Abstract
Staphylococcus aureus produces a variety of exoproteins that interfere with host immune systems. We attempted to purify cytotoxins against human leukocytic cells from the culture supernatant of S. aureus by a combination of ammonium sulfate precipitation, ion-exchange chromatography on a CM-cellulose column and HPLC on a Mono S 5/50 column. A major protein possessing cytotoxicity to HL60 human promyelocytic leukemia cells was purified, and the protein was identified as α-hemolysin (Hla, α-toxin) based on its molecular weight (34 kDa) and N-terminal amino acid sequence. Flow cytometric analysis suggested differential cytotoxicity of Hla against different human peripheral blood leukocyte populations. After cell fractionation with density-gradient centrifugation, we found that peripheral blood mononuclear cells (PBMCs) were more susceptible to Hla than polymorphonuclear leukocytes. Moreover, cell surface marker analysis suggested that Hla exhibited slightly higher cytotoxicity against CD14-positive PBMCs (mainly monocytes) than CD3- or CD19-positive cells (T or B lymphocytes). From these results, we conclude that human leukocytes have different susceptibility to Hla depending on their cell lineages, and thereby the toxin may modulate the host immune response.
INTRODUCTION
The Gram-positive bacterium Staphylococcus aureus is a common human pathogen that causes infectious diseases in various tissues, with the potential for severe morbidity and mortality, especially in compromised patients. The emergence of multidrug-resistant strains of S. aureus, including methicillin-resistant S. aureus (MRSA), has become a serious threat to global public health. It is known that S. aureus produces numerous virulence factors that play crucial roles in the pathogenesis of the infection. These factors include hemolysins, leukocidins, enterotoxins, and toxic shock syndrome toxin-1.1–4) Several studies including ours have shown that S. aureus also secretes various exoproteins that cause perturbations of the host immune system, such as complement inhibitors, immunoglobulin-binding proteins, chemotaxis inhibitory proteins, and superantigen-like proteins (SSL).5–8) The SSL family of exoproteins have been reported to influence a wide range of host immune responses (e.g., Toll-like receptor antagonist, immunoglobulin receptor antagonist, and leukocyte trafficking inhibitors).9–12) Thus, exoproteins from S. aureus are thought to interact with host immune cells and thereby facilitate the development of infections. One of the most direct actions disturbing the host immunity seems to be the secretion of cytotoxins causing serious damage to immune cells.
In this study, we attempted to purify cytotoxins from a culture supernatant of S. aureus using HL60 human promyelocytic leukemia cells as a target, and a major cytotoxic protein with a molecular weight of ca. 34 kDa was isolated. Based on its N-terminal amino acid sequence analysis, the cytotoxin was identified as α-hemolysin (Hla; also known as α-toxin). We then examined the cytotoxicity of Hla against human peripheral blood leukocytes, and found that each leukocyte population exhibited differential susceptibility to Hla. We further analyzed the selective cytotoxicity of Hla by cell fractionation and flow cytometry.
MATERIALS AND METHODS
Reagents and AntibodiesHeparin (porcine) was purchased from Mochida Pharmaceutical Co., Ltd. (Tokyo, Japan). Dextran 200000, sodium dodecyl sulfate (SDS), acrylamide, N,N′-methylenebis(acrylamide), N,N,N′,N′-tetramethyl ethylenediamine (TEMED), and 2-mercaptoethanol were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Casamino acids, yeast extract, and 7-aminoactinomycin D (7-AAD) were purchased from BD Biosciences (San Jose, CA, U.S.A.). RPMI 1640 medium and fetal bovine serum (FBS) were supplied by Sigma-Aldrich (St. Louis, MO, U.S.A.) and Biosera (Boussens, France), respectively. Lymphocyte Separation Solutions were purchased from Nacalai Tesque (Kyoto, Japan). CM-cellulofine C-200 m (CM-cellulose) was a product of Seikagaku Corp. (Tokyo, Japan). Mono S 5/50 GL and Superose 12 10/300 GL columns were purchased from GE Healthcare (Piscataway, NJ, U.S.A). A mixture of molecular weight markers (thyroglobulin, γ-globulin, ovalbumin, myoglobin, and vitamin B12) (gel filtration standard) was purchased from Bio-Rad (Hercules, CA, U.S.A.). Coomassie brilliant blue (CBB) R250 was purchased from Merck (Darmstadt, Germany).
Anti-α-hemolysin antibody and horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) antibody were purchased from Sigma-Aldrich and Kirkegaard & Perry Laboratories (Gaithersburg, MD, U.S.A), respectively. Phycoerythrin (PE)-labeled anti-CD3 antibody, Alexa Fluor 488-labeled anti-CD14 antibody and PE-Cy7-labeled anti-CD19 antibody were purchased from BD Biosciences.
CellsHL60 (a human promyelocytic leukemia cell line), THP-1 (a human monocytic leukemia cell line), U937 (a human monoblastic leukemia cell line), NALL-1 (a human acute lymphoblastic leukemia cell line) and K562 (a human chronic myelogenous leukemia cell line) were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) at 37°C under humidified 5% CO2.
Human peripheral blood leukocytes were prepared essentially as described previously.13) Briefly, human blood collected from healthy volunteers using heparin as an anticoagulant was mixed with an equal volume of 3% dextran 200000/saline. After the mixture was left to stand for 30 min to sediment most of the erythrocytes, the supernatant was centrifuged at 900 rpm for 5 min. Leukocytes were purified from the pelleted cells by hypotonic lysis of the remaining erythrocytes. The preparation of mononuclear leukocytes (lymphocytes and monocytes) and polymorphonuclear leukocytes (granulocytes) was conducted by density gradient centrifugation using Lymphocyte Separation Solutions (d = 1.077 and 1.119) according to the manufacturer’s instructions. These experimental protocols were approved by the Hoshi University Institutional Review Board (#29-002).
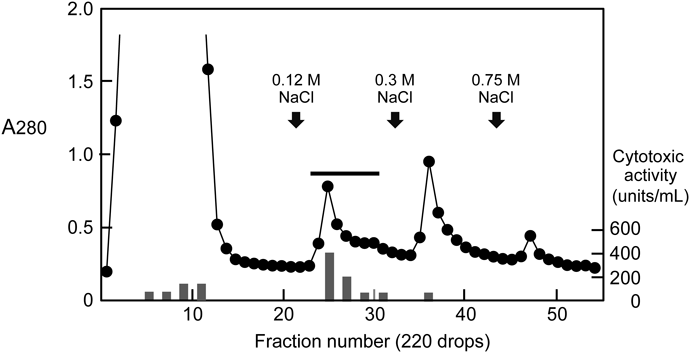
Purification of Cytotoxins from the Bacterial Culture SupernatantThe S. aureus strain (ATCC 27733) was grown in CCY medium (30.0 g/L yeast extract, 20.0 g/L casamino acids, 23.2 g/L sodium pyruvate, 6.25 g/L Na2HPO4·12H2O, 0.41 g/L KH2PO4, 20.0 mg/L MgSO4·7H2O, 7.5 mg/L MnSO4·H2O, 6.4 mg/L FeSO4·7H2O, 6.4 mg/L citric acid) under aerobic conditions at 37°C for 16 h.14) The culture supernatant was centrifuged at 5000 rpm for 20 min, then fractionated by ammonium sulfate precipitation (70% saturation) and centrifuged at 8000 rpm for 20 min. The precipitate was dissolved in a small volume of 20 mM sodium phosphate buffer (pH 5.8) and dialyzed against the same buffer. After the centrifugation to remove insoluble materials, the supernatant was applied to a column (1.5 × 12 cm) of CM-cellulose (CM-cellulofine C-200 m) that had been equilibrated with 20 mM sodium phosphate buffer (pH 5.8). The column was washed with the same buffer, and the proteins bound to the column were eluted successively with the same buffer containing 0.12, 0.3 and 0.75 M NaCl. The eluate with 0.12 M NaCl was pooled and subjected to HPLC on a Mono S 5/50 GL column (GE Healthcare). The elution was conducted at a flow rate of 0.5 mL/min with a concentration gradient of NaCl (0–0.25 M) in 20 mM sodium phosphate buffer (pH 5.8).
Cytotoxic AssayCytotoxicity was measured by the water-soluble tetrazolium method (WST-8; Seikagaku Corp.). Briefly, a serial dilution of cytotoxins was prepared in a 96-well culture plate, and a cell suspension (1 × 106 cells/mL, 0.05 mL) in RPMI 1640 medium/10% FBS was added to each well. After the plate was incubated at 37°C for 5–9 h, WST-reagent (10 µL) was added to the well, and the plate was further incubated at 37°C for 90 min. The number of surviving cells was estimated by measuring A450 (reference: A620) with a microplate reader (Model MTP-450; Corona Electric, Ibaraki, Japan).
ImmunoblottingSDS-polyacrylamide gel electrophoresis and immunoblotting were conducted as described previously.15) A specimen purified from S. aureus culture supernatant was electrophoresed on 12.5% polyacrylamide gel in the presence of SDS under reducing conditions and immunoblotted using anti-Hla antibody and HRP-conjugated anti-rabbit IgG antibody. Chemiluminescence was detected with an imaging analyzer (ImageQuant LAS 500; GE Healthcare).
Amino Acid Sequence AnalysisN-terminal amino acid sequence determination of purified proteins based on the Edman degradation method was conducted using an Applied Biosystems model 492 Procise™ cLC Protein Sequencer (Thermo Fisher Scientific, Waltham, MA, U.S.A.).
Flow CytometryFlow cytometric analysis was performed with a FACSAria™ III cell sorter (BD Biosciences) essentially as described previously.16) The profile of human leukocyte populations (granulocytes, lymphocytes and monocytes) was analyzed by the forward scatter versus side scatter (FSC vs. SSC) plots. Cells positive for 7-AAD were considered dead cells. For the analysis of leukocyte cell surface markers, PE-labeled anti-CD3 antibody, Alexa Fluor 488-labeled anti-CD14 antibody and PE-Cy7-labeled anti-CD19 antibody were used. We also analyzed the expression of a disintegrin and metalloproteinase domain-containing protein 10 (ADAM 10) on human peripheral leukocytes by flow cytometry using anti-ADAM 10 monoclonal antibody (Santa Cruz Biotechnology, Dallas, TX, U.S.A.) and PE/Dazzle™ 594-labeled anti-mouse IgG antibody (BioLegend, San Diego, CA, U.S.A.).
RESULTS
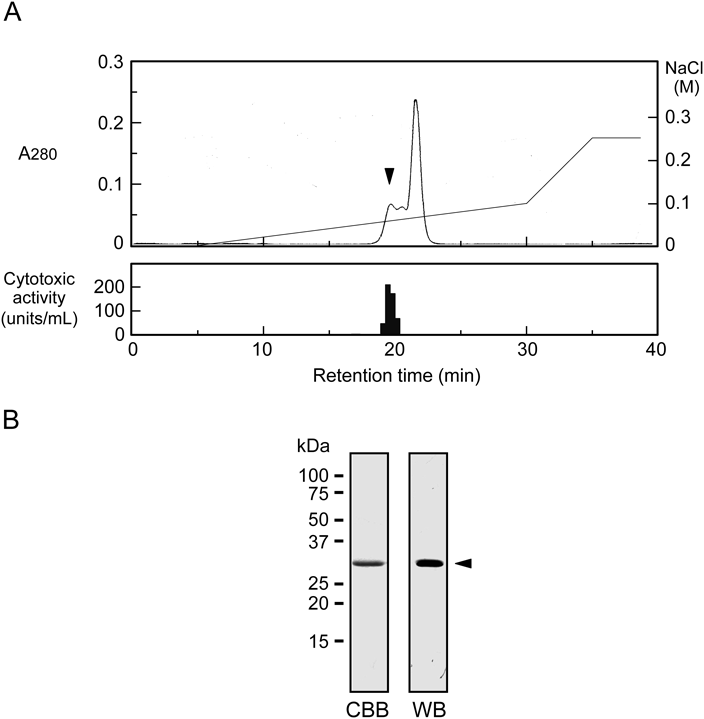
Purification of Cytotoxin from S. aureus Culture SupernatantThe crude sample after ammonium sulfate precipitation was separated by ion-exchange chromatography on a CM-cellulose column. The most active fraction for the cytotoxicity against HL60 human leukemic cells was found to be an eluate with 0.12 M NaCl (Fig. 1). This fraction was estimated to account for approximately 58% of the total cytotoxic activity. We next applied the active fraction to HPLC on a Mono S column eluted with a concentration gradient of NaCl. As shown in Fig. 2A, we detected three consecutive protein peaks at an approximate elution time of 20 min. The cytotoxicity assay revealed that the first peak eluted from the column exhibited strong activity but that no significant activity was associated with the other two protein peaks. After the active fraction was rechromatographed under the same conditions, the proteins in the corresponding fraction were analyzed by SDS-polyacrylamide gel electrophoresis. When the polyacrylamide gel was stained with CBB, a single band at ca. 34 kDa was observed (Fig. 2B). We then analyzed the N-terminal amino acid sequence of the purified protein by a protein sequencer, and the sequence of the first 17 amino acid residues was deduced to be ADSDINIKTGTTDIGSN. The NCBI-BLAST search indicated that this sequence was identical with that of S. aureus α-hemolysin (Hla, α-toxin) (Sequence ID: WP_084927791.1). When the protein sample was subjected to immunoblotting analysis using anti-Hla antibody, it gave a single band with the same electrophoretic mobility as the band detected by CBB staining (Fig. 2B). To estimate the approximate molecular weight without denaturation, the sample was applied to size exclusion chromatography on a Superose 12 10/300 GL column. Compared with the elution positions of standard proteins, the molecular weight of the purified protein was estimated to be 30–35 kDa (data not shown), suggesting that the protein was composed of a monomer with ca. 34 kDa. Based on these results, we concluded that the cytotoxin purified from the S. aureus culture supernatant was Hla.17,18)
Susceptibility of Human Leukemia Cell Lines to HlaWe then examined several human leukemic cell lines for their susceptibility to Hla. Among the cell lines tested, NALL-1 lymphoblastic leukemia cells were most susceptible (IC50 = 0.008 µg/mL), followed by HL60 promyelocytic leukemia cells (IC50 = 0.025 µg/mL), U937 monoblastic leukemia cells (IC50 = 0.072 µg/mL), and THP-1 monocytic leukemia cells (IC50 = 0.082 µg/mL) (Fig. 3A). By contrast, K562 myelogenous leukemia cells appeared to be resistant (IC50 > 3.0 µg/mL). Next, we examined the time period required for exhibiting cytotoxicity by Hla. As shown in Fig. 3B, incubation of HL60 cells for 6 h or longer in the presence of Hla was required for efficient cytotoxicity, but no substantial cytotoxicity was observed after the incubation for 3 h or shorter periods.
Differential Susceptibility of Human Leukocyte Populations to HlaWe next examined the cytotoxicity of Hla against human peripheral blood leukocytes. The dose response of cytotoxicity to THP-1 cells by Hla showed a typical sigmoid curve (Fig. 4A). By contrast, the cytotoxicity of Hla to human blood leukocytes was rather complicated; i.e., the cytotoxicity was dose-dependently increased up to 0.3 µg/mL of Hla but was almost unchanged at concentrations between 0.3–9 µg/mL (the approximate cytotoxicity was 40%). This result suggests that human leukocytes consist of multiple cell populations with different susceptibility to Hla. To further examine this possibility, we utilized flow cytometry to analyze the cell profile after treatment with various concentrations of Hla. Figure 4B (right panel) shows the profile of human leukocytes without Hla treatment. After the treatment with Hla (0.3 µg/mL), the lymphocyte and monocyte populations had almost completely disappeared (Fig. 4B, middle panel). When the concentration of Hla was increased to 27 µg/mL, a significant population of granulocytes was injured in addition to the lymphocytes and monocytes (Fig. 4B, left panel). The cell viability of each leukocyte population after the treatment with various concentrations of Hla is summarized in Table 1. The viability of monocytes or lymphocytes was markedly decreased after the treatment with Hla at 0.3 µg/mL, and more than 95% of these leukocyte populations were injured by Hla. By contrast, the viability of granulocytes was not substantially influenced by concentrations up to 3.0 µg/mL and was decreased to 45% when the Hla concentration was increased to 27 µg/mL.
Table 1. Viability of Human Leukocytes after the Treatment with Hla
| Hla (µg/mL) | Cell viability (%) |
|---|
| Leukocyte (whole) | Monocyte | Lymphocyte | Granulocyte |
|---|
| 0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 0.3 | 87.7 | 1.8 | 4.4 | 103.5 |
| 3.0 | 86.4 | 2.7 | 3.3 | 102.1 |
| 27.0 | 37.5 | 0.0 | 0.0 | 44.5 |
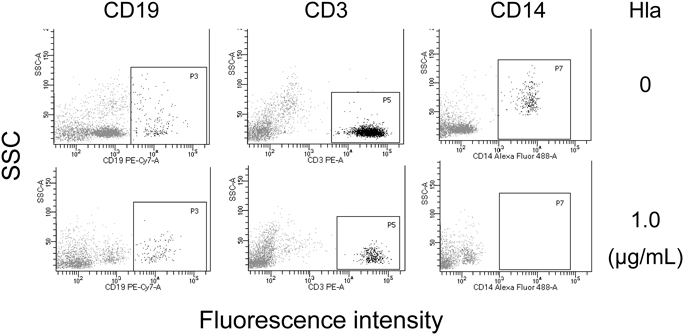
We next isolated mononuclear cells (PBMCs) and polymorphonuclear leukocytes (granulocytes) from human peripheral blood and separately examined their susceptibility to Hla. The flow cytometric analysis indicated that the granulocyte fraction consisted mainly of granulocytes with a small percentage (approximately 3%) of lymphocytes, and that the PBMC fraction was composed mainly of lymphocytes and monocytes (Fig. 5A). The results of the cytotoxicity assay shown in Fig. 5B clearly indicated that these two cell populations were quite different in their susceptibility to Hla; i.e., PBMCs were susceptible (IC50 = 0.19 µg/mL) but granulocytes were almost resistant at 9 µg/mL or lower concentrations. We next confirmed the susceptibility of lymphocytes and monocytes to Hla by analyzing their cell surface markers. The viable leukocytes were analyzed by flow cytometry after the treatment with Hla for CD19 (a marker for B lymphocytes), CD3 (a marker for T lymphocytes), and CD14 (a marker for monocytes). The cell profiles with or without Hla treatment (0 or 1.0 µg/mL) are shown in Fig. 6. We observed that all three populations (CD19-, CD3-, and CD14-positive cells) were considerably decreased after the Hla treatment. The results summarized in Table 2 indicated that viable CD3- or CD19-positive lymphocytes and CD14-positive monocytes were decreased after the Hla treatment in a dose-dependent manner. In addition, CD14-positive monocytes appeared to be more susceptible to Hla as compared to CD3- or CD19-positive lymphocytes; the percentages of viable cells (Hla: 0.3 µg/mL) were 7.3% (monocytes) and 73.9% (lymphocytes).
Table 2. Surface Marker Analysis of Viable Cells after the Treatment with Hla
| Hla (µg/mL) | CD3 (+) or CD19 (+) | CD14 (+) |
|---|
| Cell number | % | Cell number | % |
|---|
| 0 | 2086 | 100.0 | 259 | 100.0 |
| 0.3 | 1542 | 73.9 | 19 | 7.3 |
| 1.0 | 448 | 21.5 | 0 | 0.0 |
| 3.0 | 358 | 17.2 | 0 | 0.0 |
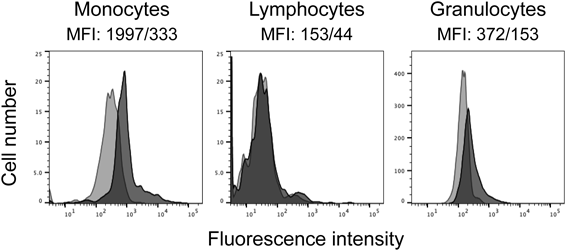
Previous studies have demonstrated that a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) serves as a high affinity receptor for Hla and that its surface expression and the binding of Hla to the cells were well correlated.19–21) We therefore analyzed ADAM10 expression of the leukocyte populations by flow cytometry. The results shown in Fig. 7 indicated that all populations (monocytes, lymphocytes, and granulocytes) expressed ADAM10 but that monocytes exhibited slightly higher expression as compared to lymphocytes and granulocytes. The higher ADAM10 expression on monocytes seems to parallel their higher susceptibility to Hla, but the ADAM 10 expression on lymphocytes was slightly lower than that on granulocytes.
DISCUSSION
In the present study, we purified a major cytotoxin against HL60 leukocytic cells from an S. aureus culture supernatant and identified the cytotoxin as Hla, based on its molecular weight (ca. 34 kDa) (SDS-polyacrylamide gel electrophoresis and size exclusion chromatography), N-terminal amino acid sequence, and immunoblotting using anti-Hla antibody. The purified protein had a strong hemolytic activity against rabbit erythrocytes but not against human erythrocytes, which was consistent with the previous studies.1,17) Hla is known as a pore-forming exotoxin that assembles cylindrical oligomers on target cell membranes, causing cytolysis of various mammalian cells, including platelets, leukocytes, respiratory epithelial cells, keratinocytes, and endothelial cells.18,22,23) The present study demonstrated selective cytotoxicity toward human leukocyte populations; i.e., monocytes and lymphocytes were much more susceptible to Hla than granulocytes. The importance of Hla in the leukocidal activity of S. aureus was suggested by a previous study showing correlation between the levels of Hla production and cytotoxicity against human PBMCs among community-associated S. aureus strains.24) Nygaard et al. also reported that the culture supernatant of a Hla-deletion mutant of a community-associated MRSA strain caused a much lower level of cell membrane damage to CD3-positive lymphocytes than the culture supernatant of the wild-type strain, suggesting that Hla plays a critical role in the membrane damage of T lymphocytes caused by S. aureus infection.25)
In addition to leukocidal activity, Hla was reported to modulate various types of immune responses—e.g., induction of neutrophil adhesion to endothelial cells,26) production of pro-inflammatory cytokines by macrophages and monocytes,23) interleukin (IL)-1β- and IL-18-mediated inflammasome activation in microglia,27) and upregulation of Toll-like receptor-2 expression on monocytes.28) Thus, Hla may cause serious immune perturbation in association with S. aureus infection.
The cause of the selective susceptibility of leukocyte populations to Hla is not clear at present, but the higher susceptibility of monocytes to Hla may result from higher expression of ADAM 10 on their cell surfaces. The observation that CD14-positive monocytes expressed ADAM 10 at a moderate level is in good agreement with a previous study.29) By contrast, the marked difference in the susceptibility of lymphocytes and granulocytes cannot be explained by ADAM 10 expression levels on these leukocyte populations, because both cell types expressed comparable levels of ADAM 10. It was also reported that phosphocholine groups of sphingomyelin clustered in the cell membranes were crucial for stable attachment of the toxin to target cells.30) In addition to the expression levels of these receptors for Hla on the cell surface, differences in oligomerization and conformational changes of Hla in cell membranes and/or intracellular processes including signaling pathways leading to cell death may be involved in the differential cytotoxicity by Hla. Characterization of the molecular mechanism underlying the toxin activity would thus appear to be important, since it would provide valuable information on the prevention of Hla-dependent cytotoxicity in infectious diseases by S. aureus.
It has also been established that so-called two-component toxins such as γ-hemolysin/PV-leukocidin possess potent leukotoxic activity.1,31) Because these toxins are secreted from S. aureus as two non-associated subunits, we did not detect their cytotoxicity during the purification process in this study. Diep et al. reported that PV-leukocidin showed strong cytotoxicity against human polymorphonuclear leukocytes (IC50 values between 0.1 and 1 nM),32) suggesting that Hla and PV-leukocidin have different target specificities for cytotoxicity. Therefore, it appears that Hla and leukocidins cooperatively injure leukocytes to suppress the host immune responses. They also demonstrated in animal models that a monoclonal antibody cross-reacting with Hla and PV-leukocidin effectively protected the animals from pneumonia induced by a community-associated MRSA.32) However, the contribution of these cytotoxins to the pathogenesis by MRSA infection may depend on the strain of S. aureus and the animal model. In a mouse model of S. aureus pneumonia, the expression levels of Hla in S. aureus strains were shown to directly correlate with their virulence, and the vaccination of mice with Hla afforded protection against staphylococcal pneumonia.33) We are hopeful that Hla vaccination or immunotherapy may also prevent S. aureus pneumonia in humans.
Acknowledgments
We would like to thank Mr. Yoshiki Otomura, Mr. Ikumi Mizumura, Ms. Manami Sato, Ms. Aiko Saito, Ms. Haruka Sato, Ms. Juri Takei, Mr. Kazuma Yoshimura, and Mr. Shunsuke Nagato (Hoshi University School of Pharmacy and Pharmaceutical Sciences) for their technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by Research Grants from the Shiono Wellness Foundation and the Japan Agency for Medical Research and Development (AMED).
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev., 13, 16–34 (2000).
- 2) Yoong P, Torres VJ. The effects of Staphylococcus aureus leukotoxins on the host: cell lysis and beyond. Curr. Opin. Microbiol., 16, 63–69 (2013).
- 3) Otto M. Staphylococcus aureus toxins. Curr. Opin. Microbiol., 17, 32–37 (2014).
- 4) Seilie ES, Bubeck Wardenburg J. Staphylococcus aureus pore-forming toxins: The interface of pathogen and host complexity. Semin. Cell Dev. Biol., 72, 101–116 (2017).
- 5) Foster TJ. Immune evasion by staphylococci. Nat. Rev. Microbiol., 3, 948–958 (2005).
- 6) Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, van Wamel WJ, van Kessel KP, van Strijp JA. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat. Immunol., 6, 920–927 (2005).
- 7) Postma B, Poppelier MJ, van Galen JC, Prossnitz ER, van Strijp JA, de Haas CJ, van Kessel KP. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J. Immunol., 172, 6994–7001 (2004).
- 8) Itoh S, Hamada E, Kamoshida G, Yokoyama R, Takii T, Onozaki K, Tsuji T. Staphylococcal superantigen-like protein 10 (SSL10) binds to human immunoglobulin G (IgG) and inhibits complement activation via the classical pathway. Mol. Immunol., 47, 932–938 (2010).
- 9) Yokoyama R, Itoh S, Kamoshida G, Takii T, Fujii S, Tsuji T, Onozaki K. Staphylococcal superantigen-like protein 3 binds to the Toll-like receptor 2 extracellular domain and inhibits cytokine production induced by Staphylococcus aureus, cell wall component, or lipopeptides in murine macrophages. Infect. Immun., 80, 2816–2825 (2012).
- 10) Bardoel BW, Vos R, Bouman T, Aerts PC, Bestebroer J, Huizinga EG, Brondijk TH, van Strijp JA, de Haas CJ. Evasion of Toll-like receptor 2 activation by staphylococcal superantigen-like protein 3. J. Mol. Med. (Berl), 90, 1109–1120 (2012).
- 11) Bestebroer J, Poppelier MJ, Ulfman LH, Lenting PJ, Denis CV, van Kessel KP, van Strijp JA, de Haas CJ. Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood, 109, 2936–2943 (2007).
- 12) Itoh S, Hamada E, Kamoshida G, Takeshita K, Oku T, Tsuji T. Staphylococcal superantigen-like protein 5 inhibits matrix metalloproteinase 9 from human neutrophils. Infect. Immun., 78, 3298–3305 (2010).
- 13) Itoh S, Susuki C, Takeshita K, Nagata K, Tsuji T. Redistribution of P-selectin glycoprotein ligand-1 (PSGL-1) in chemokine-treated neutrophils: a role of lipid microdomains. J. Leukoc. Biol., 81, 1414–1421 (2007).
- 14) Finck-Barbançon V, Prévost G, Piémont Y. Improved purification of leukocidin from Staphylococcus aureus and toxin distribution among hospital strains. Res. Microbiol., 142, 75–85 (1991).
- 15) Oku T, Itoh S, Ishii R, Suzuki K, Nauseef WM, Toyoshima S, Tsuji T. Homotypic dimerization of the actin-binding protein p57/coronin-1 mediated by a leucine zipper motif in the C-terminal region. Biochem. J., 387, 325–331 (2005).
- 16) Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, Gonzalez JB, Pascual V, Stichweh D, Wardemann H, Nussenzweig MC. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc. Natl. Acad. Sci. U.S.A., 105, 9727–9732 (2008).
- 17) Hildebrand A, Pohl M, Bhakdi S. Staphylococcus aureus α-toxin. Dual mechanism of binding to target cells. J. Biol. Chem., 266, 17195–17200 (1991).
- 18) Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev., 55, 733–751 (1991).
- 19) Wilke GA, Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus α-hemolysin-mediated cellular injury. Proc. Natl. Acad. Sci. U.S.A., 107, 13473–13478 (2010).
- 20) Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, Bubeck Wardenburg J. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat. Med., 17, 1310–1314 (2011).
- 21) Ebsen H, Schröder A, Kabelitz D, Janssen O. Differential Surface expression of ADAM10 and ADAM17 on human T lymphocytes and tumor cells. PLOS ONE, 8, e76853 (2013).
- 22) Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science, 274, 1859–1866 (1996).
- 23) Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins (Basel), 5, 1140–1166 (2013).
- 24) Mairpady Shambat S, Haggar A, Vandenesch F, Lina G, van Wamel WJ, Arakere G, Svensson M, Norrby-Teglund A. Levels of alpha-toxin correlate with distinct phenotypic response profiles of blood mononuclear cells and with agr background of community-associated Staphylococcus aureus isolates. PLOS ONE, 9, e106107 (2014).
- 25) Nygaard TK, Pallister KB, DuMont AL, DeWald M, Watkins RL, Pallister EQ, Malone C, Griffith S, Horswill AR, Torres VJ, Voyich JM. Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLOS ONE, 7, e36532 (2012).
- 26) Krüll M, Dold C, Hippenstiel S, Rosseau S, Lohmeyer J, Suttorp N. Escherichia coli hemolysin and Staphylococcus aureus alpha-toxin potently induce neutrophil adhesion to cultured human endothelial cells. J. Immunol., 157, 4133–4140 (1996).
- 27) Hanamsagar R, Torres V, Kielian T. Inflammasome activation and IL-1β/IL-18 processing are influenced by distinct pathways in microglia. J. Neurochem., 119, 736–748 (2011).
- 28) Niebuhr M, Schorling K, Heratizadeh A, Werfel T. Staphylococcal α-toxin induces a functional upregulation of TLR-2 on human peripheral blood monocytes. Exp. Dermatol., 24, 381–383 (2015).
- 29) Nygaard TK, Pallister KB, Zurek OW, Voyich JM. The impact of α-toxin on host cell plasma membrane permeability and cytokine expression during human blood infection by CA-MRSA USA300. J. Leukoc. Biol., 94, 971–979 (2013).
- 30) Valeva A, Hellmann N, Walev I, Strand D, Plate M, Boukhallouk F, Brack A, Hanada K, Decker H, Bhakdi S. Evidence that clustered phosphocholine head groups serve as sites for binding and assembly of an oligomeric protein pore. J. Biol. Chem., 281, 26014–26021 (2006).
- 31) Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol., 16, 361–369 (2008).
- 32) Diep BA, Le VT, Visram ZC, Rouha H, Stulik L, Dip EC, Nagy G, Nagy E. Improved protection in a rabbit model of community-associated methicillin-resistant Staphylococcus aureus necrotizing pneumonia upon neutralization of leukocidins in addition to alpha-hemolysin. Antimicrob. Agents Chemother., 60, 6333–6340 (2016).
- 33) Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med., 205, 287–294 (2008).