2019 Volume 42 Issue 9 Pages 1581-1589
2019 Volume 42 Issue 9 Pages 1581-1589
As a bacterium used in industry for production of several amino acids, an endotoxin-free Corynebacterium (C.) glutamicum is well known. However, it is also true that the endotoxin-producing other Corynebacterium species is present. An aim of this study is to obtain a lactic acid bacterium (LAB) that produces ornithine and citrulline at high levels. We successfully isolated a strain, designated K-28, and identified it as Weissella (W.) confusa. The production of ornithine and citrulline by K-28 was 18 ± 1 and 10 ± 2 g/L, respectively, with a 100 ± 9% conversion rate when arginine was continuously fed into a jar fermenter. Although the ornithine high production using C. glutamicum is industrially present, the strains have been genetically modified. In that connection, the wild-type of C. glutamicum produces only 0.5 g/L ornithine, indicating that W. confusa K-28 is superior to C. glutamicum to use a probiotic microorganism. We confirmed that W. confusa K-28 harbors an arginine deiminase (ADI) gene cluster, wkaABDCR. The production of ornithine and the expression of these genes significantly decreased under the aerobic condition rather than anaerobic one. The expression level of the five genes did not differ with or without arginine, suggesting that the production of amino acids in the K-28 strain was not induced by exogenous arginine.
L-Ornithine is an amino acid with a variety of significant functions and is used as a healthcare supplement. Since this amino acid is important for smooth functioning of the human immune system and liver,1,2) it has been used in the form of ornithine alpha-ketoglutarate (OKG) to treat liver disorder in the urea cycle that detoxifies ammonia and maintain its safe blood levels.2,3) Ornithine is a metabolic intermediate that plays a role in the urea cycle by detoxifying the ammonia to reduce the level of ammonia in the blood.
The administration of ornithine has been reported to reduce stress and improve the sleep quality of healthy workers.4) This amino acid is also effective in humans to reduce physical fatigue and increase mental capability via metabolic action.5,6) Since ornithine also has the ability to promote the synthesis and production of collagen,7) skin aesthetic and wrinkle improvement with ornithine has satisfied demands in the cosmetic industry.7,8) Ornithine also has been regarded as an anti-obesity medicinal agent because the amino acid promotes the synthesis of body muscle through the promotion of growth hormone secretion.8)
Although mushrooms (10–170 mg ornithine/100 g) and Corbicula japonica (20 mg ornithine/100 g) are known as vital natural sources of ornithine, they do not contain a sufficient amount to satisfy the daily demand for this amino acid (400–1000 mg). Additionally, since a sufficient quantity of ornithine is not available in fish and meat,9) the oral administration of ornithine is recommended.
Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host.”10) Lactic acid bacteria (LAB), the typical probiotics, are non-pathogenic and Gram-positive bacteria.11) The LAB strains have been traditionally used to produce fermented foods such as yogurt and cheese and are generally recognized as safe (GRAS) microorganisms.11,12) The bacteria have been reported to play beneficial immunomodulation, anti-obesity, and anti-allergic inflammation roles in human health.13)
The LAB strains are roughly classified into two groups: animal- and plant-derived LABs. The animal-derived LAB strains are found in the oral cavity and intestinal tract. However, since the plant-derived LAB strains are found at the surface of plant petals, stems, and leaves, they are more adaptable to harsh conditions.
We have claimed that plant-derived LAB strains are superior to animal-derived ones with respect to immunomodulatory activity, tolerance to gastric and bile juices, and reducing constipation.14–16) More than 700 strains stored in the plant-derived LAB library established by us have been isolated from fruits, vegetables, flowers, and medicinal plants.17–19) We have found LAB strains producing a large amount of γ-aminobutyric acid,17) bacteriocin,12) and exopolysaccharide (EPS).11,18,20,21)
In the present study, we isolated a plant-derived LAB strain, designated K-28, from the flower of Senna obtusifolia and identified it as Weissella (W.) confusa. The strain produces a large amount of ornithine when grown in the de Man, Rogosa, and Sharpe (MRS) medium supplemented with arginine. We also established the culture condition to produce a large amount of ornithine in a fed-batch culture supplemented with arginine. To confirm whether the ornithine production is mediated by the arginine deiminase (ADI) pathway in the K-28 strain, we determined the ADI gene cluster using the primer walking method and evaluated the expression level.
As a liquid medium to culture the LAB strain, MRS medium (Becton, Dickinson and company, Franklin Lakes, NJ, U.S.A.) was used. When necessary, a 1.5% (w/v) agar was added to the medium.
Isolation of Ornithine-Producing LAB from Plant SourcesTo obtain the LAB candidates, a plant sample (leaf, stalk, and flower) was suspended in the MRS medium and incubated anaerobically at the given temperature (28, 37, and 45°C) for 2–3 d. An aliquot of the cultured broth was spread on the MRS agar plate and incubated at the given temperature. Each colony that appeared on the MRS agar plate was spread on a fresh MRS agar plate for purification. Gram-staining and catalase production tests were performed for the purified colony, followed by taxonomical identification. The ornithine production by the isolated LAB strains was investigated by culturing for 72 h in the MRS medium supplemented with 0.5% (w/v) arginine.
The HPLC Chromatographic Conditions for Ornithine, Arginine, and CitrullineThe concentrations of ornithine, arginine, and citrulline were determined using the phenyl isothiocyanate (PITC) derivatization method22) with a Wakopak Wakosil-PTC HPLC column (4.6 × 250 mm; Wako Pure Chemical Industries, Ltd., Osaka, Japan): after a 10 µL aliquot of the culture supernatant in a microcentrifuge tube was dried in vacuo, 20 µL of pre-reaction reagent (ethanol/water/triethylamine (TEA) = 2/2/1) was added to the dried preparation. After drying it in vacuo, a 20 µL portion of the reaction reagent (ethanol/water/TEA/PITC = 7/1/1/1) was added and kept at room temperature for 1 h for the complete reaction. The reaction mixture was dried completely, dissolved in 1 mL of distilled water, filtered with a 0.22 µm pore-size filter, and then stored at −20°C until use.
The analytical conditions were as follows: the mobile phase was a mixture of (A) PTC-Amino Acids Mobile Phase A (Wako Pure Chemical Industries, Ltd.) and (B) PTC-Amino Acids Mobile Phase B (Wako Pure Chemical Industries, Ltd.) with a linear gradient. The programed gradient was as follows: 0 to 20 min, 0–70% B. The column chromatography was performed at 40°C and a flow rate of 1.0 mL/min, and the eluates from the column were monitored to detect amino acids with the UV detector at 254 nm.
Chromosomal DNA Extraction from LAB StrainsThe bacterial cells were harvested from the 2 mL of culture broth by centrifugation at 13000 ×g for 1 min at 4°C. The cell pellet was resuspended into 500 µL of a glucose–ethylenediaminetetraacetic acid (EDTA) solution containing 40 mg/mL lysozyme (Wako Pure Chemical Industries, Ltd.) and 4 mg/mL achromopeptidase (Wako Pure Chemical Industries, Ltd.). After the resulting cell lysate was incubated for 3 h at 37°C, a 20 µL aliquot of 10% (w/v) sodium dodecyl sulfate (SDS) was added to the lysate. The proteins from the lysate were denatured by adding a 50 µL aliquot of 5 M NaClO4 and removed by chloroform extraction. The chromosomal DNA was purified using an ethanol precipitation method. The resulting DNA was dissolved into 50 µL of TE buffer (10 mM tris(hydroxymethyl)aminomethane (Tris)–HCl and 0.1 mM EDTA, pH 8.0) and stored at 4°C until use.
DNA Sequencing and Identification of LAB StrainsTo identify the isolated LAB strains, the entire 16S ribosomal DNA (rDNA) gene fragment was amplified by PCR using 27f (5′-AGA GTT TGA TCT GGC TCA G-3′) and 1525r (5′-AAA GGA GGT GAT CCA GCC-3′) primers, and the sequence of the fragment was determined using the method described previously.23,24) The nucleotide sequence was determined with the ABI PRISM 3130xl Genetic Analyzer using the BigDye Terminator v3.0 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, U.S.A.) according to the manufacturer’s instructions. The obtained sequence raw data were analyzed using ATG C software (GENETYX Corporation, Tokyo, Japan). The sequence data were compared with those of LAB species registered in the DNA Data Bank of Japan (DDBJ) database (https://www.ddbj.nig.ac.jp/index-e.html). LAB species of the isolates were identified using the BLAST algorithm-based25) homology search and the ClustalW program-based sequence alignment (http://clustalw.ddbj.nig.ac.jp/ index.php?lang=en).
Acute Oral Toxicity and Mutagenicity TestsAn acute oral toxicity test on the K-28 cells was performed at Japan Food Research Laboratories in accordance with the OECD Guidelines for the Testing of Chemicals, Guidelines 420 (2001). Slc:Wistar/ST male rats (five weeks old) were purchased from Japan SLC, Inc. (Shizuoka, Japan). The rats were divided into two groups of five rats each and housed in polycarbonate cages in a temperature-controlled room (20–26°C) with 12 h light–dark cycles. Rats had free access to drinking water and a Labo MR Stock diet (Nosan Co., Yokohama, Japan). After one week of acclimation, one group was assigned to be a reference group and the other a LAB-fed group. After cultivation, the LAB cells were collected and resuspended in 15% (w/v) skim milk (2 × 1010 CFU/mL). The cell suspension (50 mL/kg) was divided into three doses (20, 20, and 10 mL/kg) and orally administered to the LAB-fed group of rats using a sterile stomach tube at 1 h intervals. In a control group, 15% (w/v) skim milk without LAB cells was administered. The behavior and health status of the rats were recorded every day for 14 d, and the body weight was measured at 1, 7, and 14 d after the animal experiment began. The rats were euthanized after the experimental period, and some extracted organs were analyzed histologically. The body weight difference between rats was analyzed using Welch’s t-test. The same research was carried out using female rats.
According to the manufacturer’s protocol, the mutagenicity test (umu-test) of the K-28 culture broth was performed using a Umulac AT test kit (Protein Purify Ltd., Maebashi, Japan). In the kit, the induction of the umu gene is responsible for DNA damage that was calculated by the umuC-lacZ fusion gene expression in the Salmonella enterica serovar Typhimurium NM2009. If the sample enhanced the activity of β-galactosidase more than twofold over the background, the sample was considered to exhibit the mutagenic property at the given concentration.
Culture Conditions for High Ornithine ProductionTo measure the initial pH and the added initial arginine concentration effects for ornithine production, the K-28 strain was cultured in an MRS medium supplemented with the given concentration of arginine at the given pH condition at 28°C. Under the determined optimum initial pH and arginine concentration, the K-28 strain was cultured at different temperatures to evaluate the effect of cultivation temperature on the ornithine production. The OD600 nm of the culture broth was measured to monitor the bacterial cell growth.
Effect of Fed-Batch CultivationFor the test tube–scale cultivation test, the seed culture of the K-28 strain was inoculated [final 1% (v/v)] into the MRS medium (pH 6.5) supplemented with 0.5% (w/v) arginine at 28°C without agitation. At each 12 h interval, a 1 mL aliquot of culture broth was taken and used for ornithine production analysis. The same volume of a 10% (w/v) arginine solution was added to the remaining culture broth after the 96 h cultivation. The citrulline was highly produced until 96 h of cultivation, whereas the conversion to ornithine was reduced and the cell growth was decreased after 96 h.
For the cultivation test at a jar-fermenter scale, the K-28 strain was grown in 1 L of MRS medium supplemented with 0.5% (w/v) arginine using a 3 L jar fermenter with agitation at 28°C for 96 h. During the experimental period, the pH of the culture medium was maintained at pH 6.5 by adding HCl or NaOH. After 12 h of cultivation, a 1 mL aliquot of cultured broth was taken, and 10% (w/v) arginine containing fresh MRS medium (pH 6.5) was added to the final concentration of 0.5% (w/v) arginine. Sampling of the culture broth and arginine supplementation were continued at 12 h intervals for 96 h.
Sequencing Analysis of the ADI Gene ClusterThe ADI gene cluster of the K-28 strain was amplified by PCR using the ADI-F (5′-TAG AGA ACC ACT AAA GAT C-3′) and ADI-R (5′-CTT TTT TGC ATC AGT TCC GA-3′) primers, which were designed based on conserved regions of the available ADI clusters from four W. confusa strains: AB3E41, DSM20196, LBAE C39-2, and MBF8-1 (RefSeq accession numbers NZ_FUWE01000018, NZ_JQAY01000001, NZ_CAGH01000001, and NZ_MNBZ01000002, respectively). The PCR was performed using a PrimeSTAR Max DNA Polymerase (TaKaRa Bio, Inc., Shiga, Japan) under the following conditions: 35 cycles of 10 s at 98°C, 5 s at 60°C, and 30 s at 72°C. The nucleotide sequence of the amplified fragment was determined by direct sequencing with ADI-F, ADI-R, and additionally designed inner primers. The open reading frames (ORFs) were predicted by using an ORF Finder program provided by NCBI at website (https://www.ncbi.nlm.nih.gov/orffinder/). The homology searches of the predicted ORFs were performed on the NCBI Web BLAST site (https://blast.ncbi.nlm.nih.gov/Blast.cgi) using the BLAST algorithm25) utilizing the non-redundant database. The determined nucleotide sequence was deposited to DDBJ (accession number LC479518).
RNA Extraction and RT-PCR Analysis of ADI Gene ClusterTotal RNA extraction from W. confusa K-28 cell was performed using a NucleoSpin RNA II kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany) according to the manufacturer’s protocol at each 12 h growth interval. The cDNA was synthesized from total RNA using the ReverTra Aca qPCR RT Master Mix with gDNA remover (TOYOBO, Osaka, Japan) according to the manufacturer’s instructions. In the RT-PCR analysis, the target genes, wkaA, wkaB, wkaC, wkaD, and wkaR, were amplified using the synthesized cDNA as a template, with each primer set designed individually (Table 1). The PCR reaction was performed using a PrimeSTAR Max DNA Polymerase under following conditions: 2 cycles of 5 s at 98°C, 10 s at 68°C, and 5 s at 72°C; 2 cycles of 5 s at 98°C, 10 s at 66°C, and 5 s at 72°C; 2 cycles of 5 s at 98°C, 10 s at 64°C, and 5 s at 72°C; 2 cycles of 5 s at 98°C, 10 s at 62°C, and 5 s at 72°C; and 27 cycles of 5 s at 98°C, 10 s at 60°C, and 5 s at 72°C.
| Target gene | Description | Forward primer (5′→3′) | Reverse primer (5′→3′) |
|---|---|---|---|
| wkaA | Arginine deiminase | CACTGATGAAAAGGTTCGTG | CGAACAATTCCTTAGCCAAC |
| wkaB | Ornithine carbamoyltransferase | TAGGACGTATGTTTGATGCC | GATCAATTCATCATCAGGCG |
| wkaC | Carbamate kinase | GAAGAAGCAAAGACTGTTCG | GTAATTACCGTACCATCCCC |
| wkaD | Arginine ornithine antiporter | ACAACTTGTCTTTTCAAGCG | GATTGATGCGGTACACAATG |
| wkaR | Predicted transcriptional regulator | GTTGTTGTTAAAGGGGAGAC | AACTCACCGACTAGAATGTC |
| 16S rRNA | TTGCTCAGATATGACGATGG | CCAATAAATCCGGATAACGC |
In the present study, twenty LAB strains were isolated from several plants. Among those, a LAB strain designated K-28, which was isolated from the flower of Senna obtusifolia, produced a large amount of ornithine in the MRS medium supplemented with arginine. The entire 16S rDNA sequence of the strain was compared with those of LABs registered in the DDBJ/EMBL/GenBank database. From the result of a homology search, the K-28 strain was identified as W. confusa.
Safety Evaluation of the K-28 StrainAn acute toxicity test of the K-28 cells by oral administration in mice shows that no significant activity changes or intake-related illnesses or deaths were observed. Inflammatory symptoms and obvious differences did not appear in any organs of animals during the histological analysis. Furthermore, the umu-test demonstrates that the culture broth of the K-28 strain did not induce any mutagenesis.
Effects of Cultivation Conditions on Ornithine and Citrulline ProductionWe evaluated the initial pH of the medium (4.5–9.0 at 0.5 intervals) for efficient ornithine production (Fig. 1), showing that the initial pH for efficient production was excellent between pH 5.0 and 8.0, but the ornithine production was lower at pH 4.5 and pH 8.5–9.0.

The culture was done at 28°C for 72-h. The data were expressed as mean ± standard error (S.E.) (n = 3). Different characters on the top of the bars indicate statistically significant differences between means of values obtained in different groups (Tukey’s HSD test, p < 0.05).
We also evaluated the effect of the culture temperature and the culture period on the high ornithine production. As shown in Fig. 2, after 48 h or more cultivation, the K-28 strain produced the large amounts of ornithine at 20–28°C but not at 37°C. Figure 3 reveals that the ornithine production and the cell growth may be independent. In addition, the cultivation at 28°C is better for cell growth as well as ornithine production.

The data were expressed as mean ± S.E. (n = 3). Different characters on the top of the bars indicate statistically significant differences between means of values obtained in different groups (Tukey’s HSD test, p < 0.05) at each culture period.

The data were expressed as mean ± S.E. (n = 3). Different characters beside the markers indicate satistically significant difference between means of values obtained in different temperatures (Tukey’s HSD test, p < 0.05) at each culture period.
Ornithine is converted from arginine through the two enzymatic reactions: 1) arginine deiminase (EC 3.5.3.6), converting arginine into citrulline and ammonia, and 2) ornithine carbamoyltransferase (EC 2.1.3.3), converting citrulline into ornithine and carbamoyl phosphate. Therefore, the initial concentration of arginine added to the medium may affect the production of ornithine. In this study, the effect of initial concentration of arginine added to the medium was examined within a range from 0.5 to 3.0% (w/v). Table 2 shows that the production of ornithine is parallel with the initial concentration of the added arginine. However, the conversion ratio of arginine to ornithine seems to be inversely related to the initial arginine concentration after the 48 h cultivation, suggesting that the high concentration of arginine added to the medium gave rise to the saturation of arginine conversion to ornithine. In addition, a significant increase of ornithine production was not observed at more than 96 h of cultivation (data not shown). Further, during the 24–48 h cultivation, at least about 80% of arginine was converted into ornithine.
| Cultivation time (h) | Initial Arg concentration (w/v %) | ||||||
|---|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | ||
| 24 | Orn (mM) | 20.2 ± 0.5 | 40 ± 1 | 52 ± 4 | 81 ± 3 | 94 ± 3 | 123 ± 3 |
| Ratio (%) | (85 ± 3) | (84 ± 2) | (73 ± 5) | (86 ± 3) | (79 ± 2) | (87 ± 2) | |
| 48 | Orn (mM) | 22.9 ± 0.9 | 39 ± 1 | 54 ± 2 | 82 ± 5 | 103 ± 3 | 119 ± 3 |
| Ratio (%) | (92 ± 4) | (83 ± 4) | (76 ± 3) | (86 ± 5) | (87 ± 3) | (84 ± 2) | |
| 72 | Orn (mM) | 18.9 ± 0.7 | 36 ± 1 | 52.9 ± 0.2 | 79 ± 3 | 85 ± 3 | 115 ± 5 |
| Ratio (%) | (80 ± 3) | (76 ± 3) | (74.3 ± 0.3) | (83 ± 3) | (71 ± 3) | (81 ± 4) | |
| 96 | Orn (mM) | 23 ± 3 | 39 ± 3 | 51 ± 5 | 79 ± 8 | 94 ± 3 | 109 ± 2 |
| Ratio (%) | (100 ± 10) | (81 ± 5) | (71 ± 6) | (83 ± 8) | (80 ± 3) | (77 ± 1) | |
W. confusa K-28 was cultured in the MRS broth supplemented with arginine, with an initial pH of 6.5 at 28°C. The data were expressed as mean ± S.E. (n = 3).
We expected increased ornithine production in the K-28 strain in the fed-batch culture supplemented with arginine (Table 3). As shown in Table 3, when the K-28 strain was cultured in the medium supplemented with arginine, the ornithine production was significantly increased. Furthermore, after the production of ornithine, a higher amount of citrulline was produced. The production of ornithine seemed to be saturated at 48 h, whereas the production of citrulline would be started at 36–48 h. By keeping the concentration of arginine low, the added arginine was converted to citrulline as an intermediate in the bioconversion of arginine to ornithine.
| Cultivation time (h) | Amino acid concentrations in cultured broth (mM) | Total added Arg (mM/w/v %) | Amino acid conversion ration (%) | OD600 | ||||
|---|---|---|---|---|---|---|---|---|
| Orn | Cit | Arg (unreacted) | Arg to Orn | Arg to Cit | Arg to (Orn + Cit) | |||
| 12 | †13.6 ± 0.1a | †2.2 ± 0.6a | 5.91 ± 0.06 | 23.7/0.5 | 58 ± 4 | 9 ± 3 | 67 ± 2 | 1.63± 0.02 |
| 24 | 71.6 ± 0.8b | 5.1 ± 0.6a | 1.1 ± 0.4 | 71.2/1.5 | 101 ± 1 | 7.2 ± 0.9 | 108 ± 2 | 2.17 ± 0.04 |
| 36 | 101 ± 9b, c | 12 ± 1b | 0.3 ± 0.1 | 118.7/2.5 | 85 ± 8 | 10.1 ± 0.9 | 95 ± 9 | 1.950 ± 0.008 |
| 48 | 120 ± 10c, d | 31.2 ± 0.5c | 0.19 ± 0.01 | 166.1/3.5 | 74 ± 6 | 18.7 ± 0.3 | 92 ± 6 | 1.58 ± 0.02 |
| 60 | 127 ± 8c, d | 57 ± 2d | 1.3 ± 0.1 | 213.6/4.5 | 60 ± 4 | 26.7 ± 0.8 | 86 ± 3 | 1.6 ± 0.1 |
| 72 | 139 ± 7d | 73 ± 2e | 0.5 ± 0.3 | 261.1/5.5 | 53 ± 3 | 27.9 ± 0.8 | 81 ± 4 | 1.741 ± 0.009 |
| 84 | 143 ± 7d | 106.9 ± 0.5f | 0.5 ± 0.2 | 308.6/6.5 | 46 ± 2 | 34.7 ± 0.2 | 81 ± 2 | 1.64 ± 0.02 |
| 96 | 128 ± 6c, d | 125.8 ± 0.8g | 0.34 ± 0.08 | 356.0/7.5 | 36 ± 2 | 35.4 ± 0.3 | 71 ± 2 | - |
W. confusa K-28 was cultured in the MRS broth supplemented with initial 0.5 (w/v) % arginine, with an initial pH of 6.5 at 28°C. The 1.0 w/v % arginine was added to the broth at each 12-h interval. The data were expressed as mean ± S.E. (n = 3). †The values followed by different characters in the same column are statistically significantly different (Tukey’s HSD test, p < 0.05).
We investigated the arginine-fed-batch effect using a 3 L jar fermenter under the control condition at pH 6.5. The conversion ratio from arginine to ornithine drastically increased to over 100% during the cultivation period from 24 to 48 h (Table 4). The added arginine was immediately consumed to be converted to ornithine, whereas the production of citrulline was enhanced in the long cultivation.
| Cultivation time (h) | Amino acid concentrations in cultured broth (mM) | Total added Arg (mM/w/v %) | Amino acid conversion ration (%) | CFU/mL (× 109) | ||||
|---|---|---|---|---|---|---|---|---|
| Orn | Cit | Arg (unreacted) | Arg to Orn | Arg to Cit | Arg to (Orn + Cit) | |||
| 12 | †21 ± 7a | †3.4 ± 0.4a | 10 ± 5 | 23.7/0.5 | 90 ± 30 | 14 ± 2 | 100 ± 30 | 8.2 ± 0.4 |
| 24 | 67 ± 4b | 9 ± 2a | 3 ± 2 | 47.5/ 1.0 | 142 ± 8 | 19 ± 5 | 160 ± 4 | 10 ± 2 |
| 36 | 80 ± 10b | 14 ± 2a, b | 0.9 ± 0.3 | 71.2/1.5 | 120 ± 20 | 19 ± 2 | 140 ± 20 | 9 ±1 |
| 48 | 96 ± 9b, c | 21 ± 2a, b, c | 1.2 ± 0.5 | 94.9/2.0 | 100 ± 10 | 22 ± 2 | 120 ± 10 | 8.3 ± 0.3 |
| 60 | 113 ± 7b, c | 31 ± 3b, c, d | 1.7 ± 0.8 | 118.7/2.5 | 96 ± 6 | 26 ± 3 | 122 ± 7 | 7.8 ± 0.6 |
| 72 | 115 ± 9b, c | 39 ± 5c, d, e | 2 ± 1 | 142.4/3.0 | 81 ± 6 | 27 ± 4 | 110 ± 10 | 7 ± 1 |
| 84 | 123 ± 7c | 47 ± 6d, e | 0.9 ± 0.2 | 166.1/3.5 | 74 ± 4 | 28 ± 3 | 103 ± 8 | 6 ±1 |
| 96 | 135 ± 8c | 56 ± 9e | 1.0 ± 0.2 | 189.9/4.0 | 71 ± 4 | 29 ± 5 | 100 ± 9 | 5.3 ± 0.9 |
W. confusa K-28 was cultured in the MRS broth supplemented with 0.5 (w/v) % arginine, with an initial pH of 6.5 at 28°C. The 0.5 w/v % arginine was added to the broth at each 12-h interval. The pH was maintained at 6.5 during the experimental period. The data were expressed as mean ± S.E. (n = 3). †The values followed by different characters in the same column are statistically significantly different (Tukey’s HSD test, p < 0.05).
To characterize the ADI gene cluster of the high-ornithine-producing W. confusa K-28, we determined the gene organization (Table 5) of the cluster. As shown in Fig. 4, the gene cluster, which is 5.7 kb long, consists of five genes for encoding arginine deiminase (wkaA), ornithine carbamoyltransferase (wkaB), carbamate kinase (wkaC), the arginine–ornithine antiporter (wkaD), and the putative transcriptional regulator (wkaR). The deduced amino acid sequence of WkaA has high identity with arginine deiminase (EC: 3.5.3.6), which is a key enzyme in the ADI pathway and catalyzes the conversion of arginine to citrulline. The wkaB and wkaC genes were predicted to be ornithine carbamoyltransferase (EC 2.1.3.3) and carbamate kinase (EC 2.7.2.2), respectively. The former enzyme catalyzes the conversion of citrulline to ornithine, and the latter one converts carbamoyl phosphate to ammonia and carbon dioxide. The wkaD gene is likely to play a role as an arginine–ornithine antiporter. The WkaR is predicted to be a transcriptional activator homologous with a PucR family regulator protein. The PucR is a transcriptional activator involved in the catabolism of purine and its intermediates, such as uric acid, allantoin, or allantoic acid, and activates the purine degradation pathway.26,27)
| Gene | nt position | aa | Best blast homology (source) | Accession no. | Identity (%) |
|---|---|---|---|---|---|
| wkaA | 194–1432 | 412 | Arginine deiminase (Weissella confusa LBAE C39-2) | CCF30537 | 410/412 (99.5) |
| wkaB | 1464–2510 | 348 | Ornithine carbamoyltransferase (Weissella confusa LBAE C39-2) | CCF30536 | 348/348 (100) |
| wkaD | 2513–3946 | 477 | Arginine ornithine antiporter (Weissella confusa DSM 20196) | KRN24519 | 477/477 (100) |
| wkaC | 3966–4907 | 313 | Carbamate kinase (Weissella confusa 32) | RAU08366 | 313/313 (100) |
| wkaR | 5017–5718 | 233 | PucR family transcriptional regulator (Weissella confusa 32) | RAU08366 | 233/233 (100) |

The nucleotide sequence shows wkaA and the upstream region containing an inverted repeat (13–38 bp) and two direct repeats composed of 48–54 bp or 79–85 bp. Another nucleotide sequence contains wkaR and the upstream region containing the inverted repeat ranges composed of 4916–4952 bp.
We evaluated the expression of each gene in the ADI gene cluster during the fed-batch culture using a jar fermenter and the RT-PCR method (Fig. 5). We observed that the expression level of all genes gradually decreased with the increase of cultivation time. In addition, the expression levels of those five genes did not differ in the presence or absence of the initially added arginine (data not shown), revealing that the ornithine and citrulline production by the K-28 strain does not depend on the exogenous arginine.

In the cultivation, a jar fermenter (working volume: 1-L) was used.
The expression level of each gene in the ADI cluster under the aerobic or anaerobic condition was evaluated (Fig. 6). The RT-PCR analysis shows that the expression of five genes in the ADI gene cluster is higher under the anaerobic condition than under the aerobic one. The result suggests that ornithine production was significantly high under the anaerobic condition (20.2 ± 0.3 mM) at 24 h of cultivation (Fig. 7) but not under the aerobic condition (4.1 ± 0.4 mM ornithine).

In the figure, the left-side expression profile indicates aerobic condition. The right-side profile indicates anaerobic one. The cultivation was done at initial pH 6.5 and at 28°C using the medium supplemented with 0.5% (w/v) arginine.
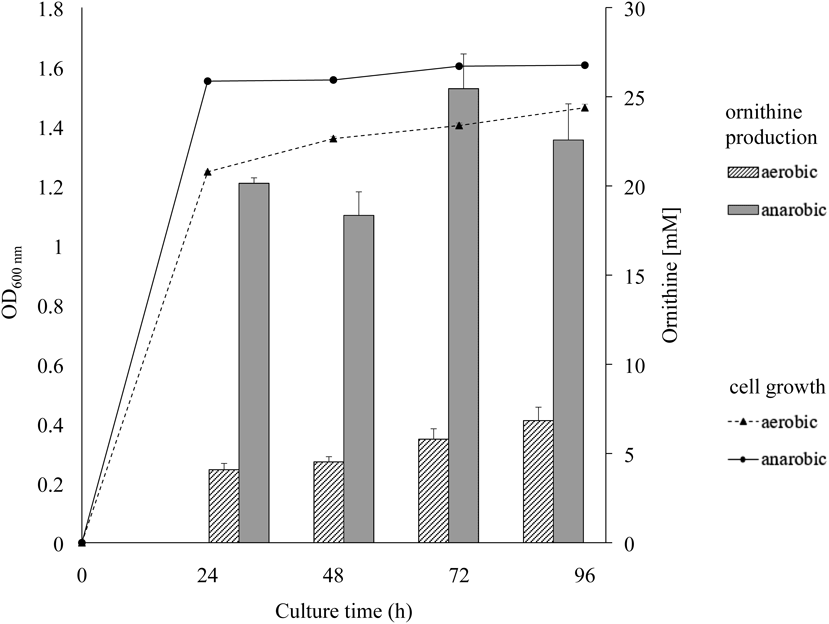
The cultivation was done at initial pH 6.5 and at 28°C using the medium supplemented with 0.5(w/v) % arginine. The data were expressed as mean ± S.E. (n = 3).
Amino acids play a critical role in deriving energy in a nutrient-limited environment.8) Through an acute toxicity test and a mutagenicity experiment, W. confusa K-28 was confirmed to be a safe and reliable bacterium. Interestingly, we observed that the K-28 strain produces ornithine and citrulline at high levels. Since the LAB strain has been isolated from plants, the strain might be adapted to harsh conditions. In fact, the K-28 strain is tolerant to gastric and bile acids (data not shown), suggesting that the strain may also useful for immunomodulation and improving constipation as well as our previous isolates.15,16) Therefore, the K-28 strain would be good candidate as a potential probiotic useful for manufacturing functional food and healthcare supplements.
The production of L-ornithine by microbial fermentation still is not to an industrially satisfactory level. The limited strains, which are C. glutamicum mutants modified genetically, have been proven to produce ornithine with satisfactory efficacy.28) In contrast to the mutant strains, the productivity of the wild-type C. glutamicum produce only 0.5 g/L ornithine.28,29) To produce ornithine, in general, arginine is decomposed by arginase urease or arginine deiminase.30) The present study shows that W. confusa K-28, harboring the ADI pathway, has significant potential to produce ornithine at a high level. In fact, it has been observed that the strain produces a high amount of ornithine (67 ± 4 mM) via a continuous arginine-feeding method for 24 h (Table 4). As shown in Fig. 2, the production is fivefold greater than that via the stand-cultivation method (12.7 ± 0.6 mM) for 24 h.
L-Citrulline stimulates the muscle protein synthesis in a short-term low-protein diet.31) This amino acid is a promising pharmaconutrient to provide nutritional support in malnourished patients, especially those who are aging and have sarcopenia.32) L-Citrulline has been reported to retard high glucose-induced endothelial senescence in combination with arginine.33)
Arginine is considered as a potential source of energy in ADI pathway by bacteria, because it forms one molecule of ATP from per mole arginine consumed during the production of ornithine and ammonia.34) A research group has reported that a function of the ADI pathway is acid-based physiology within the bacteria.35) Due to the production of ammonia through the ADI pathway, the increase of pH has been observed in Streptococcus sangria.36) During the production of citrulline, the concomitant production of ammonia by W. confusa K-28 may cause the rising pH, which could help the K-28 strain to survive in acidic environment.
Although vigorous cell growth was observed, ornithine production gradually decreased with the progression of the cultivation time, whereas the accumulation of citrulline significantly increased. The formation of citrulline from ornithine may be due to equilibration between the substrate and product, because the OTC enzyme catalyzes the reciprocal conversion between ornithine and citrulline.37) On the other hand, the ADI catalyzes the reaction toward the citrulline biosynthesis.37) Therefore, during the fermentation, arginine is converted to ornithine, and after the accumulation of the excess amount of ornithine, citrulline is gradually produced.
In the human body, citrulline is contributed as an intermediate of ureagenesis38,39) and is also the precursor of arginine for nitric-oxide synthesis. Moreover, almost all of the orally administrated arginine is metabolized in the gastrointestinal tract (GIT) and the hepatic tissue. These metabolisms are due to the expression of arginase (first-pass effect),40–43) resulting in poor oral bioavailability. Because citrulline is not the substrate of arginase, the amino acid can easily pass through the GIT and hepatic tissue. Therefore, the intake of citrulline may contribute to the increase of arginine oral bioavailability.32) Additionally, when arginine is orally administrated in combination with citrulline, the arginine can also pass through the GIT and hepatic tissue without undesirable metabolization by those organisms.32) This is because L-citrulline inhibits the arginase activity by acting as an allosteric inhibitor.44)
Arginine catabolism via the ADI pathway has been reported in some LABs and other bacteria. We compared the arginine catabolism and ornithine production by W. confusa K-28 with that by other strains. One research group has reported that W. koreensis MS1-3 and W. koreensis MS1-14 produced extracellular ornithine at 45 and 46 mg/L, respectively, when arginine was supplemented with a final concentration of 1.0% (w/v).8) However, W. confusa K-28 produced 8.85 g/L ornithine (Table 4) under the same culture conditions.
When cultured with the MRS medium supplemented with arginine, the fermented product of the K-28 strain cannot be orally administrated from the standpoint of food safety. Therefore, in the present study, carrot juice supplemented with arginine was used as a culture medium to generate ornithine. The high production of ornithine was observed at 28°C for 60 h when 100% (v/v) carrot juice supplemented with 0.5% (w/v) arginine as a culture medium (pH 6.5) was employed. The ornithine production was 21 ± 2 mM, with a conversion rate of 88 ± 8%, suggesting that the carrot juice is suitable for growing the plant-derived LAB strain.
The present study suggests that the gene expression of the ADI cluster in W. confusa K-28 is enhanced under the anaerobic condition rather than the aerobic one (Fig. 6). The production of ornithine converted from exogenous arginine, which is induced under the anaerobic condition, may be mediated by some transcriptional activator. The nucleotide sequence analysis of the ADI gene cluster in the K-28 strain suggests that there are two predicted transcriptional units, wkaA–D and wkaR. The upstream region of wkaA and wkaR contains the predicted inverted repeat sequences (Fig. 4). Twice-repeated direct-repeat sequences were also observed on the upstream region of wkaA, suggesting that the expressions of wkaA–D and wkaR are likely to be regulated by some transcriptional regulators.
This work was financially supported in part by The Satake Research Foundation and The Hiroshima University Education and Research Support Foundation. We also thank the Analysis Center of Life Science, Hiroshima University, for the use of their facilities.
The authors declare no conflict of interest.
The GenBank/EMBL/DDBJ accession numbers for the sequences reported in this paper is LC479518.