2020 Volume 43 Issue 11 Pages 1693-1698
2020 Volume 43 Issue 11 Pages 1693-1698
Cisplatin is a widely used chemotherapy for solid tumors; however, its benefits are limited by serious nephrotoxicity, particularly in proximal tubular cells. The present study investigated the renoprotective effect and mechanisms of germacrone, a bioactive terpenoid compound found in Curcuma species on cisplatin-induced toxicity of renal cells. Germacrone (50 and 100 µM) attenuated apoptosis of human renal proximal tubular cells, RPTEC/TERT1 following treatment with 50 µM cisplatin and for 48 h. Co-treating RPTEC/TERT1 cells with cisplatin and germacrone significantly reduced cellular platinum content compared with cisplatin treatment alone. The effect of germacrone on organic cation transporter 2 (OCT2) which is a transporter responsible for cisplatin uptake was determined. Germacrone showed an inhibitory effect on OCT2-mediated methyl-4-phenylpyridinium acetate (3H-MPP+) uptake with IC50 of 15 µM with less effect on OCT1. The germacrone’s protective effect on cisplatin-induced cytotoxicity was not observed in cancer cells; cisplatin’s anti-cancer activity was preserved. In conclusion, germacrone prevents cisplatin-induced toxicity in renal proximal tubular cells via inhibition OCT2 transport function and reducing cisplatin accumulation. Thus germacrone may be a good candidate agent used for reducing cisplatin-induced nephrotoxicity.
Cisplatin (cisplatinum or cis-diamminedichloroplatinum (II), CDDP) is a highly effective chemotherapeutic drug. Cisplatin was the first U.S. Food and Drug Administration (FDA)-approved platinum compound for cancer treatment.1) The pharmacological effects of platinum(II) and other metal containing compounds are now attracting interest as potential anticancer drugs.2) Currently, cisplatin is used as front-line therapy for many types of solid tumors including non-small cell lung, ovarian, head and neck, advanced cervical, bladder, and breast cancers.3–5) Although it is an effective anticancer drug, cisplatin’s therapeutic benefits are limited by serious nephrotoxicity affecting proximal tubular cells.6) A relationship between cisplatin-induced nephrotoxicity and membrane transporters such as copper transporter 1 (Ctr1) and organic cation transporter 2 (OCT2) has been observed.7–11) Cisplatin is also transported into renal tubular cells by these membrane transporters especially at the basolateral membrane.9) Interestingly, cisplatin showed weak tubular secretion into the urine by multidrug and toxin extrusion (MATE) transporters.11) This evidence might support high cellular accumulation of cisplatin. Presumably, the renal-selective toxicity of cisplatin was a result of extensive basolateral transport by OCT2 and weak efflux by MATE1/2. When cisplatin is present in cytosol, chloride dissociates from platinum becoming positively charged and pharmacologically active. Cisplatin induces cellular damage and nephrotoxicity through several mechanism including oxidative stress, DNA damage, cell apoptosis, and inflammation.12)
To improve the therapeutic efficiency of cisplatin, minimizing nephtotoxicity is very important. At present, only hydration protocols are used to reduce nephrotoxicity allowing increased doses.13) Hydration protocols, however, are not entirely effective at reducing cisplatin-induced nephrotoxicity; because only one-third of patients treated with this protocol are effective.14) Therefore, new therapeutic interventions to reduce nephrotoxicity are still necessary. Inhibiting cellular accumulation of cisplatin as introduced by Tanihara et al., showed inhibition of OCT2 by imatinib and cimetidine, substrates of OCT2, reduced cisplatin-induced nehrotoxicity in renal proximal tubular cells.15) Accordingly, inhibiting OCT2 may be a treatment for cisplatin-induced nephrotoxicity.
Germacrone is a terpenoid compound found in Curcuma species are widely used herbal medicines in China, India, and other Asian countries. It has also been shown to have medicinal attributes and it has been shown to exhibit different curative roles, which include antibacterial, antiulcer, antifungal, antitumor, anti-inflammatory, hepatoprotector, antitussive, anti-feedant, depressant vasodilator, and choleretic effects.16–18) The pharmacokinetic data revealed that rapid absorption and long half-time of germacrone.19) Since, germacrone has been reported to be a protective agent for acute liver injury induced by xenobiotics in mice,20) it is interesting whether germacrone has a protective effect on renal proximal tubular cells toxicity induced by cisplatin. The present study investigated the effects and mechanism of germacrone on cisplatin-induced toxicity of human renal proximal tubular cells.
Cisplatin, thiazolyl blue tetrazolium bromide (MTT), and tetrapentylammonium (TPeA) were purchased from Sigma (MO, U.S.A.). Radiolabeled methyl-4-phenylpyridinium acetate (3H-MPP+) was purchased from American Radiolabeled Chemicals, Inc. (MO, U.S.A.). Apoptosis Detection Kit (Annexin V-fluorescein isothiocyanate (FITC)/ propidium iodide) was purchased from BD biosciences (CA, U.S.A.). Anti-Bcl-2 and β-actin antibodies were obtained from Cell Signaling technology (MA, U.S.A.). Germacrone was isolated from rhizomes of Curcuma elata by our group.21) The rhizomes of Curcuma elata Roxb. were collected from Sawangdaendin district, Sakon Nakhon province, Thailand. The plant was identified by Puangpaka Soontornchainaksaeng and Thaya Jenjittikul, Department of Plant Science, Faculty of Science, Mahidol University, Bangkok, Thailand. The voucher specimen (SCMU, No. 305) is deposited at the Department of Plant Science, the Faculty of Science, Mahidol University.
Cell CultureRabbit (rb) OCT1 and rbOCT2 transfected in CHO-K1 cells were cultured in F12 Ham Kaighn’s modification (F12K) medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin. RPTEC/TERT1, human renal proximal tubular cell and HepG2 cells, hepatocellular carcinoma cells, were obtained from American Type Culture Collection (ATC C). RPTEC/TERT1 cells were cultured in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) medium supplemented with 5 µg/mL insulin, 5 µg/mL transferrin, 5 ng/mL sodium selenite, 100 U/mL penicillin, 100 µg/mL streptomycin, 10 ng/mL epithelial growth factor, and 36 ng/mL hydrocortisone. HepG2 cells were cultured in DMEM (high glucose) supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. All cells were incubated at 37°C in a humidified 5% CO2 and 95% air atmosphere, and subcultured according to the ATC C’s protocols.
Cell Viability AssayCell viability was evaluated by MTT assay, using the reduction of MTT substrate to a purple formazan dye. Cells were seeded in 96-well tissue culture and incubated with different treatments. After incubation, cells were added at 100 µL/well of MTT solution (0.5 mg/mL in serum-free medium) and incubated for a further 4 h. Medium was removed and the formazan salt formed was dissolved with dimethyl sulfoxide (DMSO). Absorbance at 530 nm was measured by microplate reader. The data was shown as a percentage of cell viability compared with control (vehicle-treated cells).
Cell ApoptosisRPTEC/TERT1 cells were treated with vehicle or test compounds for 48 h followed by staining the cells with Annexin V-FITC and propinium iodide (PI) in the dark for 15 min. Apoptic and necrotic cells were determined by flow cytometric measurements using a BD Accuri C6 flow cytometer. A minimum of 20000 events/sample were analyzed and apoptotic cells were counted and expressed as a percent of total cells.
Uptake Assay in Cell Culture StudiesThe uptake study was performed as described by our previous studies.22,23) Cells were seeded on a 24-well plate and grown until they become confluent monolayer. Confluent cells were washed twice with transport buffer (NaCl 137 mM, KCl 3 mM, Na2HPO4·7H2O 0.5 mM, KH2PO4 1 mM, MgCl2·6H2O 0.5 mM, CaCl2·2H2O 1 mM, D-glucose 5.6 mM at pH 7.4), and then were incubated in 37°C for 30 min. After this preincubation period, the cells were incubated with D-phosphate buffered saline (PBS) containing 3H-MPP+ (approx. 10 nM) alone or with a test compound at 37°C for 1 min. Uptake was stopped by removing the transport buffer and washing three times with ice-cold D-PBS. Lysis solution (10% sodium dodecyl sulfate (SDS) in 0.4 N NaOH) was added overnight, and neutralized with HCl. Accumulated radioactivity was measured by liquid scintillation beta counter. Transport of 3H-MPP+ was calculated as fmol/min/cm2 of the confluent monolayer surface and expressed as a percentage of control.
MATEs-Mediated 3H-MPP+ TransportCell monolayers were washed twice with 1 mL of transport buffer containing 30 mM NH4Cl in 37°C for 20 min in order to manipulate the intracellular acidification.24,25)
The uptake experiment was performed by incubating the cells with 200 µL of K+ based-buffer (pH 8.5; KCl 130 mM, MgSO4.7H2O 1.2 mM, CaCl2·2H2O 1 mM, K2HPO4 2 mM, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 20 mM and D-glucose 5 mM) containing 10 nM of 3H-MPP+ for 20 min. Then, cells were washed three times with ice-cold buffer to stop transport activity. MATEs-mediated 3H-MPP+ transport was calculated as fmol/min/cm2 of the confluent monolayer surface and expressed as percentage of control.
Intracellular Accumulation of Platinum in Human Renal Proximal Tubular CellsRPTEC/TERT1 cells were grown on 6-well plates until confluence and then incubated with 100 µM cisplatin alone or with a test compound for 4 h. At the end of this incubation period, cells were washed three times with PBS. Cells were removed by incubation with 0.25% trypsin for 20 min at 37°C and then centrifuged at 1500 ×rpm for 10 min. Subsequently, cells were solubilized overnight in 0.5 mL of concentrated nitric acid. Samples were subjected to flame furnace atomic absorption spectrophotometer (FFAAS) to determine platinum content. Data were expressed as ng of platinum/mg of total protein. OCT2-mediated platinum accumulation was calculated by subtracting platinum accumulation in the presence of 1 mM TPeA, an inhibitor of OCT, from the total platinum accumulation.
Western Blot AnalysisEqual amounts of protein lysates were separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE) then transferred onto nitrocellulose membranes. The blots were blocked by non-fat dry milk (5%) for 1 h, and they were then overnight incubated with primary antibodies. The blots were then washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h. Expression of proteins were detected using chemiluminescent HRP substrate. Data are shown as the ratio of the intensity of interested proteins normalized by loading proteins.
Statistical AnalysisAll data were expressed as mean and standard deviation (mean ± S.D.). Statistical analysis was performed using the statistical software package, GraphPad Prism, Version 4.0. Statistically significant differences among groups were compared using one-way ANOVA followed by Tukey’s comparison of pairs. Statistical significance was considered at p < 0.05.
The protective effect of germacrone on cisplatin toxicity was determined in human renal proximal tubular cell line, RPTEC/TERT1 cells. The cells were incubated with vehicle, cisplatin (50 µM), germacrone (50 and 100 µM), or cisplatin plus germacrone for 48 h. Cells treated with cisplatin plus germacrone were pretreated with germacrone for 1 h. As shown in Fig. 1A, RPTEC/TERT1 cells treated with 50 µM cisplatin alone significantly reduced cell viability compared with vehicle-treated cells. Cells co-treated with cisplatin and germacrone at 50 and 100 µM significantly attenuated the effect of cisplatin; revealing germacrone’s protective effect in renal proximal tubular cells. Next, the protective effect of germacrone on cisplatin-induced cell death was confirmed. Data obtained from apoptosis analysis revealed that germacrone reduced cisplatin-induced cell apoptosis of RPTEC/TERT1 cells confirming cisplatin’s cytotoxicity was significantly attenuated by co-treatment with germacrone in human renal proximal tubular cells (Fig. 1B). Cisplatin significantly decreased expression of Bcl-2, an anti-apoptotic protein. This effect was restored by co-treating with germacrone (Fig. 1C).

RPTEC/TERT1 cells were treated with vehicle, cisplatin (50 µM), germacrone (50–100 µM), or cisplatin plus germacrone for 48 h followed by measurement of cell viability (A), apoptotic cells (B), and Bcl-2 expression. * p < 0.05 vs. vehicle-treated cells and # p < 0.001 vs. cisplatin-treated cells. Data were obtained from three independent experiments and expressed as mean ± S.D.
Cisplatin is transported into renal proximal tubular cells via renal OCT28); therefore protective effect of germacrone might be mediated by reduced accumulation of cisplatin. Germacrone’s effect on cellular cisplatin accumulation in human renal proximal tubular cells was examined. RPTEC/TERT1 cells were treated with the following conditions: 1) 100 µM cisplatin for 4 h; 2) 100 µM germacrone for 1 h, followed by 100 µM cisplatin plus 100 µM germacrone for 4 h; and 3) 1 mM TPeA, a non-selective OCT inhibitor, for 1 h, followed by 100 µM cisplatin plus 1 mM TPeA for 4 h. As shown in Fig. 2A, cellular platinum content was significantly inhibited by 1 mM TPeA indicating OCT-mediated cisplatin uptake. Cellular platinum levels in cells co-treated with germacrone were significantly lower than cells treated with cisplatin alone. These data indicated that germacrone inhibited cisplatin uptake in human renal proximal tubular cells and its effect was mediated by inhibition of OCTs. Next, the effect of germacrone on function of OCTs in renal proximal tubular cells was determined. RPTEC/TERT1 cells were incubated with vehicle containing 3H-MPP+ or in combination with 50 and 100 µM germacrone for 5 min. As shown in Fig. 2B, germacrone significantly inhibited cellular accumulation of 3H-MPP+. Next, the effect of germacrone on transport activity of MATEs, transporters responsible for efflux of cisplatin, was determined. Interestingly, incubation the cells with high concentration of germacrone (200 µM) did not inhibit MATEs-mediated 3H-MPP+ transport (Fig. 2C).

(A) Effect of germacrone on cellular platinum content in human renal proximal tubular cells; RPTEC/TERT1 cells were incubated under the following conditions: 1) 100 µM cisplatin; 2) 100 µM germacrone for 1 h followed by medium containing 100 µM cisplatin plus 100 µM germacrone for 4 h.; 3) 1 mM TPeA for 1 h followed by medium containing 100 µM cisplatin plus 1 mM TPeA for 4 h. (B) Effect of germacrone on 3H-MPP+ uptake in RPTEC/TERT1 cells; cells were incubated with 3H-MPP+ alone, or with germacrone for 5 min. (C) Effect of germacrone on MATEs-mediated 3H-MPP+ transport in RPTEC/TERT1 cells. Data were expressed as percent of control as obtained from three independent experiments. * p < 0.05 vs. vehicle-treated cells.
To determine whether germacrone inhibits OCT2, cellular 3H-MPP+ accumulation in cells expressing OCT2 was measured. CHO-K1-OCT2 cells were incubated with medium containing (approx. 10 nM) 3H-MPP+ alone (control) or various concentrations (0–1000 µM) germacrone for 5 min. As shown in Fig. 3, germacrone inhibited OCT2-mediated 3H-MPP+ uptake in dose-dependent manners. The IC50 of germacrone for OCT2 was 15.5 ± 1.4 µM. In addition, the inhibitory effect of germacrone on OCT1 was determined. The results showed that germocrone showed low inhibitory effect on OCT1-mediated 3H-MPP+ uptake with IC50 of 155.7 µM.
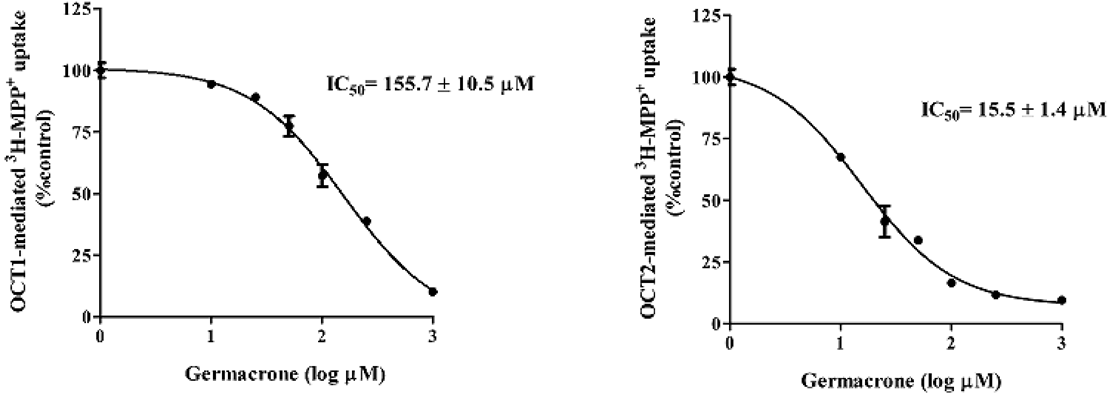
CHO-K1 cells expressing OCT1 or OCT2 were incubated with 3H-MPP+ alone, or with various concentrations of germacrone for 1 min. 3H-MPP+ uptake was measured; calculated as percentage of control; and expressed as mean ± S.D. The IC50 values were calculated from three independent experiments.
Whether germacrone’s attenuation of cisplatin-induced cytotoxicity in renal proximal tubule cells decreased cisplatin-induced cancer cell death was investigated next. Hepatocellular carcinoma cells (HepG2 cells) were treated with following conditions for 48 h: 1) vehicle; 2) 100 µM germacrone; 3) 50 µM cisplatin; and 4) cisplatin plus germacrone. Cisplatin alone reduced cell viability in HepG2 cells relative to control. Co-treating the cells with cisplatin and 100 µM germacrone further decreased cell viability compared with cisplatin-treated cells (Fig. 4).

HepG2 cells were treated with medium containing vehicle, 50 µM cisplatin alone, 100 µM germacrone alone, or cisplatin plus germacrone for 48 h. * p < 0.05 vs. vehicle-treated cells and #p < 0.05 compared with cisplatin-treated cells. Data were obtained from three independent experiments and expressed as mean ± S.D.
The major adverse effect of cisplatin is nephrotoxicity affecting proximal tubular cells.6) As cisplatin is transported into renal proximal tubular cells via OCT2 and CTR1.7,26) Inhibition of cisplatin uptake into the renal cells such as inhibition of OCT2 might be a logical target for minimizing nephrotoxicity.6) Inhibition of OCT2 transport function by OCT2 inhibitors, such as cimetidine and imatinib, reduces cisplatin-induced nephrotoxicity in both in vitro and in vivo experimental models.15) The present study demonstrates the protective effect of germacrone, a terpenoid compound, on cisplatin-induced toxicity of renal proximal tubular cells via inhibition of OCT2-mediated cisplatin uptake into cells.
Germacrone’s protective effect against cisplatin’s cytoxicity was observed in human renal proximal tubular cell lines, RPTEC/TERT1 cells, that expresses xenobiotic transporters such as OCT2, MATE1, MATE2,27,28) and CTR1 (unpublished data). Cisplatin-induced cytotoxicity of renal proximal tubular cells was mediated by increase in apoptotic cells with reduction of anti-apoptotic protein (Bcl-2) expression which were similar with previous studies.29) The cytotoxic effect of cisplatin was inhibited by germacrone. Since, intracellular level of cisplatin is a factor determining its severity, it is possible that the protective effect of germacrone was mediated by reducing cisplatin content in the cells. Cellular platinum content was determined by treating human renal proximal tubular cells with cisplatin alone or in concert with germacrone. Germacrone significantly reduced cellular platinum accumulation compared with cisplatin treatment alone supporting the hypothesis that germacrone’s protective effect was, at least partially, mediated by reduced cisplatin accumulation. Total cellular platinum accumulation was partly (about 70%) mediated by OCT2. As cellular platinum accumulation was not completely abolished by co-treatment with TPeA, an OCT inhibitor, other pathways, such as CTR1, may have a role in the remaining of cisplatin cellular accumulation.10,30)
Next, to prove whether germacrone inhibited OCT2 function, the inhibitory effect of germacrone on OCT2 transport function in cells expressing OCT2 alone was delineated. Since OCT2 shares high homology with OCT1, cations that interact with OCT2 might interact with OCT1 as well, the effect of germacrone on OCT1 was determined. Our data revealed that germacrone showed a potent inhibitory effect on OCT2 with a low inhibitory effect on OCT1. The IC50 value for OCT2 was ten-fold less than the IC50 value of OCT1. Although germacrone showed an inhibitory effect on OCT2, it is unclear whether germacrone acts as an inhibitor or a substrate of OCT2. In addition, the present study also revealed that germacrone did not inhibit transport activity of MATEs indicating gernacrone selectively inhibited OCT2.
Germacrone could attenuate cisplatin-induced cytotoxicity in human renal proximal tubular cells via reducing cisplatin accumulation, this mechanism might be important for cisplatin’s anticancer activity. To evaluate this hypothesis, human hepatocellular carcinoma cells were co-treated with cisplatin and germacrone and toxicity was assessed. Treating the HepG2 cells with germacrone alone showed significantly reduced cell viability. Co-treatment with germacrone did not reverse cisplatin’s antitumor activity. These data indicate that the uptake of cisplatin in HepG2 is mediated by OCT2-independent pathway. Therefore, germacrone might be targeted approaches to reduce cisplatin-induced renal cell toxicity. The study in animal model is required to support whether germacrone could be a candidate agent for preventing nephrotoxicity of cisplatin.
In conclusion, germacrone inhibits OCT2-mediated cisplatin uptake into renal proximal tubular cells as well as reduces cisplatin-induced cytoxicity in renal proximal tubular cells. As such, germacrone may be a candidate agent for reducing nephrotoxicity during cisplatin chemotherapy. The therapeutic potential of germacrone needs to be proved in animal models in future studies.
We thank Prof. Stephen H. Wright for providing CHO-K1-OCT1 and CHO-K1-OCT2 cells. This research project has been supported by The Thailand Research Fund (TRF, Grant No. IRN58W0004).
The authors declare no conflict of interest.