are necessary to test this possibility. Since it has been shown that stimulation of G protein-coupled receptors by their specific agonists leads to the activation of the PI3K/Akt signaling pathway,20) it is possible that adenosine derivatives, including ANO, activate the PI3K/Akt signaling pathway through G-protein coupled adenosine receptors.
It would be interesting to examine whether adenosine activates the PI3K/Akt/GSK-3β signaling pathway via A2B receptor. It is shown that the adenosine/A2B receptor/cAMP/PKA axis blocks multiple signaling pathways including ERK1/2 and Akt.21) In fact, our preliminary study showed that addition of adenosine resulted in down-regulation of Akt phosphorylation in LPS-stimulated RAW264.7 cells (data not shown). Furthermore, treatment with H-89 resulted in the promotion of ANO-induced suppression of both TNF-α and IL-6 release by LPS-stimulated peritoneal macrophages (Fig. 6). These results suggest that PKA acts as a blocker of the PI3K/Akt signaling pathways in the ANO-treated macrophages. Thus, the PI3K/Akt/GSK-3β signaling pathway may not be involved in the anti-inflammatory effects exerted by adenosine, although further studies are necessary to address this issue.
MSCs are capable of differentiating into several lineages, including bone, cartilage and fat. The PI3K/Akt signaling pathway plays important regulatory roles in the growth, metabolism, angiogenesis, and differentiation of MSCs.11,22) In osteogenic differentiation, the involvement of the PI3K/Akt signaling pathway has been demonstrated.12,13) In this study, we showed that ANO effectively promoted osteogenic differentiation of MC3T3-E1 cells as determined by ALP activities and calcium deposition, but was attenuated by the addition of Wortmannin, suggesting the involvement of the PI3K/Akt signaling pathway in the ANO-induced osteogenic differentiation. Rao et al. reported that adenosine promotes osteogenic differentiation of human embryonic stem cells on mineralized materials through A2B AR.23) These findings suggest a possibility that ANO may promote osteogenic differentiation by activating the PI3K/Akt signaling pathway through A2B AR.
Furthermore, we showed that ANO induced the differentiation of 3T3-L1 into adipocytes when cultured with a combination of dexamethasone and insulin. The important role of PKB/Akt in the adipocyte differentiation has been previously established.24) The combined targeted disruption of PKBα/Akt1 and PKBβ/Akt2 in mice resulted in impaired adipogenesis. Furthermore, knockdown of PKBα/Akt1 in 3T3-L1 cells blocked their ability to differentiate.25) From these results together with our findings that ANO stimulated phosphorylation of Akt, it is possible that ANO promotes adipocytic differentiation via the PI3K/Akt signaling pathway, although this issue should be addressed in future studies. Studies to establish promising techniques for adipogenic differentiation is still underway.26) Since ANO is a natural product found in royal jelly, our results further suggest the usefulness of ANO in regenerative medicine from the point of view of safety as well as efficacy.
In summary, we have shown that ANO activates the PI3K/Akt signaling pathway, leading to phosphorylation of GSK-3β. Since phosphorylated GSK-3β acts as an inhibitory regulator for inflammatory responses, we suggest that the above pathway represents an alternative anti-inflammatory mechanism used by ANO in addition to up-regulation of the anti-inflammatory transcription factor, c-Fos. GSK-3β has been implicated in the pathogenesis of diverse diseases including neurodegenerative diseases, heart failure, diabetes mellitus and inflammation.27–30) Recently, it has been shown that GSK3 may be a therapeutic target for aging.31) Our results suggest that ANO and its derivatives may be potential lead compounds for the treatment of GSK-3β-related disorders. ANO-induced promotion of osteogenic and adipocyte differentiation further indicates its application for regenerative medicine.
The authors are employees of Hayashibara Co., Ltd. and declare no conflict of interest.