2020 Volume 43 Issue 12 Pages 1815-1822
2020 Volume 43 Issue 12 Pages 1815-1822
Blood transport proteins are biogenic molecules with unique and interesting inherent characteristics that make up living organisms. As the utilization of their inherent characteristics can be a groundbreaking strategy to resolve and improve several clinical problems, attempts have been made to develop pharmaceutical and biomedical preparations based on blood transport proteins for the treatment and diagnosis of disorders. Among various blood transport proteins, we focus on the immense potential of hemoglobin and albumin to serve as carriers of biomedical gases (oxygen and carbon monoxide) and anticancer agents (low-molecular compounds and antisense oligodeoxynucleotides), respectively, for the development of innovative drug delivery systems (DDS) to treat intractable disorders and solid cancers. In this review, I introduce the pharmaceutical technology, strategies, and application of DDS carriers that have been designed on the basis of the structure and function of hemoglobin and albumin. In addition, the prospect of using hemoglobin and albumin as materials for DDS carriers is discussed.
Several researchers have evaluated blood transport proteins for use as biomaterials in drug delivery systems (DDS) using genetic engineering and biological techniques, such as polymerization, modification, and conjugation. An advantage of this approach is that clinical issues can be resolved or improved by the utilization or application of inherent characteristics of blood transport proteins. On the basis of this approach, our research group focuses on the immense possibilities of two blood transport proteins, hemoglobin and albumin, to develop innovative DDS carriers for the delivery of medical gases, low-molecular compounds, and genes. In this review, I would like to introduce our unique DDS strategies based on hemoglobin and albumin, and their pharmaceutical and biomedical applications for the treatment of intractable disorders and solid cancers. In addition, the prospect of using hemoglobin and albumin as materials for DDS carriers is discussed.
Biological active gaseous molecules, such as oxygen, nitric oxide, carbon monoxide (CO), and hydrogen sulfate, possess physiological activities and play a central pathophysiological role. Recently, exogenously administered physiologically active gaseous molecules were found to exert therapeutic effects against several disorders such as inflammatory disorders and cancer, suggesting that biological gases have great potential as medicinal seeds. However, it is difficult to directly administer these gases into the body, which indicates the need for developing carriers (donors) to achieve the medical application of these gaseous molecules.
Hemoglobin has a unique tetramer structure consisting of four pairs of heme moieties and globin proteins (two alpha globin proteins and two beta globin proteins). In the body, hemoglobin packed in red blood cells plays an essential role in systemically delivering oxygen from the lungs by reversibly binding oxygen to heme moieties. In addition to oxygen, hemoglobin molecules contribute to the delivery of other endogenous gaseous molecules, such as CO. These facts indicate that hemoglobin is an ideal material to serve as a carrier of medical gaseous molecules. However, there are some challenges when using hemoglobin as a material for medical gas carriers from the viewpoint of safety and pharmacokinetics: free hemoglobin induces adverse effects (nephrotoxicity and hypertension),1) and it has a short biological half-life in the blood circulation due to its rapid clearance via CD163 after forming a complex with haptoglobin.2) Therefore, pharmaceutical manufacturing is required to develop a hemoglobin-based biomedical gas carrier.
2.1. Hemoglobin Vesicles (Hb-V)Hb-V have been developed as artificial red blood cell that encapsulate human hemoglobin into liposomes, the surfaces of which are modified with polyethylene glycol (PEG) (Fig. 1). Hb-V is designed to mimic the structure, but not the size, of red blood cells (Hb-V: approx. 250–280 nm, red blood cell: approx. 8000 nm). The cellular structure of Hb-V makes it possible to impart the desired gas-carrying capacity by controlling the co-encapsulation ratio of hemoglobin and an allosteric effector (pyridoxal 5′-phosphate). Although the hemoglobin in Hb-V is purified from outdated red blood cells, contaminations, such as viruses, pathogens, and endotoxins, in the Hb-V suspension are completely eliminated in the preparation process through pasteurization and nanofiltration.3) Furthermore, the Hb-V suspension is guaranteed long-term storage (over 2 years) without the need for restrictive storage conditions (storage at room temperature), while allowing restoration of the structure and oxygen-carrying capacity of the Hb-V.4,5) These characteristics of Hb-V directed us to investigate Hb-V as a medical gas carrier.

Human hemoglobin purified from outdated red blood cell preparation and pyridoxal 5'-phosphate (allosteric effector) are encapsulated into liposomes with a lipid membrane consisting of 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC), cholesterol, 1,5-bis-O-hexadecyl-N-succinyl-L-glutamate (DHSG), and 1,2-distearoyl-sn-glycero-3-phosphatidyl-ethanolamine-N-PEG (PEG-DSPE) at a molar ratio of 5/4/0.9/0.3. The diameter of the Hb-V particles is maintained between 250–280 nm. The Hb-V particles are deoxygenated and suspended in a physiological salt solution for long-term storage and are ready-to-use. (Color figure can be accessed in the online version.)
Hb-V needs to exhibit favorable biocompatibilities to be used as a medical gas carrier. According to numerous animal studies, Hb-V potentially exhibits a favorable safety profile, including blood compatibility (no platelet and complement activation) and lack of tissue damage, nephrotoxicity, and hypertension, even if repeatedly administered at high doses.3) Furthermore, as summarized in Table 1, it exhibited well-defined pharmacokinetic characteristics such as blood retention; distribution, metabolic, and excretion profiles; the influence of accelerated blood clearance phenomenon; and drug-interaction with CYP6–17). On the basis of accumulated evidence, some basic research has been conducted to investigate the possibility that Hb-V can be applied as an oxygen carrier against various ischemic or hypoxic disorders such as hemorrhagic shock,18) brain ischemia,19) and pre-eclampsia.20) In these studies, Hb-V supplied sufficient oxygen to hypoxic areas without any adverse events. Notably, investigator-initiated clinical trial (Phase Ia) on the use of Hb-V as a resuscitative fluid (artificial red blood cells) are being planned for initiation in the winter of 2020.
| Blood retention | ✓ Half-life is approximately 19, 31, 63, and 72 h in mice, rats, rabbits, and cynomolgus monkeys, respectively.6–8) |
| ✓ Half-life in humans is estimated to be 3–4 d.9) | |
| ✓ Blood retention is prolonged in conditions with cirrhosis.10) | |
| Distribution | ✓ Hb-V mainly distributes in the liver and spleen in healthy animals6,8) and in animal models of hemorrhagic shock,9) cirrhosis,11) and hyperlipidemia.12) |
| ✓ Hb-V does not transfer from mother to fetus in pregnant rats.13) | |
| Metabolism | ✓ Hb-V is mainly captured and degraded by the mononuclear phagocyte system in the liver and spleen.6) |
| ✓ Hb-V is completely metabolized even in conditions with hypometabolism.11) | |
| Excretion | ✓ Encapsulated hemoglobin and lipid components of Hb-V are excreted through the same route as the corresponding endogenous substances.6) |
| ✓ Hb-V is completely eliminated from the body within 14 d after administration in healthy animals6) and in animal models of hemorrhagic shock,9) cirrhosis,11) and hyperlipidemia.12) | |
| Others | ✓ Hb-V does not induce the accelerated blood clearance phenomenon even when repeatedly administered at putative doses.14,15) |
| ✓ Hb-V slightly inhibits the metabolic activity of hepatic CYP; however, the metabolic activity recovers within 7 d of administration.16,17) |
Recent evidence suggests that exposure to low concentrations (<500 ppm) of CO can provide beneficial effects such as anti-apoptosis, anti-inflammatory, and anti-oxidative effects in mammalian species,21) incentivizing researchers to develop CO-based pharmaceutical preparations. Under physiological conditions, most of the endogenous CO, which is produced in the body as a byproduct during the metabolism of heme by heme oxygenase,22) is scavenged and carried by hemoglobin inside red blood cells, implying that red blood cells can be utilized as inherent carriers of CO.23) In fact, it has been reported that exogenously supplied CO-bound red blood cells function as CO donors.24) Hb-V can bind to CO by virtue of its similarity in structure and function to red blood cells.25) Furthermore, CO-bound Hb-V can be easily prepared by bubbling pure CO gas through a solution of deoxyHb-V for several minutes (Fig. 2). Additionally, CO-bound Hb-V can gradually release CO.26) These facts lead us to the original strategy for CO-based medicine based on biomimetic DDS in which Hb-V, instead of red blood cells, is used as a CO carrier for the treatment of intractable disorders.

CO-bound Hb-V was prepared by bubbling deoxyHb-V solution with pure CO gas for several minutes because Hb-V possesses the ability to reversibly bind to CO. The physicochemical properties of CO-bound Hb-V, except for the carboxyhemoglobin content, are the same as those of deoxyHb-V. CO-bound Hb-V gradually releases CO after administration and functions as an oxygen carrier after releasing CO. Excess CO is excreted through exhaled air. (Color figure can be accessed in the online version.)
When exogenously exposed, CO acts as a cytoprotective gas at a low concentration and as a toxic gas at a high concentration.21) Therefore, there is concern that adverse biological responses to CO-bound Hb-V administration may be greater than beneficial effects of this treatment. However, in a safety study that evaluated a single administration of high-dose CO-bound Hb-V in healthy mice, all mice survived without any systemic adverse effects of CO, such as hypotension, abnormal behavior, and appearance changes up to 28 d after administration.27) The poisonous effects of CO are generally attributed to cell asphyxiation by CO, which inhibits systemic oxygen delivery by red blood cells. Since the oxygen delivery capacity of red blood cells can be maintained after the administration of CO-bound Hb-V, this treatment did not induce the deleterious effects of CO. Furthermore, the pharmacokinetic evaluation of CO-bound Hb-V in mice and rats showed that CO was cleared from the blood circulation within 6 h after CO-bound Hb-V administration via exhaled air26,28) (Fig. 2). On the basis of these observations, there would be no concern that CO-bound Hb-V exerts CO-derived deleterious effects on the body, at least when a single high dose is administered.
2.5. Application of CO-Bound Hb-V for the Treatment of Intractable DisordersThe benefits of CO-bound Hb-V as a CO donor for the treatment of intractable disorders have been demonstrated using animal disorder models.29) Comprehensive suppression of pathogenesis by CO released from CO-bound Hb-V has been shown to contribute to these therapeutic effects (Fig. 3). We summarize the potent therapeutic effects and mechanisms of action of CO-bound Hb-V in various intractable disorders in the following sections.
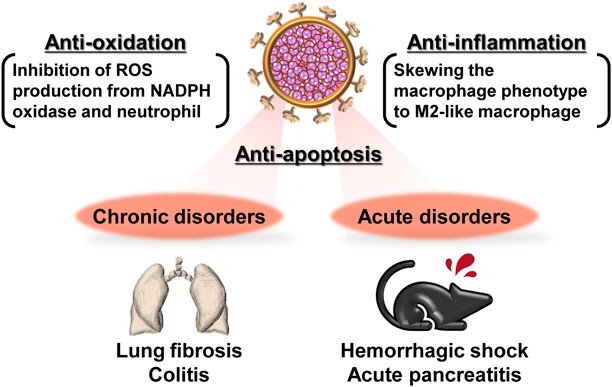
CO-bound Hb-V suppresses the pathogenesis of acute and chronic disorders via comprehensive cytoprotective effects, such as anti-oxidation, anti-inflammation, and anti-apoptosis, by virtue of CO released from CO-bound Hb-V. (Color figure can be accessed in the online version.)
In rats with hemorrhagic shock, CO-bound Hb-V resuscitation, as well as resuscitation with oxygen-bound Hb-V or red blood cells, resulted in the survival of all rats throughout the observation period (up to 6 h), with immediate recovery of hemodynamic parameters (blood pressure and heart rate) and blood gas parameters (atrial oxygen and carbon dioxide partial pressure, pH, base excess, and lactate). This result indicated that CO-bound Hb-V can serve as a novel resuscitative fluid. Furthermore, transfusion with CO-bound Hb-V suppressed the plasma levels of aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase compared to that of oxygen-bound Hb-V and red blood cells. In addition, CO-bound Hb-V transfusion resulted in lower accumulation of oxidation products (nitrotyrosine (NO2-Tyr)) in the organs than transfusion with oxygen-bound Hb-V and red blood cells. These results suggest that CO-bound Hb-V transfusion suppressed systemic ischemia-reperfusion injury following massive hemorrhage via the antioxidative effects of CO released from CO-bound Hb-V.
2.5.2. Lung Fibrosis30)Since the CO in CO-bound Hb-V is excreted from the lungs,28) CO-bound Hb-V could be potentially applied for the treatment of respiratory disorders. In a mouse model of lung fibrosis, which was induced by bleomycin, administration of CO-bound Hb-V delayed the progression of lung fibrosis and restored lung function (forced vital capacity). Quantitative analysis of cells in the bronchoalveolar lavage fluid and inflammatory cytokines in the lungs showed that CO-bound Hb-V treatment significantly suppressed the infiltration or production of these inflammatory cells, which indicated that the anti-inflammatory effects of CO contribute to the prevention of fibrosis development. Furthermore, immunostaining of the lungs showed that the accumulation of oxidation products (8-hydroxy-2′-dioxygenase and NO2-Tyr) were observed in the oxygen-bound Hb-V-treated mice, while it was suppressed in the CO-bound Hb-V treated mice. Since CO-bound Hb-V inhibited the activation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX)-4, a potent source of reactive oxygen species (ROS) in lung fibrosis,31) in the lungs of bleomycin-induced lung fibrosis model mice, the reduction of ROS production via NOX-4 by CO-bound Hb-V apparently contributed to the amelioration of lung fibrosis. These results suggested that CO-bound Hb-V suppressed the progression of lung fibrosis by virtue of the comprehensive cytoprotective effects (antioxidative and anti-inflammatory effects) of CO.
2.5.3. Colitis27)Treatment with CO-bound Hb-V improved the disease activity index (bloody stool, diarrhea, and weight loss), colon shrinkage, histological injury, and survival duration compared to oxygen-bound Hb-V treatment in a mouse model of colitis that was induced by dextran sulfate sodium (DSS). Additionally, CO-bound Hb-V treatment was found to suppress the accumulation of the oxidation product (NO2-Tyr) and infiltration of neutrophils into the colonic tissues in a DSS-induced model of colitis. It has been reported that CO forms a complex with NOX-2,32) a generator of ROS in neutrophils.33) CO-bound Hb-V was shown to suppress NOX-4 activation in a mouse model of lung fibrosis30); therefore, the suppression of oxidative injury by CO-bound Hb-V could result in the inhibition of NOX-2 activation in neutrophils. Interestingly, treatment with CO-bound Hb-V decreased the systemic levels of proinflammatory cytokines (tumor necrosis factor (TNF)-α and interleukin (IL)-6) and increased the systemic level of the anti-inflammatory cytokine (IL-10). Furthermore, the anti-apoptotic effects of CO contributed to the suppression of colitis pathogenesis, according to the observation that treatment with CO-bound Hb-V decreased the number of terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL)-positive cells in the colon of a mouse model of colitis. These findings indicate that the antioxidative, anti-inflammatory, and anti-apoptotic actions of CO can comprehensively contribute to the therapeutic effects of CO-bound Hb-V in colitis.
2.5.4. Acute Pancreatitis26,34)The therapeutic effects of CO-bound Hb-V in acute pancreatitis have been demonstrated by the suppression of increase in the plasma levels of pancreatic enzymes (amylase and lipase) and by an increase in the mortality in mouse models of acute pancreatitis, which were induced by choline-deficient ethionine-supplemented diet or cerulein. Since the administration of CO-bound Hb-V ameliorated the accumulation of oxidation products (NO2-Tyr) and neutrophils in the pancreas, the antioxidative effect of CO-bound Hb-V is believed to inhibit the progression of pancreatitis via the aforementioned mechanism (see 2.5.3 section). Furthermore, CO-bound Hb-V treatment suppressed complications associated with acute pancreatitis, especially distal organ injuries (liver, kidneys, and lungs) following systemic inflammatory response syndrome. This is because the CO-bound Hb-V increased the production of anti-inflammatory cytokines and decreased the production of inflammatory cytokines as follows: CO skews the macrophage phenotype to an M2-like macrophage phenotype, which is associated with the production of anti-inflammatory cytokines,35) as indicated by the observation that CO-bound Hb-V treatment increased the mRNA expression of M2 macrophage markers (CD163, MRC1, or IL-10) in RAW264.7 cells and the pancreas of mice with acute pancreatitis. These results indicate that CO released from CO-bound Hb-V partly exerts systemic anti-inflammatory effects by regulating macrophage polarization, thereby ameliorating both acute pancreatitis and distal organ injuries.
2.6. Future Prospective Pharmaceutical Applications of HemoglobinApart from Hb-V, attempts have been made to develop hemoglobin-based medical gas carriers for the treatment of intractable disorders.36,37) Since the lack of biological active gases, such as oxygen and CO, is associated with the pathogenesis of several disorders, the delivery of these gas molecules by hemoglobin-based preparations holds great potential for advancing the field of medicine. In addition, a few attempts have been made to develop DDS carriers that use hemoglobin as a ligand for targeting macrophages, on the basis of the fact that free hemoglobin is scavenged by macrophages via CD163.38,39) Further evidence is required regarding the efficacy of hemoglobin as a material for DDS carriers.
Albumin is the most abundant protein in the plasma with a unique structure and inherent characteristics: a single thiol group from a cysteine residue at position 34 (34Cys) and two major hydrophilic pockets, namely site I and site II40) (Fig. 4). Utilization of these characteristics of albumin makes it possible to develop albumin-based anti-cancer preparations that address the clinical problems associated with commercially available anti-cancer drugs. A successful example of such preparations is albumin-bound paclitaxel nanoparticles (nab-paclitaxel; Abraxane®), which was approved in 2006 for use in patients with metastatic breast cancer for whom combination chemotherapy had failed.41) Abraxane® accomplishes high solubility of paclitaxel without a solubilizing agent by harnessing the drug-binding characteristic of albumin. This section attempts to introduce albumin-based preparations for cancer therapy with a focus on four types of albumin-based DDS carriers (Fig. 4).
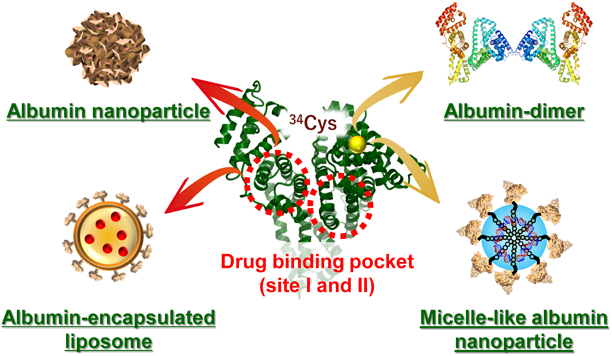
Albumin nanoparticles and albumin-encapsulated liposomes load hydrophobic drugs by utilizing the drug-binding property of albumin. Albumin dimers and micelle-like albumin nanoparticles utilize a highly reactive functional group, thiol, from a free cysteine residue at position 34, to form conjugates. (Color figure can be accessed in the online version.)
Two types of promising albumin dimers have been developed, and they exhibit favorable characteristics for application in cancer therapy.42,43) Komatsu et al. produced a human serum albumin dimer by chemically cross-linking 34Cys of two albumin molecules via 1,6-bis(maleimido)hexane44) (Fig. 5A). Matsushita et al. genetically linked two human serum albumin molecules via an amino acid linker (GGG GS)2 in the Pichia pastoris yeast system.45) Both types of albumin dimers maintain the structural and functional properties of albumin monomers with good biocompatibilities.44,46) Furthermore, the blood retention of both albumin dimers is much longer than that of albumin monomer,45,47) indicating that albumin dimers are expected to deliver anticancer agents to solid tumors via the enhanced permeability retention (EPR) effect. In fact, S-nitrosated albumin dimer, which loaded nitric oxide (anticancer agent), exhibited superior anti-tumor activity to S-nitrosated albumin monomer in colon cancer (C26 cell)-bearing mice by virtue of their higher accumulation at the tumor site.48,49) Moreover, albumin dimers showed sufficient blood retention in doxorubicin-induced nephrotic mice owing to the prevention of glomerular filtration,50) which suggested that albumin dimers can function as anticancer drug carriers and plasma expanders under conditions of hypoalbuminemia and renal failure caused by anticancer drug treatment.

(A) Albumin dimer: Chemically produced albumin dimer is cross-linked through thiol from a free cysteine residue at position 34 of two albumin molecules via a linker (1,6-bis(maleimido)hexane. Genetically produced albumin dimer is crosslinked at the C and N termini of two albumin molecules via an amino-acid linker (GGGGS)2 in the Pichia pastoris yeast system. (B) Albumin-encapsulated liposome: An albumin–drug complex, which is prepared by adding a hydrophobic drug into an albumin solution, is encapsulated into liposomes via the thin-film hydration method. (C) Albumin nanoparticle: A desolvating solution (ethanol) is added into an albumin solution (20 mg/mL) with doxorubicin (0.5 mg/mL) and glutaraldehyde under magnetic stirring at room temperature. (D) Micelle-like albumin nanoparticle: Albumin and cationic polymer (PDMAEMA) are conjugated via covalent binding between maleimide in maleimide-terminated PDMAEMA and thiol from a free cysteine residue at position 34 of albumin. Micelle-like albumin nanoparticle can be fabricated by adding gene into an as-prepared conjugation solution. (Color figure can be accessed in the online version.)
In general, liposomes encapsulate hydrophobic and hydrophilic substances into the lipid membrane and aqueous core of liposomes, respectively. The research concept of albumin-encapsulated liposomes involves encapsulating hydrophobic drugs into the aqueous core of liposomes by utilizing the drug-binding properties of albumin (Fig. 5B). The advantage of this method is that drugs or substances can be stably encapsulated in the aqueous core of liposomes at significant amounts. As expected, albumin-encapsulated liposomes enhanced the encapsulation of hydrophobic substances, such as warfarin, diazepam, paclitaxel, silibinin, and tacrolimus, into the aqueous core of liposomes, resulting in an increase in water solubility of these drugs through their binding to hydrophobic pockets in albumin.51,52) This is because the structure and function of albumin is preserved even after encapsulation into liposomes. In addition, in vivo studies showed that albumin-encapsulated liposomes possessed the ideal characteristics (stealth effect and biocompatibility) to serve as a carrier to target solid tumor; this result directed us to the preparation of paclitaxel-albumin complex-encapsulated liposomes (PTX-Alb liposomes). PTX-Alb liposomes exhibited comparable cytotoxic activity to nab-paclitaxel (Abraxane®) against two-dimensional (2D)- or 3D-cultured pancreatic (AsPC-1) and breast cancer (MCF-7 and MDA-MB-231) cells.53,54) Furthermore, in vivo imaging evaluation using IVIS® imaging system showed that albumin-encapsulated liposomes accumulated at the tumor site in AsPC-1-bearing mice via the EPR effect.53) Through these characteristics, PTX-Alb liposomes demonstrated their superior anticancer effects to nab-paclitaxel in AsPC-1-bearing mice,53) suggesting that albumin-encapsulated liposomes are effective carriers of albumin-binding anticancer agents.
3.3. Albumin NanoparticlesThe desolvation technique is a rapid and straightforward method to prepare protein nanoparticles. Although several researchers have prepared and evaluated the anticancer activity of anticancer agent-loaded albumin nanoparticles in vitro and in vivo,55,56) the optimal conditions for the preparation of anticancer agent-loaded albumin nanoparticles remain unclear. Using doxorubicin as a model anticancer drug, we established the optimal conditions for preparing doxorubicin-loaded albumin nanoparticles for delivery to solid tumor sites via the EPR effect: desolvating agent, ethanol; albumin concentration, 20 mg/mL; pH, 8.5; and doxorubicin concentration, 0.5 mg/mL (Fig. 5C). Under these preparation conditions, the physicochemical characteristics of doxorubicin-loaded albumin nanoparticles are as follows: diameter, approx. 108 nm; polydispersity index (PDI), 0.08; zeta-potential, −35 mV; and entrapment efficacy of doxorubicin, 95.2%.57) Furthermore, in vitro and in vivo evaluation of anticancer effect showed that treatment with doxorubicin-loaded albumin nanoparticles strongly suppressed the growth of tumors in C26 cell-bearing mice compared to free doxorubicin treatment; this observation is associated with the accumulation of doxorubicin-loaded albumin nanoparticles at the tumor site via the EPR effect. Notably, the treatment with doxorubicin-loaded albumin nanoparticles resulted in lower lung metastasis than the treatment with free doxorubicin.58)
3.4. Micelle-Like Albumin NanoparticlesMicelle-like albumin nanoparticles based on a cationic polymer, poly(2-dimethylaminoethyl methacrylate) (PDMAEMA), were designed as a gene carrier.59) This nanoparticle can be prepared in two steps: albumin is covalently conjugated to PDMAEMA through direct linkage between the maleimide-terminated PDMAEMA and the 34Cys of albumin, and in turn, the as-prepared conjugations are mixed with genes59) (Fig. 5D). The resulting nanoparticles condense the gene into the core of the nanoparticle via electrostatic interaction with PDMAEMA, the surface of which is covered with albumin. This unique structure of micelle-like albumin nanoparticles prevents the degradation of genes by nucleases. Furthermore, in vivo 2D and 3D imaging showed that micelle-like albumin nanoparticles accumulated in the tumor site of AsPC-1-bearing mice and reached the core of the tumor. This is because (i) the micelle-like albumin nanoparticles meet the criteria (molecular size, surface charge, biocompatibility, and blood retention) to reach the solid tumor site via the EPR effect, and (ii) the albumin corona facilitates tumor uptake via glycoprotein 60 and secreted protein acidic and rich in cysteine.55) Due to these advantageous characteristics, micelle-like albumin nanoparticles that condense the ISIS5132, which is a phosphorothioate antisense oligodeoxynucleotide inhibitor of c-raf-1 kinase expression with a high anti-cancer activity,60) could suppress the in vivo tumor growth of AsPC-1 compared to ISIS5132 alone.61)
3.5. Future Prospective Pharmaceutical Applications of AlbuminIn addition to Abraxane®, several drugs that utilize the inherent characteristics of albumin have received approval in the market or are in clinical development for the treatment and diagnosis of diabetes (Levemir®), γ-emitting radionuclide imaging (Nanocoll®), and hepatitis C infection (Albuferon™).42) Therefore, albumin-based nanocarriers, not limited to cancer therapy, can be applied for the treatment and diagnosis of various intractable disorders. In the future, we hope to test the usefulness and efficacy of albumin-based nanocarriers for the treatment of other intractable disorders.
I would like to acknowledge Dr. Masaki Otagiri; Dr. Toru Maruyama; the laboratory members of the Department of Biopharmaceutics, Graduate School of Pharmaceutical Sciences, Kumamoto University; and Laboratory of Pharmacokinetics, Faculty of Pharmaceutical Science, Sojo University for their valuable contributions to this work. I also thank Dr. Hiromi Sakai, Department of Chemistry, Nara Medical University; Dr. Teruyuki Komatsu, Department of Applied Chemistry, Faculty of Science and Engineering, Chuo University; and Dr. Martina Stenzel, Center for Advanced Macromolecular Design, School of Chemistry, The University of New South Wales for their valuable advice and kind consideration.
The author declares no conflict of interest.
This review of the author’s work was written by the author upon receiving the 2020 Pharmaceutical Society of Japan Award for Young Scientists.