2019 Volume 42 Issue 7 Pages 1120-1127
2019 Volume 42 Issue 7 Pages 1120-1127
Hydroxytyrosol (HT) is a simple phenol compound present in olive oil. In a previous in vitro study, we showed that HT downregulated lipopolysaccharide-mediated expression of inducible nitric oxide synthase, cyclooxygenase-2 (COX-2), tumor necrosis factor alpha, and interleukin-1β, resulting in reduced nitric oxide and prostaglandin E2 production. In the present study, we aimed to determine whether HT suppresses COX-2-induced inflammation in a carrageenan-induced rat paw edema model. Additionally, we compared its activity with those of the selective COX-2 inhibitor, celecoxib for a comparative control, and a representative nonsteroidal anti-inflammatory drug (NSAID), indomethacin for a positive control. HT, celecoxib, and indomethacin significantly suppressed swelling in carrageenan-injected rat paws. Although HT was less effective than celecoxib and indomethacin, it had a delayed onset of action. Moreover, we evaluated whether HT aggravates gastric damage, which is a typical adverse effect associated with NSAIDs and COX-2 inhibitors under low dose aspirin (LDA) treatment, in an aspirin-induced gastric damage rat model. Unlike celecoxib and indomethacin, HT did not cause gastric damage when co-administered with aspirin. Our results indicate that HT exerts a delayed but sustained anti-inflammatory effect against COX-2-mediated inflammation. Finally, the combination of short-acting conventional anti-inflammatory drugs and long-acting HT can be considered a new, safe, and effective anti-inflammatory treatment modality even when continuously administered for a long period under LDA treatment.
Inflammation results from the complex biological responses of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants. These protective responses involve immune cells, blood vessels, and molecular mediators, and facilitates recovery from infection. However, if targeted destruction and assisted repair are not properly phased, inflammation can lead to persistent tissue damage by leukocytes, lymphocytes, or collagen.1) In addition, excessive inflammation causes chronic inflammation.2) Thus, it is important to control inflammation and associated pain and hyperthermia.
Hydroxytyrosol (HT), a small phenolic molecule found in olive oil, possesses strong anti-oxidant activity and acts as an anti-inflammatory, anti-thrombotic, anti-tumor, and anti-microbial agent.3) It has been demonstrated safe in rodents.4) It is widely used as food and in nutritional supplements.5) In a previous in vitro study, we found that HT inhibits the lipopolysaccharide-mediated stimulation of inducible nitric oxide synthase,6) cyclooxygenase-2 (COX-2), and interleukin-1β expression, resulting in reduced nitric oxide and prostaglandin (PG) E2 production.7) Therefore, we aimed to evaluate the in vivo anti-inflammatory activity of HT.
The carrageenan-induced rat paw edema model is typically used as an in vivo model for evaluating the anti-inflammatory effect of drugs.8,9) Carrageenan activates COX-2, which produces PGE2 using arachidonic acid as a reaction substrate. PGE2 causes acute exudation, resulting in swelling.10,11) In this study, we used the carrageenan-induced rat paw edema model to evaluate the anti-inflammatory activity of HT because HT has the potential of inhibiting COX-2-related inflammation. Additionally, we compared its efficacy with those of indomethacin, a representative nonsteroidal anti-inflammatory drug (NSAID),12) and celecoxib,13) a selective COX-2 inhibitor.
In elderly individuals, low-dose aspirin (LDA) is an effective and commonly-prescribed treatment for preventing thrombosis, and the number of prescriptions has been increasing in recent years.14) These aged patients often develop orthopedic diseases, such as joint pain. Therefore, anti-inflammatory drugs and LDA are frequently used together.15) NSAIDs and selective COX-2 inhibitors are used for the treatment of joint damage, pain, and inflammation.16) COX-1 and COX-2 are enzymes responsible for producing PGs.17) Inhibiting these enzymes by NSAIDs and selective COX-2 inhibitors reduces levels of PGs, resulting in reduced pain and inflammation.16) However, in some circumstances, COX-2 is upregulated in the stomach, wherein it generates PGs that protect the gastric mucosa.18) COX-2 appears to play an important role in healing gastric ulcers in rats and mice.19,20) Its expression is upregulated in the stomach during ischemia and the administration of selective NSAIDs or selective COX-2 inhibitors exacerbates the gastric damage that occurs during reperfusion.21) Moreover, both NSAIDs and LDA are risk factors for gastrointestinal disorders.22) Considering these risks, we aimed to evaluate the safety of HT in a rat model of gastric damage with LDA co-administration.21–24)
HT (> 98% purity) and celecoxib were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Indomethacin, carrageenan, aspirin, and sterilized 0.5% (w/v) methylcellulose (MC) solution were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Distilled water, which was used as a vehicle, was obtained from Otsuka Pharmaceutical Factory, Inc. (Tokyo, Japan). HT (50 mg/mL) and aspirin (5 mg/mL) solutions were prepared by dissolving in distilled water. Celecoxib (10 mg/mL) and indomethacin (3 mg/mL) suspensions were prepared in 0.5% (w/v) MC. Carrageenan solution (1.0% (w/v)) was prepared in distilled water.
AnimalsFive-week-old male Sprague–Dawley rats [Crl:CD (SD)] were purchased from Charles River Laboratories Japan, Inc. (Yokohama, Japan). During the acclimation period, clinical observations (once daily), and body weight measurements (twice weekly) were conducted. The animals were housed at a temperature of 20–26°C, humidity of 30–70%, air ventilation at approximately 17 air changes per hour, and a 12-h photoperiod (07:00 to 19:00).
All animal experiments were conducted according to the animal care guidelines of Kaken Pharmaceutical Co., Ltd. (Shizuoka, Japan).
Effects of HT, Celecoxib, and Indomethacin on Carrageenan-Induced Rat Paw EdemaTwenty SD rats were divided equally into four groups (n = 5/group) and treated with 0.5% (w/v) MC (control), 500 mg/kg HT, 100 mg/kg celecoxib, or 30 mg/kg indomethacin. All treatments were administered orally at a dosing volume of 10 mL/kg.
With regard to the rationale for selection of the dose levels, a previous study reported that no abnormal findings were obsereved in rats at 2 g/kg HT.4) Therefore, 500 mg/kg HT was set as the dose at which the anti-inflammatory activity can be expected without causing any toxic effect. In this study, we also intended to evaluate the effects of HT, celecoxib, and indomethacin on aspirin-induced gastric damage. For a comparative control, 100 mg/kg celecoxib was set as the dose at which the anti-inflammatory activity can be expected without gastric damage when no aspirin is administered.25) For a positive control, 30 mg/kg indomethacin was set as the dose at which both of the anti-inflammatory activity and gastric damage can be expected.23) According to a previous study,10) the carrageenan injection and paw volume measurement were performed as follows: one hour after the administration of test compounds, carrageenan inflammation was induced by subcutaneous administration of 0.1 mL of carrageenan into the footpad of the right hind paw; foot volumes were measured up to a mark on the lateral malleolus using a plethysmometer (Model 7141, Ugo Basile, Milan, Italy) before and 1, 2, 3, 4, and 5 h after carrageenan administration. The swelling ratio (% swelling) and swelling inhibition ratio (% inhibition) were calculated using the following formulas from a previously published study9):
 |
 |
where V0 and VX are the hind paw volumes before (0 h) and 1, 2, 3, 4, and 5 h after carrageenan injection, respectively, Control and Test are the mean volumes in the control and test groups, respectively.
All animals were sacrificed by exsanguination under isoflurane anesthesia after the final measurement of paw volume. Paws were removed by cutting at the tibiotarsal level and were subsequently processed for histopathologic examination. Paw tissues were fixed in neutral buffered 10% formalin, dehydrated and defatted, embedded in paraffin, and sectioned. Tissue sections were stained with hematoxylin and eosin. Histopathological grading of rat hind paw tissue sections was performed as follows: 0, negative; 1, minimal; 2, slight; 3, moderate.
PGE2 Concentration in the Rat FootpadTwenty-four SD rats were divided equally into four groups (n = 6/group) and orally administered 500 mg/kg HT, 100 mg/kg celecoxib, 30 mg/kg indomethacin, and 0.5% (w/v) MC. One hour after administration, inflammation was induced by subcutaneous administration of 0.1 mL carrageenan into the footpad of rats. Rats (n = 3) not undergoing any intervention were used as negative controls. The rats were euthanized under isoflurane anesthesia 1 and 3 h after carrageenan-induced inflammation (n = 3/point). The footpads were collected and lysed with CelLytic™ MT (Sigma-Aldrich, MO, U.S.A.) containing a protease inhibitor cocktail (Sigma-Aldrich). The PGE2 concentrations of the lysates were determined using a PGE2 enzyme-linked immunosorbent assay kit (Enzo Life Sciences Inc., NY, U.S.A.) and were compared with PGE2 concentrations of the control rats.
Effects of HT, Celecoxib, and Indomethacin on Aspirin-Induced Gastric DamageForty SD rats were divided equally into eight groups (n = 5/group). The rats were fasted for 16 h and orally administered HT (Groups 1 and 5), celecoxib (Groups 2 and 6), indomethacin (Groups 3 and 7), and 0.5% (w/v) MC (Groups 4 and 8) at the same doses used in the carrageenan-induced rat paw edema model study. One hour after administration of the drugs, all animals were orally administered 50 mg/kg aspirin (Groups 1–4) or 0.5% (w/v) MC (Groups 5–8). Four hours after aspirin or 0.5% (w/v) MC administration, the rats were sacrificed by exsanguination under isoflurane anesthesia for assessing gastric damage. The lengths of all hemorrhagic lesions were measured with digital calipers, and the “% gastric damage area” was calculated for each rat using the following formula:
 |
At the time of necropsy, blood samples were collected from the abdominal aorta under isoflurane anesthesia, and samples treated with ethylenediaminetetraacetic acid-2K were used for hematology examinations performed using a hematology system (ADVIA120, Bayer Healthcare, NY, U.S.A.).
PGE2 Concentration in the Rat StomachTwenty-four SD rats were divided equally into four groups (n = 6/group) and orally administered 500 mg/kg HT, 100 mg/kg celecoxib, 30 mg/kg indomethacin, and 0.5% (w/v) MC. One hour after administration, all the animals were orally administered 50 mg/kg aspirin. The rats (n = 3) without any intervention were used as negative control. The rats were euthanized under isoflurane anesthesia 1 and 4 h after aspirin administration (n = 3/point). The stomach specimens were collected and PGE2 concentrations were evaluated in the same way as the carrageenan-induced rat paw edema study.
Statistical AnalysisThe results are represented as the mean ± standard error of the mean. The quantitative data were analyzed using Bartlett’s test for homogeneity of variance. Dunnett’s test for multiple comparisons and a Dunnett-type test for multiple comparisons were performed to compare between the control and intact groups and each test group when the variance was homogenous and heterogeneous, respectively. The qualitative data (histopathological grading) were analyzed using a cumulative χ2 test to compare between the vehicle control group and each test group. A two-sided significance level of 5% was set for the statistical analyses.
First, the anti-inflammatory activity of HT in the carrageenan-induced rat paw edema model was assessed. The mean hind paw volumes were increased after the injection of carrageenan solution in all groups. In the HT-treated group, the swelling ratio was significantly less than that in the control group (p < 0.05) at 3, 4, and 5 h after carrageenan administration. In the celecoxib-treated group, the swelling ratio was significantly less than that in the control group (p < 0.05) at 1, 2, and 3 h after carrageenan administration. In the indomethacin-treated group, the swelling ratio was significantly less than that in the control group (p < 0.05) at all time points after carrageenan administration (Fig. 1A).
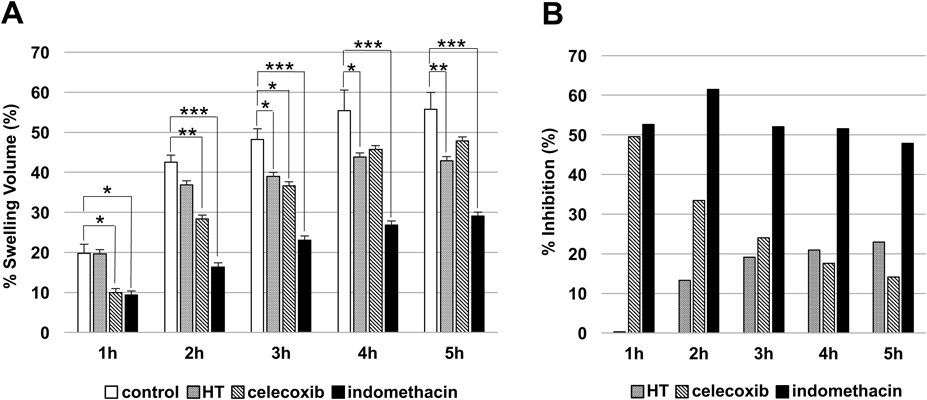
(A) The % swelling volume after carrageenan injection and treatment with hydroxytyrosol (HT), celecoxib, and indomethacin of rat paw edema 1, 2, 3, 4, and 5 h after carrageenan injection. * p < 0.05, ** p < 0.01, *** p < 0.001 as compared to control (Dunnett’s test for multiple comparisons). Each bar represents the mean ± standard error of data from five animals, except for the control group, in which data from only four animals were used. (B) % inhibition by hydroxytyrosol (HT), celecoxib, and indomethacin in the rat paw edema model.
The calculated swelling inhibition ratios at 1, 2, 3, 4, and 5 h after carrageenan administration were respectively 0.34, 13.3, 19.2, 20.9, and 22.9% in the HT-treated group, 49.6, 33.4, 24.0, 17.6, and 14.2% in the celecoxib-treated group, and 52.7, 61.5, 52.1, 51.6, and 47.9% in the indomethacin-treated group (Fig. 1B).
One animal in the control group could not be administered carrageenan properly; therefore, this animal was excluded from the evaluation. However, a sufficient quantity of data was able to be secured for statistical analysis despite this occurrence, and it was concluded that the data deficiency did not affect the reliability of this study.
HT Attenuates Carrageenan-Induced Inflammatory Response in the Hind PawSlight mixed-cell infiltration and moderate edema at the acrotarsium were observed in all rats. However, mixed-cell infiltration was significantly lower in the HT (p < 0.05), celecoxib (p < 0.01), and indomethacin (p < 0.05) groups than in the control group. Additionally, edema was significantly lower in the celecoxib (p < 0.01), and indomethacin (p < 0.01) groups than in the control group (Figs. 2A, B). These results indicate that HT exerts an anti-inflammatory effect by suppressing cellular infiltration.

(A) Histological staining of rat hind paw tissue sections 5 h after carrageenan injection. (a) 0.5% (w/v) methylcellulose (control), (b) hydroxytyrosol (HT), (c) celecoxib, and (d) indomethacin. (a) shows evident dermal edema (*) and subcutaneous mononuclear cell infiltration (↑). They were weaker in (b), (c), and (d) (de: dermis, sc: subcutis). (B) Histopathological grading of rat hind paw tissue sections 5 h after carrageenan injection. * p < 0.05, ** p < 0.01 compared to control (cumulative χ2 test). Each bar represents the mean ± standard error of data from five animals, except for the control group, in which data from only four animals were used.
At 1 h, carrageenan significantly increased the level of PGE2 in the rat footpad compared to that in the intact group. The addition of HT, celecoxib, and indomethacin suppressed this PGE2 production (Fig. 3A). At 3 h, the level of PGE2 in the control group decreased to the same level as that in the control group. Indomethacin reduced the level of PGE2 significantly (Fig. 3B).

PGE2 production (A) 1 h after and (B) 3 h after carrageenan-induced inflammation followed by the administration of 0.5% (w/v) methylcellulose as control, hydroxytyrosol (HT), celecoxib, and indomethacin. The group without carrageenan-induced inflammation served as negative control. * p < 0.05 as compared to the control group (Dunnett’s test for multiple comparisons). # p < 0.05 as compared to the control (Dunnett-type test for multiple comparisons). Each bar represents the mean ± standard error of data from three animals.
Next, the risk of gastric damage when HT was combined with aspirin was evaluated. Under aspirin-free conditions, no gastric damage was found in the control, HT, and celecoxib groups. Only indomethacin caused mild gastric damage (Figs. 4A, 4B). When co-administered, aspirin did not lead to gastric damage in the control group as demonstrated by the absence of any hemorrhagic erosion. Similarly, the combined administration of aspirin and HT did not induce gastric damage. In contrast, the combined administration of aspirin and celecoxib or indomethacin caused detectable gastric damages (Fig. 4C). A significant increase in the % gastric damage area was observed when indomethacin was co-administered with aspirin (Fig. 4D).

(A) Photographs of rat stomachs 4 h after administration of 0.5% (w/v) methylcellulose with (a) 0.5% (w/v) methylcellulose as control, (b) hydroxytyrosol (HT), (c) celecoxib, and (d) indomethacin. (B) The % gastric damage area of rat stomach 4 h after 0.5% (w/v) methylcellulose with 0.5% (w/v) methylcellulose as control, hydroxytyrosol (HT), celecoxib, and indomethacin. Each bar represents the mean ± standard error of data from five animals. (C) Photographs of rat stomachs 4 h after aspirin co-administration (50 mg/kg) with (a) 0.5% (w/v) methylcellulose as control, (b) hydroxytyrosol (HT), (c) celecoxib, and (d) indomethacin. (D) The % gastric damage area of rat stomach 4 h after aspirin co-administration (50 mg/kg) with 0.5% (w/v) methylcellulose as control, hydroxytyrosol (HT), celecoxib, and indomethacin. ## p < 0.01 as compared to the control (Dunnett-type test for multiple comparisons). Each bar represents the mean ± standard error of data from five animals.
In the hematology examination, when co-administered aspirin, the number of RBCs and WBCs increased (not significant) in the celecoxib and indomethacin groups. However, they did not increase in the HT group (Figs. 5A, 5B). The number of reticulocytes increased (not significant) only in the indomethacin group (Fig. 5C). The number of neutrophils significantly increased in the celecoxib and indomethacin group, but not in the HT group (Fig. 5D). There was no change in the hematological parameter under aspirin free condition (data not shown).

After aspirin co-administration (50 mg/kg) with 0.5% (w/v) methylcellulose as control, hydroxytyrosol (HT), celecoxib, and indomethacin, the number of (A) RBCs, (B) WBCs, (C) reticulocytes, and (D) neutrophils in blood samples collected from the abdominal aorta was examined. * p < 0.05 as compared to the control (Dunnett’s test for multiple comparisons). Each bar represents the mean ± standard error of data from five animals.
These results indicate that HT does not cause gastric damage even when co-administered with aspirin.
HT Decreases PGE2 Production in the Rat StomachAt 1 h following administration, aspirin reduced the level of PGE2 in the rat stomach compared to that in the intact group. Additionally, the combined administration of aspirin and celecoxib or indomethacin reduced the level of PGE2 (Fig. 6A). This result was consistent with the degree of gastric damage. On the other hand, combined administration of aspirin and HT significantly reduced PGE2 production whereas HT did not cause gastric damage. At 4 h, the level of PGE2 in the control group recovered to the same level as the intact group. Indomethacin, however, significantly reduced the level of PGE2 (Fig. 6B).

After aspirin co-administration (50 mg/kg) with 0.5% (w/v) methylcellulose as control, hydroxytyrosol (HT), celecoxib, and indomethacin, PGE2 production (A) 1 h after and (B) 4 h after aspirin administration. Intact group (without aspirin) served as the negative control. ** p < 0.01 as compared to the control (Dunnett’s test for multiple comparisons). # p < 0.05, ## p < 0.01 as compared to the control (Dunnett-type test for multiple comparisons). Each bar represents the mean ± standard error of data from three animals.
In the present study, we evaluated the anti-inflammatory activity of HT, and compared its efficacy with that of celecoxib, and indomethacin in a carrageenan-induced rat paw edema model. The results showed that HT significantly suppresses the swelling ratio compared to control, beginning at 3 h after carrageenan injection, with the maximum inhibition of carrageenan-induced rat paw edema (22.9%) at 5 h. In contrast, celecoxib and indomethacin significantly suppressed the swelling ratio from 1 h (49.6 and 52.7%, respectively) after carrageenan injection. Although HT was less effective than celecoxib and indomethacin, its effect increased with time, whereas effects of celecoxib and indomethacin decreased with time. In fact, HT more strongly suppressed the swelling ratio than celecoxib (comparative control) at 4 and 5 h. Thus, HT may have a delayed onset of action, indicating that the combination of HT and celecoxib or indomethacin may provide a sustained anti-inflammatory effect.
Moreover, the extent of mixed-cell infiltration was significantly lower in the HT, celecoxib, and indomethacin groups as compared to that in the control group. The infiltrated cells consisted of lymphocytes, neutrophils, eosinophils, and typical mononuclear cells. Cell infiltration and fluid extravasation at the site of inflammation were caused by the release of histamine, serotonin, and PGs.26) Thus, it was considered that HT exerts anti-inflammatory effects by inhibiting inflammatory mediators, such as histamine, serotonin, and PGs; and reducing the production of PGE2.27) Although HT inhibited mixed cell infiltration, it did not inhibit edema. Edema is caused by capillary dilation in the acute phase of inflammation, but mixed cell infiltration is caused by the migration of inflammatory cells to the site of inflammation in the late phase of inflammation.28) It was considered that HT could not suppress acute phase response (within several tens of minutes) of inflammation. Whereas HT suppressed carrageenan-induced PGE2 production in the rat footpad similar to celecoxib and indomethacin at 1 h after administration, HT exerted the anti-inflammatory effects a few hours later. Further studies are needed to elucidate the differences in the molecular mechanisms of HT and the existing anti-inflammatory drugs.
While evaluating the degree of gastric damage, there was no damage in the control, HT, and celecoxib groups under aspirin-free conditions; while indomethacin caused mild damage. On the other hand, combined administration of aspirin and celecoxib or indomethacin-induced detectable gastric damages. In contrast, the combined administration of aspirin and HT did not induce gastric damage which was consistent with our observations for the control group. A significant increase in the % gastric damage area and neutrophil counts in the blood was observed after administration of celecoxib and indomethacin, but not HT.
LDA is an effective and commonly-prescribed treatment for preventing thrombosis, and the number of prescriptions of LDA in the elderly has increased in recent years.14) Celecoxib and indomethacin are used for the treatment of inflammatory disorders, such as rheumatoid arthritis, which is also a common disease in the elderly. However, aspirin can induce gastrointestinal mucosal damage even at a low dose.23) Further, long-term use of both COX-2 inhibitors and NSAIDs can cause gastrointestinal adverse effects in aged patients treated with LDA.22,29–32) They can induce mucosal damage which is mainly caused due to suppression of PG synthesis via inhibition of COX-2 activity.16,30,33) However, the present study showed that co-administration of HT with aspirin did not cause gastric damage in spite of its PGE2-suppressive activity. This finding suggests that HT prevents gastric mucosal injury even in a low PGE2 condition, which facilitates anti-inflammatory treatment without adverse effects caused by the existing anti-inflammatory drugs.
In conclusion, the results of the present study suggest that HT exerts delayed but sustained anti-inflammatory effects against COX-2-mediated inflammation. Additionally, HT effectively prevents gastric damage even in a low PGE2 condition when co-administered with aspirin. HT is commercially available in foods and nutritional supplements,5) and shows no adverse effects even at high doses (2 g/kg).4) Although further studies are needed to elucidate the molecular mechanisms underlying its anti-inflammatory activities, HT displays the potentials of a long-acting anti-inflammatory drug. Finally, the combination of HT with the existing anti-inflammatory drugs, which means short-acting conventional anti-inflammatory drugs and long-acting HT, may be considered as a novel, safe, and effective anti-inflammatory treatment modality even when continuously administered for a long period with LDA treatment.
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declare no conflict of interest.