2019 Volume 42 Issue 8 Pages 1253-1267
2019 Volume 42 Issue 8 Pages 1253-1267
Systemic platelet behaviors in experimental animals are often assessed by infusion of isotope-labeled platelets and measuring them under anesthesia. However, such procedures alter, therefore may not reveal, real-life platelet behaviors. 5-Hydroxytryptamine (5HT or serotonin) is present within limited cell-types, including platelets. In our studies, by measuring 5HT as a platelet-marker in non-anesthetized mice, we identified stimulation- and time-dependent accumulations in liver, lung, and/or spleen as important systemic platelet behaviors. For example, intravenous, intraperitoneal, or intragingival injection of lipopolysaccharide (LPS, a cell-wall component of Gram-negative bacteria), interleukin (IL)-1, or tumor necrosis factor (TNF)-α induced hepatic platelet accumulation (HPA) and platelet translocation into the sinusoidal and perisinusoidal spaces or hepatocytes themselves. These events occurred “within a few hours” of the injection, caused hypoglycemia, and exhibited protective or causal effects on hepatitis. Intravenous injection of larger doses of LPS into normal mice, or intravenous antigen-challenge to sensitized mice, induced pulmonary platelet accumulation (PPA), as well as HPA. These reactions occurred “within a few min” of the LPS injection or antigen challenge and resulted in shock. Intravenous injection of 5HT or a catecholamine induced a rapid PPA “within 6 s.” Intravenous LPS injection, within a minute, increased the pulmonary catecholamines that mediate the LPS-induced PPA. Macrophage-depletion from liver and spleen induced “day-scale” splenic platelet accumulation, suggesting the spleen is involved in clearing senescent platelets. These findings indicate the usefulness of 5HT as a marker of platelet behaviors, and provide a basis for a discussion of the roles of platelets as both “defenders” and “guardians.”
In the 1970s–1980s, we succeeded in measuring various biogenic amines, including 5-hydroxytryptamine (5HT or serotonin), in animal tissues.1,2) We found that lipopolysaccharide (LPS, a cell-wall component of Gram-negative bacteria) increased histamine and polyamines in various tissues a few hours after its systemic injection,3–5) while 5HT was increased, predominantly in the liver.6,7) Various inflammatory substances, including the cytokine interleukin (IL)-1, also increased hepatic 5HT.6–10) The increases in histamine and polyamines were mediated by the induction of the enzymes responsible for forming them. So, at first, we thought that the hepatic 5HT increase might likewise be due to its enhanced synthesis.11,12) However, it became clear that the hepatic 5HT increase actually reflects an accumulation of platelets.13) This finding led us to become interested in studying platelet behaviors. By measuring 5HT as a marker of platelets and by making electron microscopic observations, we found that platelets accumulate in liver, lung, and/or spleen with various time-courses, depending on the type and degree of stimulation. Here, we review these and other findings as a basis for a discussion of the real-life roles of platelets.
Platelets are anuclear cells produced by the fragmentation or autophagy of megakaryocytes,14–16) making them the smallest discoid cells (diameter 2–5 µm and thickness 0.5 µm: c.f. erythrocytes’ diameter 7.5–8.5 µm and thickness 1.7 µm). In number, platelets (15–40 × 107/mL) are second to erythrocytes (450–500 × 107/mL) in blood. The life-span of platelets in the circulation (about 10 d in humans and 4–5 d in mice) is regulated by mechanisms including apoptosis.17)
2.2. Contents of PlateletsUpon activation, platelets release their contents and stimulate both themselves and other cells. Platelets contain dense-granules, α-granules, lysosomes, mitochondria, tubular inclusions, and glycogen-containing glycosomes.18,19) Dense-granules contain bioactive amines, cations, nucleotides, and phosphates. α-Granules contain a variety of proteins, including growth factors (hepatocyte growth factor, etc.), cytokines (IL-1, etc.), chemokines, complements, etc. Lysosomes contain enzymes that hydrolyze proteins, carbohydrates, and phosphates. The platelet cell membrane expresses a variety of receptors,20) including receptors for the platelet’s own constituents such as 5HT, ADP,19) and IL-1.21,22) Platelets express receptors for exogenous substances, too, such as the Toll-like receptor for LPS (TLR4).23) Despite their lack of nuclei, platelets contain mRNA, microRNA, and components necessary to perform translation. Hence, platelets can produce proteins (including IL-1).24,25) Activated platelets release mitochondria, and fatty acids and lysophospholipids are released from the mitochondrial membrane by phospholipase A2, promoting inflammation.26)
2.3. Platelet MicroparticlesAlthough apoptosis has been attributed to nucleated cells, it also occurs in platelets.27) Platelet apoptosis produces microparticles, in which a variety of constituents are enclosed, including transcription factors and mRNA.25,28,29) Among the microparticles in human blood, platelet-derived microparticles (0.1–1 µm) are the most numerous (approx. 240 × 103/mL),30,31) although they are far fewer in number than platelets themselves. Recent in vitro studies have demonstrated that platelet-derived microparticles can deliver platelet transcripts to other cells (endothelial cells, monocytic cells, and neutrophils),32–36) suggesting a role in regulating protein synthesis in these cells.25) Incidentally, various cells have also been reported to produce particles (called “microvesicles” or “exosomes,” containing mRNAs and microRNAs), and these particles are transferred horizontally to other cells and modify the functions of the target cells.37,38)
2.4. Platelet Shape ChangesPlatelet shape changes occur dramatically even when the concentration of the stimulant is too low to induce platelet aggregation or adhesion,39,40) with the production of spiny spheres with lamellipodia and long, thin filopodia.39) Unlike the roles of aggregation or adhesion, the roles played by platelet shape changes are poorly understood.39)
2.5. Roles of PlateletsIn addition to their roles in hemostasis and thrombosis, platelets are coming to be recognized as active participants in immune or self-defense responses.41,42) Platelets have been implicated in allergy,43) diabetes mellitus,44,45) infection,46) inflammatory diseases,47–49) and cancer.50,51) Platelets also play roles in various physiological reactions, e.g., tissue healing,52,53) vascular integrity,54) and lymphatic flow and vessel maturation.55)
2.6. Platelet TranslocationJohnson et al.56) suggested that “incorporation” of platelets into endothelial cytoplasm might be involved in supporting the endothelium. Lellouch-Tubian et al.57) obtained evidence of extravascular platelet recruitment in the guinea-pig lung within 3 min of an intravenous (i.v.) injection of platelet-activating factor (PAF). Such a rapid pulmonary platelet translocation also occurs in rats after an i.v. injection of PAF or ADP.58) Kirschbaum et al.59) recently found that platelets stimulate hepatocyte proliferation via an “internalization” of platelets into hepatocytes. However, with the exception of the studies reviewed hereafter, there had been no kinetic and/or quantitative analyses of these platelets behaviors. Thus, it was not clear how many platelets might translocate into the lung or liver, or what the time-scale might be.
Our studies have been based on measurements of 5HT as a marker of platelets. Because this methodology may not be familiar to the reader, we will briefly explain it. In contrast to histamine and polyamines, 5HT is a substance that is easily degraded via oxidation. In our method, ethylenediaminetetraacetic acid (EDTA) and cysteine are used to reduce such oxidation, and HClO4 is used to precipitate proteins, viz, pre-weighed test-tubes containing 3 mL of a mixture of 0.4 M HClO4, 2 mM EDTA-2Na, and 0.1% cysteine–HCl are used to collect blood for the measurement of 5HT. The blood is dropped directly into the tube from the neck of a stunned and decapitated mouse, rapidly mixed and weighed. The tubes are quickly transferred into a dry-ice/ethanol bath, then stored in a dry-ice box. In addition, tissues are rapidly removed from the mice, weighed, and stored in the dry ice box. Because the 5HT in blood is unstable, blood 5HT is determined within 48 h, while tissue 5HT is measured within a few days of sampling. 5-HT is isolated by a column-chromatographic method, and then measured by its intrinsic fluorescence,2) allowing simultaneous measurement of many samples. For measuring platelets themselves, several drops of blood from stunned and decapitated mice are collected into a pre-weighed test-tube containing 0.5 mL of 4 mM EDTA in 0.01 M phosphate-buffered saline. The tube is weighed and the number of platelets is counted in a Celltac MEK-4150 cell-counter.
The level of hepatic 5HT increases after an intraperitoneal (i.p.), i.v., or intragingival injection of various mitogenic substances including LPS and concanavalin A (ConA).6,7,60) Such a hepatic 5HT increase is induced by LPS at as low a dose as 0.2 µg/kg (i.v).7) In C3H/HeJ mice—now known to be mice lacking the LPS receptor Toll-like receptor-4 (TLR4)—the LPS-induced hepatic 5HT increase was much less than in control mice.7) LPS is a typical stimulator of macrophages, and at the time when we found that LPS increases hepatic 5HT (viz. around 1984), macrophages had been shown to produce a lymphocyte-activating factor called IL-1 that stimulates helper T-cells.61,62) Later, we found that IL-1-like molecules—purified from the culture medium of a murine macrophage cell line—could induce a hepatic 5HT increase,11) and this was confirmed by using recombinant human IL-1.9) Recombinant IL-1α or IL-1β induced a hepatic 5HT increase at doses as low as 0.1 µg/kg (i.p.).9) Although recombinant human TNF-α also induced a hepatic 5HT increase, it required doses greater than 10 µg/kg (i.p.).9) The hepatic 5HT increase induced by LPS and the above cytokines was prevented by neither α-monofluoromethyldopa (irreversible inhibitor of 5HT synthesis) nor pargyline (inhibitor of monoamine oxidases).9,12)
4.2. Hepatic 5HT Increase Reflects Platelet AccumulationThe intestine contains a large amount of 5HT, and the liver is directly linked to this organ via the portal vein. Hence, we supposed that intestinal 5HT might be transported into the liver. In 1992, we reported the following results.13) In reserpine-treated mice, in which the 5HT in the blood (but not in the intestine) is mostly depleted, LPS did not increase hepatic 5HT. In addition, the LPS-induced hepatic 5HT increase was associated with reductions of both circulating platelets and blood 5HT (Fig. 1A). These findings encouraged us to do electron microscopic observation,13) leading to the conclusion that such a hepatic 5HT increase reflects hepatic platelet accumulation (HPA). Numerous platelets were located in the sinusoidal and perisinusoidal (Disse) spaces between hepatocytes and endothelial cells (Fig. 1B). These platelets retained dense granules and microtubules, indicating that they retained their intact structure. In addition, there was no apparent deposition of fibrin in the perisinusoidal spaces. Well-developed cell processes from Kupffer cells surrounded the majority of platelets, and there were electron-dense sites between platelets and Kupffer cells (Figs. 1B, C), indicating their direct interaction. All of the Kupffer cells were Mac-1 positive, indicating that they had been activated. Surprisingly, a direct contact of platelets with hepatocytes was also evident (Fig. 1C). Similar profiles were observed in mice given IL-1α and/or TNF-α. In addition, we found that (i) in macrophage-depleted mice, the LPS- or IL-1/TNF-induced HPA was abolished, (ii) several platelets were found inside some hepatocytes and these hepatocytes displayed no visible damage (Figs. 1D, E), and (iii) there were many polysomes around the degranulated platelets within the hepatocytes.63) These results indicate that, in response to LPS or IL-1 and/or TNF, platelets translocate into the liver in a way that is different from aggregation, some enter hepatocytes, and enhance protein synthesis, and that during this process, hepatic macrophages play a crucial role.
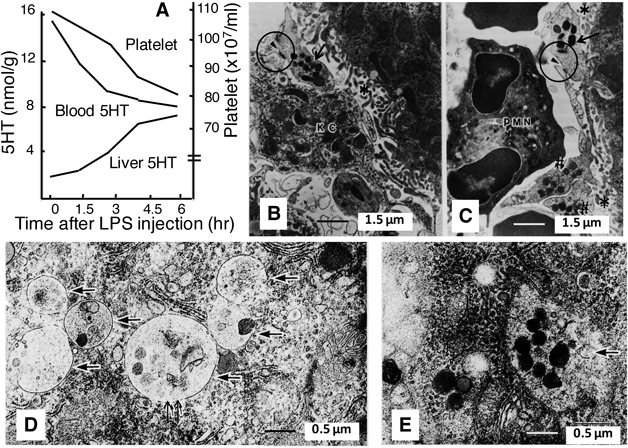
A, B, and C: Effects of LPS (0.1 mg/kg, i.v.).13) In A, the temporal relationship among the levels of liver 5HT, blood 5HT, and platelets is outlined. To provide a clear impression of the patterns of change in the measured variables, this and several subsequent Figures omit the data-points and statistical symbols. However, these can be seen in the original research articles, which are cited in each legend. In B, it is notable that a platelet (arrow) is located within a Disse Space (*), and that there is an electron-dense site (arrowhead within circle) suggesting contact between the platelet and a Kupffer cell (KC). In C, in addition to contact between Kupffer cell and platelet, it is notable that the platelet is extending its pseudopod into the hepatocyte, leading us to suppose this indicates a process for its translocation into the hepatocyte. Two platelets (#) are penetrating into a space of Disse. A polymorphonuclear neutrophil (PMN) is also present in the sinusoidal space. D and E: Platelets inside hepatocytes.63) The liver was removed 4.5 h after i.p. injection of LPS (0.1 mg/kg). In D, most granules within platelets (single arrows) have been lost, although the platelet in the center (small double arrow) has retained its microtubules. Many polysomes are also evident around platelets. In E, the platelet membrane (single arrow) is in the process of fusing, and there are many polysomes around this platelet too.
Peripherally administered 5HT cannot enter the central nervous system across the blood brain barrier, and the liver is the central organ regulating metabolism. In studies performed largely in the 1980s, we noted that there is a close parallelism between hepatic 5HT and hypoglycemia in terms of both time-course and dose-dependence.7,9,10) 5-Hydroxytryptophan (the direct precursor of 5HT) has long been known to induce hypoglycemia,64) and our experiments using this substance revealed a close correlation between the 5HT increase in the liver (but not in the brain) and hypoglycemia.10,12) Later, in 2002, we found that IL-1 is a prerequisite for the hypoglycemic action of LPS.65) These results suggest that 5HT may cause hypoglycemia following its selective increase in the liver.
Insulin, a typical inducer of hypoglycemia, has been subcutaneously injected in many animal experiments. So, in the 1990 s, we compared hypoglycemic effects between insulin and IL-1 following their subcutaneous injection into mice.9) Although insulin-induced hypoglycemia was not significant at 20 µg/kg, it was profound at 50 µg/kg or more, resulted in convulsions at 200 µg/kg or more, and occurred more rapidly than that induced by IL-1. However, IL-1 induced hypoglycemia even at 0.1 µg/kg, with its extent being almost constant across the dose range 10 to 200 µg/kg (without accompanying convulsions). These results indicate that the IL-1-induced hypoglycemia is different from the insulin-induced hypoglycemia.
The effects of 5HT given by systemic injection on blood glucose are controversial because 5HT stimulates various receptors throughout the body (except the brain). In studies by other groups, 5HT1 or 5HT2 receptors have been shown to contribute to hypoglycemia via insulin release (5HT1 and 5HT2),66,67) hepatic glycogen synthesis (5HT1 and 5HT2),68) or glycolysis in skeletal muscles (5HT2),69,70) while 5HT2A and 5HT2B receptors contribute to hyperglycemia via adrenaline release (5HT2A),71) gluconeogenesis (5HT2B), or inhibition of glucose uptake (5HT2B).72) Moreover, whereas i.p. injected 5HT induces hypoglycemia in mice,66,67) it has the opposite effect, hyperglycemia, in rats.71)
4.4. Hepatitis and Hepatic PlateletsAn i.p. injected mixture of LPS and D-galactosamine induces hepatitis with severe hepatic congestion in mice, and an involvement of platelets has been suggested.73) We found that there was a marked 5HT/platelet accumulation in the liver (but not in the lung, spleen, kidney, or intestine) only in those mice in which congestion had occurred.74)
Platelets, when stimulated, express Fas ligand (FasL) on their surface and then release it.75) Fas, the FasL receptor, is ubiquitously expressed in various organs, and the Fas/FasL interaction, which induces apoptosis in Fas-positive cells,76) has been postulated to be involved in various inflammatory diseases.77) Jo2 monoclonal antibody, which acts as a Fas agonist, induces focal hemorrhage and necrosis in the liver, but not in other tissues.78) We found that i.v. injected Jo2 induces platelet accumulation predominantly in the liver as well as entry of platelets into hepatocytes.79) Jo2 also induced HPA and hepatitis in mice deficient in both IL-1 and TNF-α. In platelet-depleted mice, the Jo2-induced hepatitis was not reduced, and actually hepatitis markers were significantly augmented, although the survival time of mice given a lethal dose of Jo2 was significantly increased. Interestingly, prior induction of HPA by a low dose of LPS markedly reduced the Jo2-induced hepatitis.
Intrahepatic hypercoagulation has been suggested to be indispensable for the development of the hepatitis induced by i.v. injected ConA.80,81) In our study,82) LPS-induced HPA depended on macrophages, while in the case of ConA-induced HPA such dependency was only partial. Platelet-depletion significantly exacerbated ConA-hepatitis. Blockade of the receptor for P-selectin (adhesion molecule for leukocytes on platelets and endothelial cells) or use of anti-P-selectin antibody reduced both the HPA and hepatitis induced by ConA. Prior HPA induction by a low-dose of LPS powerfully reduced both the ConA-induced HPA and hepatitis. Such protection by LPS-pretreatment did not occur in macrophage-depleted mice. In platelet-depleted mice, LPS-pretreatment severely exacerbated ConA-hepatitis. In mice depleted of both platelets and macrophages, ConA did not induce hepatitis. In IL-1-KO mice, ConA-hepatitis was mild, and protection by LPS was not detected. Translocation of platelets into Disse spaces and their entry into hepatocytes were confirmed in mice pretreated with LPS and then given ConA. These results suggested that (i) HPA is both causal and protective in the development of hepatitis, (ii) aggregation of platelets is causal, and this may be induced by unknown platelet stimulants leaked from injured hepatocytes, (iii) the HPA inducible in a macrophage-dependent manner by prior low-dose treatment with LPS is protective, and (iv) IL-1 is involved in both the causal and protective effects.
HPA has also been reported in various other models, including the residual liver after hepatectomy,83) ischemia-reperfusion-induced hepatitis,84) viral hepatitis,85) bile duct ligation-induced liver injury,86) and cirrhosis (or cholestasis).87) In those studies, too, both exacerbating and protective effects of platelets were reported. Intrasinusoidal or extravasated aggregation of platelets is causally involved in bile duct ligation-induced liver injury86) and in ischemia reperfusion-induced hepatocellular damage.88) In the latter case, 5HT-induced vasoconstriction has been suggested to promote hepatic dysfunction.88)
4.5. Platelets and 5HT in Repair and RegenerationAccumulating evidence suggests that platelets play a critical role in the repair and regeneration of various tissues.53,89) It has also been suggested that platelets may act as gatekeepers of the vascular wall to preserve vascular integrity.54) Concerning the liver, Ohkohchi’s group demonstrated that HPA is involved in liver regeneration via direct contact with hepatocytes, Kupffer cells, and sinusoidal endothelial cells.90) As described above, we found platelets within hepatocytes in mice treated with LPS,63,82) Jo2,79) or ConA.82) Such internalization of platelets or platelet-like particles was also observed in both in vivo and in vitro experiments by Kirschbaum et al., and the platelet RNA transferred to hepatocytes was shown to mediate hepatocyte proliferation.59)
Tryptophan hydroxylase (TPH) is the rate-limiting enzyme in 5HT synthesis. In 2003, the existence of two forms of TPH (TPH1 and TPH2) was confirmed.91,92) In TPH1-KO mice, 5HT was deficient in the blood, gut, and pineal gland, but its level in the brain stem was normal, indicating that platelet 5HT is produced by TPH1 in the gut.91,92) Since then, it has been suggested that 5HT serves as a paracrine factor, an endocrine hormone, or a growth factor, and that 5HT plays important roles in gastrointestinal motility, enteric neurogenesis, mucosal growth/maintenance, intestinal inflammation, osteogenesis, and hepatic regeneration.93,94) Moreover, platelet 5HT has been shown to affect immune responses by modulating the functions of monocytes, macrophages, lymphocytes, and endothelial cells.95) In the liver, platelet-derived 5HT reportedly mediates liver regeneration.96) Such a positive effect of 5HT has also been reported in murine models of hepatitis induced by ischemia and reperfusion97) or acetaminophen.98) In contrast, viral hepatitis is aggravated by platelet-derived 5HT.85) Lesurtel et al.99) and Papadimas et al.100) have reviewed these positive and negative effects of platelet 5HT.
In anesthetized rabbits and dogs, infused 51Cr-labeled platelets display a biphasic response: a rapid drop “within a few minutes” of i.v. injection of LPS and then a slow drop lasting for several hours (see review by Morrison and Ulevitch).101) Although electron microscopy demonstrated pulmonary and hepatic platelet aggregation with extensive platelet destruction, no quantitative evaluation was made, and no studies had been performed on non-anesthetized animals at that time.
In 1996, by measuring 5HT we kinetically and quantitatively evaluated such rapid platelet behavior in non-anesthetized mice.74) As shown in Fig. 2, i.v. injected Escherichia coli (E. coli) LPS induced drastic 5HT changes in BALB/c mice, with the maximum decline of blood 5HT at 2.5–5.0 min after the injection. Corresponding to this decline, 5HT increased markedly in the lung and liver (but only slightly in the spleen—a result discussed in Section 7). In that study, 70% or more of the 5HT that had disappeared from the blood accumulated in the lung, and anaphylaxis-like shock followed. This type of shock is often called “anaphylactoid shock” since although it resembles anaphylactic shock in occurring rapidly after an injection of (or exposure to) certain substances, it is a reaction to non-antigenic substances, rather than to antigenic ones. The rapid pulmonary and hepatic 5HT accumulations were induced only by high doses of LPS (more than 100 µg/kg), while the slow (hour-scale) hepatic 5HT accumulation could be induced by much lower doses (1 µg/kg or less), even when given by i.p. injection. Interestingly, such marked 5HT changes did not occur when E. coli LPS was injected into C3H/HeN mice, although similar 5HT changes and shock occurred when a Prevotella intermedia (P. intermedia) LPS preparation was injected into such mice, indicating a strain difference in LPS-induced anaphylactoid shock. Figure 3 shows electron microscopic illustrations of platelets accumulated in the lung of C3H/HeN mice.102) A large number of aggregated platelets were evident in the lung removed 3 min (before shock) after the injection (Fig. 3A). At 8 min (during shock), there were many degranulated platelets (Fig. 3B). Although the shock severity had weakened by 30 min, the mice still looked exhausted. In the lung removed at this time, platelet structure had mostly been ruptured (Fig. 3C). These results indicate that in addition to hour-scale behaviors, i.v. injected LPS and/or some component(s) included in the LPS preparations induce minute-scale platelet behaviors in a manner dependent on both the dose and the strain of mouse, leading to shock.74)

E. coli LPS (0.5 mg/kg) was i.v. injected into non-anesthetized BALB/c mice, and the blood and tissues were collected or removed at 2.5, 5, 10, 15, and 30 min (insets) with additional sampling up to 4.5 h after the injection. The 5HT levels after LPS injection are outlined.74)

P. intermedia LPS (4 mg/kg) was i.v. injected into C3H/HeN mice. Arrow, platelet or its remnant. A: Lung removed after 3 min (before shock). Many platelets are aggregating inside blood vessels, but they retain their intact structure and contain many granules. B: Lung removed after 8 min (during anaphylactoid shock). Cytoplasm of aggregated platelets has become electron-lucent, and degranulating or degranulated platelets and remnants of degraded platelets are seen. C: Lung removed after 30 min (after shock). Most platelets have lost their granules, and cell membrane and other structures are ruptured.
Muramyl dipeptides (MDP) are central constituents of the peptidoglycans that compose bacterial cell walls, and MDP is the minimal structural unit of peptidoglycans required to induce immune responses.103) In 1987, Takada and Galanos104) reported that mice given an i.v. injection of MDP followed by injection of LPS from various bacteria exhibited both rapid shock (anaphylactoid shock) and slow shock (called “endotoxic shock,” a reaction that becomes evident in mice several hours after an injection of LPS). LPS-induced enhancement of rapid shock was maximal when LPS was administered 4 h after an MDP injection. Anaphylactoid shock occurred even in C3H/HeJ mice, an endotoxic shock-resistant strain.104,105) Later (1998–1999), C3H/HeJ mice were shown to have mutated Toll-like receptor 4 (TLR4).106,107) Takada et al. also suggested a possible involvement of 5HT in the anaphylactoid shock.105)
Collaboration between Endo’s and Takada’s groups clarified the following. MDP-pretreatment sensitizes mice to LPS-induced anaphylactoid shock.102) Accumulations of platelets and their degradation in lung and/or liver (especially in the lung) are causal to induce anaphylactoid shock.102,108) K-76 (complement C5 inhibitor) prevents such platelet responses and anaphylactoid shock.108–111) Aspirin (anti-platelet drug), glycyrrhizin (anti-hepatitis drug), and dexamethasone (steroid) reduce the anaphylactoid shock.112,113) Anaphylactoid shock is also induced by i.v. injected whole cells of oral streptococci.114) In mice depleted of their macrophages or platelets, LPS-induced anaphylactoid shock is completely prevented.113,115) However, endotoxin shock is augmented in platelet-depleted mice.113) These results suggest that platelets are important in innate immunity against bacterial infection.
5.2. Platelet Responses to i.v. Injected AntigensAnaphylaxis is induced in individuals sensitized to an antigen. Histamine is a well-known shock-inducing substance, and mast cells and basophils contain a large amount of histamine. Although basophils are scarce in mice, antigens increase FcεR-positive basophils in mice.116,117) Interestingly, anaphylaxis occurs even in mice deficient in mast cells118–121) or immunoglobulin E (IgE).122) Murine platelets contain much 5HT, while human platelets contain both histamine and 5HT.123) In 2002, we examined the platelet contribution to anaphylaxis using ovalbumin (OVA) as an antigen.124) As shown in Fig. 4, platelets accumulated in the lung and liver in OVA-sensitized mice 15 to 30 s after i.v. injection of OVA, and platelet degranulation occurred. These platelet responses were dramatic and more rapid than histamine release. In addition, platelet responses occurred at doses of OVA lower than those inducing histamine release. In platelet-depleted mice, anaphylactic shock was markedly reduced. Cyproheptadine (antagonist against both histamine and 5HT) almost abolished shock signs, while (in contrast to the situation in anaphylactoid shock) K-76 and aspirin were not effective. These results indicate that in addition to their role in anaphylactoid shock, platelets are involved in anaphylactic shock, but via a different mechanism.

Measurements were made at 0, 15, 30, 60, and 120 s after i.v. injection of OVA (12.5 µg per mouse) into sensitized BALB/c mice. The levels of platelets, 5HT, and/or H are outlined.124) There was no significant difference in platelet count at time 0 between sensitized and non-sensitized mice. In non-sensitized mice, even 50 µg per mouse of OVA induced no detectable changes either in 5HT levels in the lung and liver or in platelet count in the blood (not shown).
In rabbits, such platelet accumulations in the lung and liver in anaphylaxis had already been reported by Pinckard et al.,125) and platelet activating factor (PAF) was suggested to mediate it. However, PAF activates the platelets of humans, rabbit, dog, guinea pig, horse, and cat, but not those of mouse, rat, or hamster.126) Thus, in mice, an unknown mediator(s), other than PAF, may be involved in the antigen-induced platelet responses. Another possibility is that platelet activation by an antigen may itself induce platelet accumulation in the lung and/or liver.
In the 1990s, studies in mice showed that the time–course of histamine increase in the plasma is similar to that of PAF,121) and that PAF is involved in anaphylactic shock.121,127) Pharmacological studies suggest that histamine and 5HT mutually augment shock signs in mice.128,129) Thus, all of these substances may be involved in anaphylaxis.
5.3. Roles of Minute-Scale Platelet Behaviors in InfectionInfection or bacterial components reportedly cause either protection or exacerbation of allergic disorders.130–132) Even daily oral hygiene procedures may cause exposure to micro-organisms to occur repeatedly.133) P. intermedia is the dominant Gram-negative bacterium in periodontal pockets. We found that i.p. co-injection of P. intermedia LPS and OVA into normal BALB/c mice promoted both anaphylaxis and histamine-release following the OVA-challenge.134) Injection of the LPS before the OVA-challenge augmented shock-responses by augmenting pulmonary platelet accumulation (PPA), but not histamine release. As described above, platelets accumulated in lung and/or liver are involved in the anaphylactoid shock induced by bacterial components, too.
The blood that has passed through parts of the body other than the digestive tract passes first to the lungs before being re-circulated by the heart, and the blood that has passed through the digestive tract passes next to the liver. We suppose that platelets may play important roles in both innate (in a complement-dependent manner) and acquired immunity (in a complement-independent manner), and that the lung and liver may be the defensive fronts at which platelets cope with exogenous materials, including pathogens.
In the 1960s, it was reported that the lung of the anesthetized dog can remove more than 90% of i.v. injected 5HT during “a single circulation.”135,136) Although it was not clear what cells were involved in such a rapid removal, endothelial cells and/or mast cells were suggested. In the 1980 s, by monitoring 111In-labeled platelets in anesthetized guinea pigs and rats, ADP, collagen, PAF, or 5HT were found to induce PPA which, in the case of the ADP- or 5HT-induced PPA, was transient, although anesthesia was supposed to interfere with such platelet behaviors.137,138)
These observations led us to examine the effects of i.v. injected 5HT on platelets in non-anaesthetized BALB/c mice. We confirmed that 5HT and ADP each induced a rapid and transient PPA, reaching maximum within 6 s and disappearing within 18 s of the injection139) (Fig. 5), although the exact peak time was unclear because of technical difficulties. Our data, shown in Fig. 6, indicate the following. 5HT induces such a rapid PPA at ≥0.04 µmol/kg. The 5HT level in the blood is not elevated even when 5HT is injected at doses of up to 1 µmol/kg. On the contrary, the 5HT level in the blood is decreased at doses of 0.2 or 1 µmol/kg, while the 5HT levels in the lung and liver are increased. The pulmonary 5HT reached a maximum level after 5HT injection at 1 µmol/kg, and 5HT levels in the blood, liver, and kidney are dose-dependently increased after 5HT injection at 1 µmol/kg or more. These results indicate that the platelets accumulated in the lung rapidly take up the injected 5HT, and this uptake becomes saturated when the 5HT injection dose reaches 1 µmol/kg.

Mice were killed at 0 (no injection), 6, 12, and 18 s after the injection of 5HT (0.2 µmol/kg) or ADP (1 µmol/kg). The levels of platelets and/or 5HT in the blood and lung are outlined.139)

The mice were killed at 6 s after the injection. The levels of platelets and/or 5HT are outlined.139)
In that study, we also observed the following.139) Ketanserin (5HT2-receptor antagonist) inhibits the pulmonary 5HT uptake. Fluoxetine (5HT-uptake inhibitor) raises the 5HT level in the blood, but significantly depresses it in the lung, at 6 s after 5HT injection. In platelet-depleted mice, the pulmonary 5HT uptake is much reduced. The half-life of 5HT in the lung, when 5HT is given at 10 µmol/kg (i.v.), is <20 s. Unlike 5HT, i.v. injected histamine is largely excreted by the kidney without any uptake by the lung. These results demonstrate that platelets rapidly translocate into the lung upon stimulation of 5HT2 receptors, take up 5HT and swiftly degrade it, and then return to the circulation.
6.2. Platelet Dense Granules and 5HT UptakeDense granules and melanosomes belong to a family of lysosome-related organelles. Hence, common genes affect their formations. Mutations of the genes encoding the proteins named “biogenesis of lysosome-related organelles complex (BLOC)-1, -2 and -3” have been shown to cause both a dense-granule defect and albinism.140,141) Various mice with such mutations have been established,140) including “cappuccino mice” with cappuccino-like colors in the skin and eye. Our collaborators also established cappuccino mice,142) and found that the mice exhibited a profound defect in platelet dense granules (i.e., lacking 5HT in platelets), although platelet levels were similar to those in control C57BL/6 mice. In 2009, we found that in such mice there was no 5HT increase in the lung after i.v. injection of 5HT,139) indicating that platelet dense granules take up 5HT.
6.3. Platelet Responses to CatecholaminesLittle is known about in vivo effects of epinephrine and norepinephrine on platelets. Although their actions on platelets in vitro are weak, they include pro-aggregatory effects.143–145) Since recent studies have demonstrated that macrophages and neutrophils release catecholamines,146,147) we examined the effects of catecholamines and macrophages on platelet behaviors.148)
First, we found that i.v. injected epinephrine, norepinephrine, and dopamine all induced PPA within 6 s of the injection148) (Fig. 7), leading to dose-dependent shock (occurring 10–20 s after the injection). Their minimum PPA- and shock-inducing doses were at nmol/kg levels. Notably, although i.v. injected 5HT or ADP induces PPA, shock-signs are not observed even when the injection is at 10 µmol/kg. Secondly, our pharmacological studies using platelet- or macrophage-depleted mice indicated that (i) epinephrine and norepinephrine induced PPA via stimulation of both α1- and α2-receptors, while dopamine induced PPA via α1-receptors, and (ii) the α2-mediated PPA and shock depended on both macrophages and complements, while the α1-mediated PPA and shock depended on neither macrophages nor complements.148)

Epinephrine (75 nmol/kg) or Klebsiella O3-LPS (2 mg/kg) was i.v. injected, and blood and lung were taken at various time-points. The levels of blood platelets and lung 5HT are outlined.148)
As described in Section 5, i.v. injected LPS induces “minute-scale” PPA (Fig. 2) and anaphylactoid shock in mice. Macrophages and neutrophils reportedly produce and release catecholamines within 15 min of stimulation with LPS in vitro.146,149,150) Hence, in the third part of the above study, we examined the involvement of catecholamines in the LPS-induced minute-scale PPA.148) We found that (i) within 30 s of i.v. injection, Klebsiella O3 LPS increased catecholamines in the lung, but reduced them in the blood (Fig. 8), (ii) these responses preceded the PPA, and (iii) both α1- and α2-receptor antagonists prevented these responses and the anaphylactoid shock.148) These results suggest that LPS-induced PPA is mediated by catecholamines derived from macrophages, and that in the lung, platelets instantly take up the catecholamines, resulting in their decrease in the blood.

Klebsiella O3-LPS (1 mg/kg) was i.v. injected, and blood and lung were taken at various time-points for measurements of plasma epinephrine (E), norepinephrine (NE), and dopamine (DA). The levels of these amines are outlined,148) the values being expressed as a percentage of the value at time 0 (without injection). The actual level at time 0 (expressed in ng/g or ng/mL) is shown at the top of each panel. There was no significant change in catecholamine levels in the lung of mice given saline within 1 min of its injection (not shown).
We also noted that neither prazosin (α1 blocker) nor yohimbine (α2 blocker) was effective at reducing the anaphylactic shock induced by OVA.148) As described in Section 5, LPS-induced (but not OVA-induced) anaphylactoid shock depends on complement C5. These results indicate that the mechanisms underlying anaphylactoid shock and anaphylactic shock are fundamentally different, although platelets are involved in both of them.
6.5. Roles of Second-Scale Platelet BehaviorsAs described above, we found that 5HT induces PPA in a second-scale manner (Fig. 5), and platelets in the lung take up 5HT and swiftly degrade it.139) Moreover, when 5HT was injected at 1 µmol/kg or less, the 5HT level in the blood was decreased even at 6 s after the injection (Fig. 6). If 5HT were not somehow eliminated from the blood, this injection would result in blood 5HT increasing to around 15 nmol/g. In a different study, we noted that i.v. injected Klebsiella O3 LPS increased catecholamines in lung, but reduced them in blood, again in a second-scale manner (Fig. 8), and these responses preceded the PPA (Fig. 7). In the following paragraphs, we discuss these findings.
First, it should be remembered that the blood that has passed through the systemic circulation passes first to the lungs before being re-circulated. In our experiments, blood was taken from the neck (i.e., mainly from the carotid artery) by decapitation. Thus, the blood we used is the blood that has just exited the pulmonary circulation (see Section 3).
The plasma level of 5HT reportedly regulates the density of 5HT transporters on platelet membranes, and the 5HT transporters regulate plasma 5HT levels.151) Although there have been few studies on transporters for catecholamines in platelets, it is known that platelets take up serum catecholamines,152–154) and adrenergic antagonists reportedly inhibit the uptake of epinephrine by platelets.155) The pharmacological profiles of these transporters in platelets resemble those in adrenergic neurons.156) Recent studies on neurons have demonstrated that autoreceptors functionally couple to transporters for monoamines,157–159) and that the balance between the synthesis of catecholamines and their uptake is tilted toward the latter.160) Thus, in platelets that have accumulated in the lung, stimulation of 5HT receptors or adrenergic receptors might be expected to stimulate the uptake of 5HT or catecholamines by their respective transporters, leading to their rapid removal/filtration from the circulation. The PPA-associated removal of 5HT and catecholamines would seem to be an excellent mechanism for the prevention of their systemic delivery.
Incidentally, a significant number of platelets are already present in the lung (we call them “resident platelets”) as evidenced by the lungs of normal mice containing 5HT at a level much higher than those in the liver and kidney, whereas such pulmonary 5HT is largely absent in platelet-depleted mice.13,139) It has been reported that 20–50% of the mature megakaryocyte population reaches the lungs in humans, and that 7–17% of the platelets are released there.161,162) Thus, it is possible that such pulmonary resident platelets may be first activated by 5HT or catecholamines and then rapidly take up these substances from the blood.
The lifespans of human and murine platelets are 7–10 and 4–5 d, respectively.163,164) As reviewed by Grozovsky et al.,165) known platelet clearance mechanisms include antibody-mediated clearance by spleen macrophages. At present, however, this clearance is considered to be “classical.”165) Recent studies, mostly based on the observation of transfused platelets, are emphasizing that hepatic macrophages and hepatocytes themselves (ingestion of platelets) are involved in the clearance of both transfused and senescent platelets.166–168)
7.2. Contribution of Macrophages in SpleenVan Rooijen and colleagues demonstrated that liposomes encapsulating dichloromethylene bisphosphonate (Cl2MBP-liposomes) deplete phagocytic macrophages: their i.v. injection eliminates hepatic and splenic macrophages, while their intratracheal injection eliminates alveolar macrophages.169–171) In the spleen, the majority of macrophages are located in the red pulp, and they are completely eliminated within 24 h of a single i.v. injection of Cl2MBP-liposomes, with the complete lack of macrophages continuing for 3 d and several days being needed for recovery.171)
We confirmed such a macrophage depletion in the spleen and liver in mice given an i.v. injection of Cl2MBP-liposomes.172) In spite of such a drastic macrophage depletion, the mice showed no apparent abnormality, and we found a 5HT increase in the spleen and a 5HT decrease in the blood, with the effects reaching nearly maximum within 2 d of the injection173) (Fig. 9). The 5HT levels in the liver and lung tended to decrease. The 5HT changes in the spleen and blood corresponded well to each other in terms of both time–course and dose–response. It took about 10 d for complete recovery from these 5HT changes, a time–course similar to that of the macrophage recovery in the red pulp reported by Van Rooijen et al.171) The 5HT changes shown in Fig. 9 reflect platelet changes. Indeed, electron microscopic analysis revealed a marked accumulation of platelets in the splenic cords. To judge from the 5HT level in the blood at day 2, the platelets taken into the spleen may correspond to about 80% of the platelets lost from the blood.173) It should also be noted that the 5HT level in normal mice is highest in the spleen of all tissues tested (25-fold or more greater than in the liver).13) These findings suggest that depletion of macrophages in the spleen and liver induces accumulation of platelets in the spleen, but not in the liver, and that the accumulated platelets are eliminated by splenic macrophages. Thus, in normal mice, senescent platelets may be removed by the spleen.

Left: Time course of changes in 5HT levels after i.v. injection of Cl2MBP-liposomes. Mice were sacrificed at various time points after the liposome injection. The levels of 5HT in the blood and spleen are outlined. Right: Electron-microscopic examination of the spleen. Mice were sacrificed 2 d after the i.v. injection of Cl2MBP-liposomes. In A, a few platelets (arrows) and erythrocytes (e) can be seen. In B, in addition to erythrocytes, many platelets can be seen.
In mice given an i.v. injection of LPS, a fraction of the platelets accumulated in the lung and/or liver return to the blood within several minutes or one hour (depending on the dose and/or kind of LPS),74,102,108–113) while in mice given an i.v. injection of 5HT or ADP, most of the platelets accumulated in the lung and/or liver return to the blood within a few seconds.139) However, in macrophage-depleted mice the decrease in platelets continues for several days, as shown in Fig. 9, suggesting that the supply of platelets may be insufficient or slow, and that macrophages may play a role in supplying new platelets. This potentially important subject remains to be clarified in future.
Senescent platelets may be denatured. In Fig. 2, the large, rapid decrease in blood 5HT/platelets was accompanied by concomitant marked pulmonary and hepatic 5HT/platelet increases, but the increases in the spleen were comparatively small. Thus, in terms of its biological significance the platelet accumulation in the spleen may be different from those in the lung and liver. We suppose that the platelets accumulated in the spleen may be platelets that had been denatured by interaction with LPS, and that such denatured platelets may be selectively taken up by the spleen.
The blood stream from the digestive tract is firstly directed into the liver, and the blood returned to the heart from the systemic circulation is passed to the lungs before being sent to the rest of the body. Thus, when pathogens or toxic substances have entered or appeared in blood, they almost immediately reach either lung or liver, and these organs must function as the front-line in the body’s defense against such harmful materials. During the course of our studies on platelets, we usually kept two questions in mind: “Is it important that platelets are so small?” and “Is it important that platelets can exhibit a dramatic shape change?” We found that platelets can enter hepatocytes by extending processes into the hepatocytes. We also found that platelets accumulate in lung and liver in a second-scale manner. We suppose that the small size and considerable shape flexibility of platelets can support their rapid accumulation in lung and liver and their entry into hepatocytes.
5HT seems to be a good marker of platelets for studying platelet behaviors in mice. By measuring 5HT, we found evidence of hour-, minute-, second-, and day-scale platelet behaviors (summarized in Table 1). These findings support the view that platelets, in addition to their roles in hemostasis, play important roles as defenders against hazardous substances (both exogenous and endogenous) and as guardians of homeostasis. In their 2003 review, Weyrich et al. suggested that nucleated cell types in invertebrates (e.g., amebocytes in the arthropod horseshoe crab), nucleated thrombocytes in non-mammalian vertebrates, and platelets in mammals share innate defensive functions.174) Thus, from the viewpoint of phylogeny, the platelet accumulation in the lung and/or liver reviewed here looks like a defensive reaction that may have evolved specifically in mammals.
| Platelet behaviors | Inducers (i.v. injected)* | Sites of platelet accumulation | Tested effects |
|---|---|---|---|
| Hour-scale | LPS* (Figs. 1 and 2) | Liver | Hypoglycemia and hepatitis** |
| (>1 h) | IL-1*, TNF-α* | Liver | Hypoglycemia and hepatitis** |
| ConA | Liver | Hypoglycemia and hepatitis | |
| LPS (Fig. 2) | Spleen | Removal of denatured platelets | |
| Minute-scale | LPS (Figs. 2, 3, 7, and 8) | Lung and liver | Anaphylactoid shock |
| (>0.5 min) | Bacterial whole cells | Lung and liver | Anaphylactoid shock |
| Antigens (Fig. 4) | Lung and liver | Anaphylactic shock | |
| Second-scale | 5HT (Figs. 5 and 6) | Lung | No shock |
| (approx. <6 s) | ADP (Fig. 5) | Lung | No shock |
| Catecholamines (Figs. 7 and 8) | Lung | Shock | |
| Day-scale | Clodronate liposomes (Fig. 9) | Spleen | Removal of senescent platelets |
| (approx. <10 d) | (depletion of macrophages) |
* i.p. also effective. ** LPS exhibits both protective and exacerbating effects on hepatitis.
We are grateful to the many people who joined in this study. We are also grateful to Dr. Robert Timms, a former language-editor of J. Physiol. (Lond.), who edited our manuscripts throughout our studies and provided instructive suggestions.
The authors declare no conflict of interest.