2020 Volume 43 Issue 2 Pages 221-229
2020 Volume 43 Issue 2 Pages 221-229
Low molecular weight metabolites produced by the intestinal microbiome that have been associated with health and disease as metabolites need to be constantly absorbed from the intestinal lumen and transported to intestinal epithelial cells and blood. Polyamines, especially spermidine and spermine, are bioactive chemicals which promote autophagy and suppress inflammation. The main source of exogenous polyamines is the intestinal lumen, where they are produced by intestinal microbiome. Considering the intestinal microbiome as a manufacturing plant for bioactive substances, we developed a novel hybrid putrescine biosynthesis system strategy, in which the simultaneous intake of Bifidobacterium animalis ssp. lactis LKM512 (Bifal) and arginine (Arg) upregulates the production of the putrescine, a precursor of spermidine and spermine, in the gut by controlling the bacterial metabolism beyond its vast diversity and inter-individual differences. In a clinical trial, healthy individuals with a body mass index near the maximum “healthy” range (25 kg/m3; n = 44) were randomized to consume either normal yogurt containing Bifal and Arg (Bifal + Arg YG) or placebo (normal yogurt) for 12 weeks. The change in reactive hyperemia index determined by EndoPAT from week 0 to 12 in the Bifal + Arg YG group was significantly higher than that in the placebo group, indicating that Bifal + Arg YG intake improved vascular endothelial function. In addition, the concentrations of fecal putrescine and serum spermidine in the Bifal+ Arg YG group were significantly higher than those in the placebo group. These findings suggest that consuming Bifal + Arg YG prevents or reduces atherosclerosis risk by upregulating blood spermidine levels, which subsequently induces autophagy.
Low molecular weight metabolites produced by the intestinal microbiome are absorbed from the intestinal lumen and carried into systemic circulation. Among these metabolites, bioactive metabolites play a direct role in health and disease. The production of a bioactive substance by the intestinal microbiome is expected to be continuous, and will increase the total supply, compared to temporally providing the bioactive substance as an oral dose. However, few studies have attempted to produce bioactive metabolites using the microbiome. We previously found a very complex pattern of bacterial metabolites. Briefly, metabolomics with capillary electrophoresis/time-of-flight mass spectrometry (CE-TOFMS) identified 179 metabolites from the colonic luminal metabolome derived from mice,1) while 700 to 1500 unidentified metabolites were detected per individual. Therefore, it is very difficult to upregulate the production of a targeted bioactive metabolite in the intestinal lumen, since bacterial metabolism in this niche featuring vast diversity and inter-individual differences would need to be regulated.
Our research of the past 20 years has focused on polyamines as interesting bioactive metabolites selected from the metabolome produced by intestinal microbiome. Polyamines (for example, putrescine, spermidine, and spermine) are important metabolites of intestinal bacteria, and putrescine is present in the intestinal lumen at concentrations of 0.5 to 1 mM in healthy humans.2,3) Polyamines are required for cell growth and proliferation, and their intracellular production and concentration in tissues and organs decreases with age.4–6) Spermidine is a promising antiaging intervention.7) Our group has explored the relationship between polyamines and the prevention of senescence with the ultimate aim being to create novel foods and/or technologies that facilitate bacterial putrescine production to increase the polyamine levels in individuals who have weakened polyamine biosynthesis systems.
Polyamines (putrescine, spermidine, and spermine) are ubiquitous low-molecular weight organic cations found in all organisms. They modulate the functions of RNA, DNA, proteins, and other acidic substances.8,9) Polyamines are biosynthesized from the precursor ornithine, in the order of putrescine, spermidine, and spermine in mammalian cells, and cellular polyamine content is strictly regulated by several key enzymes in healthy cells (Fig. 1). Cellular polyamines have several important functions, including as ion channels, in cell–cell interactions, within the cytoskeleton, in signaling via phosphorylation and other mechanisms, and in the activity of eukaryotic initiation factor-4A (eIF4A) via spermidine as a precursor for hypusination, transcription, mRNA translation, and autophagy.9,10) Various physiological activities depend on the magnitude of their polarity, in which putrescine, spermidine, and spermine are mild/weak, moderate, and strong, respectively (Fig. 1).
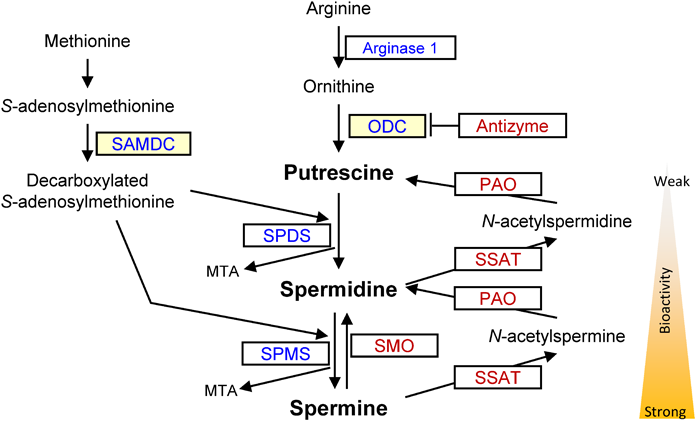
Enzymes are denoted in squares and those related to synthesis and degradation/inhibition are shown in blue and red, respectively. S-Adenosylmethionine decarboxylase (SAMDC) and ornithine decarboxylase (ODC) are rate-limiting enzymes in polyamine synthesis. SAMDC decarboxylates S-adenosylmethionine to the decarboxylated S-adenosylmethionine (dcSAM) so that dcSAM can provide aminopropyl groups to putrescine to produce spermidine by spermidine synthase (SPDS), and are added to spermidine to produce spermine by spermine synthase (SPMS). Spermine can be directly recycled to spermidine by spermine oxidase (SMO). Spermine and spermidine can be recycled to spermidine and putrescine, respectively, by spermidine/spermine-N1-acetyltransferase (SSAT) followed by oxidation by polyamine oxidase (PAO) through N-acetylspermine and N-acetylspermidine, respectively. Antizyme works as an ODC inhibitor under negative feedback regulation by polyamines. MTA: methylthioadenosine. The strength of bioactivity in descending order is spermine, spermidine, and putrescine. (Color figure can be accessed in the online version.)
Polyamines have many biological functions related to health and disease. Polyamines have strong cellular protective functions against environmental stress through antioxidant activity, antimutagenicity, and the inhibition of aberrant gene methylation.11,12) Polyamines suppress inflammation by inhibiting inflammatory cytokine synthesis in macrophages13) and the expression of leukocyte function-associated antigen-1 (LFA-1).14) Polyamines also induce the maintenance of an intestinal mucosal layer15) including the synthesis of E-cadherin16) and occludin,17) which are adhesion molecules of the intestinal epithelial tight-junction, and mucosal repair from gut injury.18,19) Collective findings indicate that polyamines are indispensable substances for healthy mammals. Intracellular biosynthesis of polyamines, especially spermidine, and their concentration in several organs decrease with aging in rodents, in organs including the heart, liver, thymus, kidney, spleen, brain, and skeletal muscle.4–6) In humans, the blood levels of polyamines are higher in centenarians (90–106 years old) than in individuals in their sixties to eighties, and are similar to those of individuals in their thirties to fifties,20) suggesting that individuals with high body polyamine levels may potentially live longer and survive to be centenarians.
Based on these collective facts, several teams, including ours, have suggested that the extension of healthy life expectancy, including the prevention of cardiovascular diseases, can be expected if exogenous polyamines are supplied to the body when the ability to synthesize polyamines begins to decline. We have obtained interesting supportive data for this at the organism level in the last 10 years. For example, an extension of lifespan via the oral administration of polyamines, especially spermidine, in model organisms, such as worms,21) fruit flies,21) and mice22) has been reported. Our team also found that the upregulation of colonic luminal polyamines produced by the intestinal microbiota delays senescence in mice.23,24) Spermidine supplementation protected against age-induced memory impairment by enhancing autophagy in aging fruit flies.25) Recent experiments using a mouse model revealed the extension of lifespan, along with cardioprotection26) and the prevention of liver fibrosis and hepatocellular carcinoma,27) by the activation of autophagy with the oral administration of spermidine. These findings were introduced in the review article in a high impact journal.10)
However, high levels of polyamines are also observed in tumors, and the selective inhibition of ornithine decarboxylase, a key enzyme in polyamine synthesis (Fig. 1), is effective against malignancies, including colon cancer.28,29) Hence, whether polyamines are harmful or beneficial for health remains controversial. Our target has been normal cells in healthy mammals. Sequential experiments basis on the hypothesis that a continuous supply of polyamines potentially promotes a healthy life are discussed in this review.
There are decisive differences between polyamines and many other food-derived functional substances. Most other functional candidates are not synthesized in the human body. Polyphenols are synthesized in plants, whereas polyamines are ubiquitous bioactive substances present in all organisms, including humans. Polyamines have markedly higher affinity for our bodies than other functional candidates because our body has absorption, transport, and degradation systems. Thus, there are no problems of absorption and overdose that have hindered the practical application of many other functional foods.
Most dietary polyamines are absorbed in the small intestine.30) We previously compared the polyamine concentration in colonic contents derived from germ-free mice and microbiome-colonized ex-germ-free mice, and confirmed that most of the putrescin and spermidine in the colonic lumen are produced by the gut microbiome.1) We also confirmed that putrescine is the most abundant polyamine in human intestinal lumen, that the fecal level of polyamine in elderly people is lower than the level in healthy adults, and that this level depends on the bacterial composition of the intestinal microbiome.2) Based on our fecal polyamine level data obtained from 230 Japanese individuals, including previous data2,3) (includes unpublished data that are being updated), and a 400 mL colonic volume,31) we determined an approximate colonic luminal level of total polyamines of approximately 300 µmol in humans. This value is much higher than polyamine levels per day derived from polyamine-rich food calculated using data on polyamine levels in food items.6) For example, approximately 50, 22, and 61.5 µmol polyamines are obtained from 50 g fermented soybean, 50 g blue cheese, and 100 g broccoli, respectively, indicating that the level of potent polyamines produced by the intestinal microbiome is more than 6-times higher than these polyamine-rich foods. Therefore, we hypothesized that if bacterial polyamine production can be induced by controlling the metabolism of the intestinal microbiome, polyamines can be produced continuously and at a higher level compared to diet-derived polyamines, and therefore, that the increased intake of intestinal bacterial polyamines could prolong lifespan. As described later, and as predicted by this hypothesis, the upregulation of intestinal bacterial polyamine (putrescine) production promotes longevity in mice and improves vascular endothelial function in humans.
Probiotics are defined as live microbial food supplements that confer a health benefit to the host by improving intestinal balance,32) such as by influencing the composition of metabolites derived from the intestinal microbiome. Supplementation of Bifidobacterium animalis ssp. lactis (strain LKM512) increased fecal polyamine concentration in small-scale clinical trials.33,34) However, the increase in polyamine concentration upon ingestion of B. animalis ssp. lactis varied between individuals, and mainly ranged from 20 to 2000 mM. Moreover, total polyamine levels did not increase at all in certain individuals. Further, although we searched for polyamine-producing bacteria using approximately 300 isolates obtained from human feces, by using polyamine-free agar including Bacteroides intestinalis,35) Bacteroides finegoldii,36) and Bacteroides dorei37) that were discovered as new species in these studies, no isolate displayed stably increased polyamine concentration with high reproducibility in human fecal cultures. These results indicate that the ingestion of a single bacterial strain, including this bifidobacterial strain per se, is insufficient to elevate the intestinal polyamine concentration. Therefore, we shifted our search to substances that stably induce polyamine production/release in the intestinal microbiome.
4.2. Arginine: A Chemical That Upregulates Polyamine Production in the Intestinal MicrobiomeIn a study designed to search for the aforementioned substances, and in order to remove the influence caused by individual diet on fecal metabolome, identical meals were prepared. This was done based on the findings from a preliminary study that showed the fecal metabolome is greatly affected by food ingredients. Fecal samples from 12 volunteers who were served identical meals were used for metabolomic analysis. Hijiki seaweed, an indigestible ingredient, was served for breakfast on day 4 to measure the food retention time in the digestive tract. Fecal part that detected seaweed was analyzed by metabolomics using CE-TOFMS, which detected 221 metabolites. The metabolites that correlated with levels of putrescine, which is the most abundant polyamine in human feces, as well as precursors of spermidine and spermine, which have strong bioactivity, were determined. Arginine (Arg) was selected among several candidates by screening using fecal cultivation.24) The effect of Arg was present in all fecal samples despite large individual differences in the intestinal microbiome. Oral administration of Arg to mice increased putrescine concentration in a dose-dependent manner (Fig. 2a). In contrast, antibiotic treatment before Arg ingestion completely eliminated the effect of Arg on putrescine production. Using a jugular catheter, changes in blood polyamine concentrations were measured in rats (Fig. 2b). Blood spermidine concentration increased at 4 and 8 h, and spermine concentration tended to increase at 8 h after oral Arg administration. However, putrescine concentration was very low and did not change, indicating that putrescine produced by the intestinal microbiome is transported into the blood and rapidly biosynthesized to spermidine and spermine. Following the injection of Arg through a proximal colon catheter, fecal putrescine concentrations increased; this was observed when Arg intake was more than 4.4 mg/kg of body weight, indicating that this dose of Arg supplementation (approximately 250–300 mg/60 kg of human) can feasibly be applied to humans by a specific coating technique. In another experiment, in which mice between 12 and 18 months of age received a 6-month treatment, putrescine production and the reduction of serum proinflammatory cytokines were enhanced by the oral administration of a combination of Arg and B. animalis ssp. lactis (LKM512) compared with Arg alone or B. animalis ssp. lactis alone. Thus, mice orally administered arginine in combination with the B. animalis ssp. lactis long-term (for 12 months, from age 14 months to 26 months) showed suppressed inflammation and extended longevity24) (Fig. 2c).
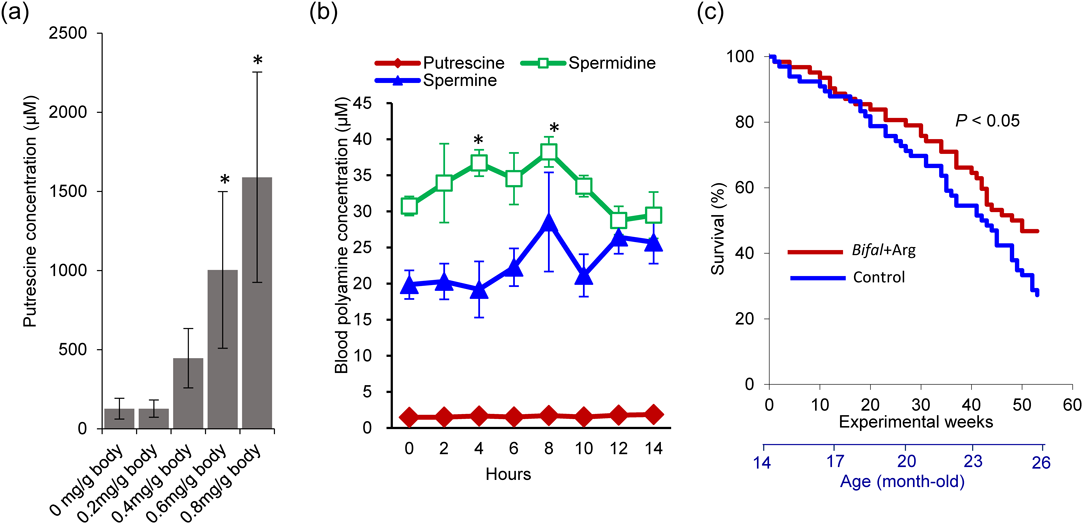
(a) Fecal putrescine concentration after oral administration of Arg in ICR mice (n = 8, 9-week-old); * p < 0.05 (vs. 0 mg/body). (b) Changes of blood polyamine concentration after oral Arg administration in rats with a jugular catheter (n = 5, 9-week-old); * p < 0.05 (vs. pre-treatment: 0 h). (c) Long-term oral administration of both Bifal and Arg delays aging in ICR mice. The mix was administered three times a week to mice beginning at 14 months of age (female Arg + Bifal group: n = 62, female control group: n = 66). Error bars represent standard error of the mean (S.E.Ms.). (Color figure can be accessed in the online version.)
Scientific evidence is essential for the use of a functional food to prevent disease. Thus, the intestinal bacterial biosynthesis pathway involved in the production and release of putrescine from Arg must be determined. Putrescine is biosynthesized by bacterial cells from agmatine, which is produced by the decarboxylation of Arg by the arginine decarboxylases SpeA and AdiA. Two metabolic pathways for the conversion of agmatine to putrescine have been reported. The first is the agmatine ureohydrolase (SpeB) pathway, where agmatine is directly converted to putrescine. The second is the N-carbamoylputrescine pathway, which involves the production of N-carbamoylputrescine from agmatine by agmatine iminohydrolase, followed by its conversion to putrescine by N-carbamoylputrescine amidohydrolase. In silico genome analysis of the 56 most abundant bacterial species in the human microbiome has predicted that many species are unable to produce putrescine from arginine using their own enzymes, due to incomplete synthetic pathways,38) suggesting the existence of a metabolic pathway spanning multiple bacterial species in the gut. Thus, we analyzed extracellular polyamines and polyamine intermediates in fecal cultures grown in the presence of isotope-labelled Arg using CE-TOFMS. This analysis demonstrated that putrescine is indeed produced and released through multiple pathways in the microbiome, with extracellular intermediates exchanged between bacterial species.39)
Next, we searched the collective metabolic pathway of the intestinal microbial community to produce putrescine from Arg. Surprisingly, putrescine concentration in the culture supernatant from a mixed microbial culture, including 14 species from the four main phyla (Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria) in the human intestine in medium containing Arg, was more than 40 times higher than the average concentration for each species cultured individually (Fig. 3a). To determine which bacteria were responsible for the induced putrescine production, we analyzed the putrescine concentration in medium from cocultured pairs of bacterial species. The highest concentration was found in medium from mixed Escherichia coli and Enterococcus faecalis coculture. This concentration was over 8 times higher than that observed when either species was cultured separately. This putrescine production in mixed culture was enhanced when the pH of the medium was weakly acidic (pH 5.0 to 6.5), suggesting that the induction of putrescine production might be attributed to a bacterial acid resistance system. Additionally, En. faecalis produced putrescine in the supernatant from the E. coli culture, although E. coli alone did not produce putrescine in the supernatant from the En. faecalis culture. During the cultivation of E. coli, Arg decreased and agmatine increased. When spent medium from E. coli cultivation was used to culture En. faecalis, agmatine decreased while putrescine increased. Based on these results and data in the literature, we proposed a new hypothesis of putrescine production by sequential reactions in E. coli and En. faecalis (Fig. 3b, a part of E. coli and En. faecalis). This hypothesis implies that the acid resistance system (using adiA and adiC) of E. coli, and the energy (ATP) production system (using aguA and aguD) of En. faecalis, can combine, resulting in induced putrescine production. Notably, agmatine is an intermediate between Arg and putrescine in these pathways. To confirm this, we prepared genetically engineered E. coli and En. faecalis. When adiA-deleted E. coli was cocultured with wild-type En. faecalis, putrescine production was abolished. Similarly, the coculture of adiA-complemented E. coli and wild-type En. faecalis completely restored putrescine production. Almost the same findings were obtained in the case of adiC of E. coli and aguD of En. faecalis, validating our hypothesis.40)
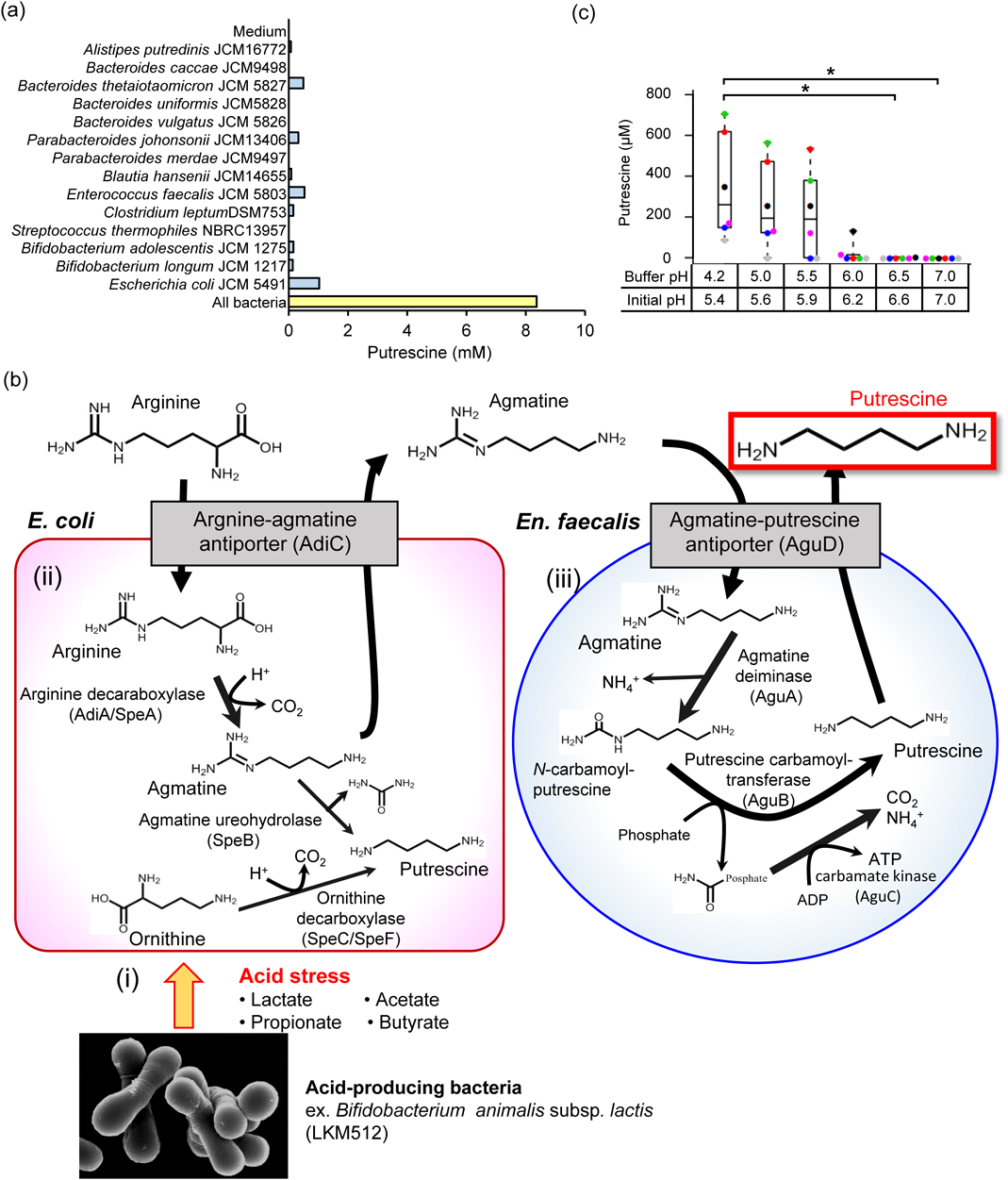
(a) Induction of putrescine production by mixed microbial cultures. Extracellular putrescine concentrations in cocultures and individual cultures of 14 bacterial strains in GAM medium supplemented with 2 mM arginine. (b) Mechanistic model of a novel pathway for putrescine production from arginine through agmatine involving the collaboration of three different bacterial species. (i) This pathway is triggered by environmental acidification due to acetate and lactate produced by acid-producing bacteria, represented by B. animalis ssp. lactis LKM512. (ii) In the second step, the acid–tolerance system of E. coli (arginine-dependent acid resistance system) is activated by acidic stress to exchange agmatine inside the cell for arginine present outside. (iii) In the third step, the energy production system of En. faecalis (agmatine deiminase system) is activated to exchange putrescine inside the cell for agmatine present outside (owing to the acid-tolerance system of E. coli). Putrescine is a byproduct of the collaboration between these different bacterial species. Abbreviations: Arg, arginine; Agm, agmatine; Put, putrescine; AdiA, arginine decarboxylase; AdiC, arginine/agmatine antiporter; AguA, agmatine deiminase; AguB, putrescine carbamoyltransferase; AguC, carbamate kinase; AguD, agmatine/putrescine antiporter. (c) Extracellular putrescine concentration in human feces incubated at different pH values. The same sample is represented by the same color; * p < 0.05. (Color figure can be accessed in the online version.)
It is still unclear why the oral administration of the mixture of Arg and B. animalis ssp. lactis results in a stronger induction of putrescine production than a single administration of each supplement. The likely answer is that acidification of the environment by B. animalis ssp. lactis triggers this putrescine production system. To test the effect of the environment created by B. animalis ssp. lactis on putrescine production in vivo, gnotobiotic mice were colonized with E. coli and En. faecalis, with or without B. animalis ssp. lactis. Comparing the fecal pH in the mice colonized with only E. coli and En. faecalis, the fecal pH in the mice colonized with B. animalis ssp. lactis was significantly lower. Additionally, in the gnotobiotic mice colonized with all three of these bacteria, the putrescine concentrations were significantly higher than those in the mice colonized with only E. coli and En. faecalis.
In conclusion, we identified a novel pathway for putrescine production from arginine through the agmatine intermediate involving the independent metabolic systems of three types of bacteria (the “hybrid putrescine biosynthesis system”) in the gut. This pathway consists of independent strategies of the individual contributing bacteria, which involve commensal bacteria possessing the arginine-dependent acid resistance system, represented by E. coli; commensal bacteria possessing agmatine deiminase-associated energy production system, represented by En. faecalis; and the acid-producing bacteria including probiotics, represented by B. animalis ssp. lactis40) (Fig. 3b).
4.4. Universality of This Biosynthetic Pathway in the Human IntestineBecause En. faecalis is a dominant bacterial species in humans, it is expected to play a significant role in putrescine production in the intestinal lumen. However, E. coli is a relatively minor species in the intestine. However, we confirmed that putrescine production is induced by collaboration with En. faecalis and other intestinal bacterial species, such as Fusobacterium varium, Citrobacter youngae, etc., which possess homologs of AdiA and AdiC, as determined by in silico search. To confirm the expression of genes related to the hybrid putrescine biosynthesis system in the colonic lumen of humans, we analyzed the number of RNA sequences of these genes using previously reported metatranscriptome data obtained from eight human feces samples.41) Expression of the adiA, adiC, and aguA genes derived from various bacterial species was found in all fecal samples, indicating that these three key genes of the hybrid putrescine biosynthesis system are universally activated in the human intestine. In fact, by suspending the culturing human feces in various pH buffers, the putrescine concentrations increased in most samples at pH <5.940) (Fig. 3c). Therefore, there is a strong possibility that this system works in the human intestine by simultaneous supplementation of Arg and B. animalis ssp. lactis LKM512. This novel strategy appears to provide polyamines to the body by controlling intestinal bacterial metabolism, irrespective of the vast diversity and inter-individual differences.
Several articles have indicated that oral supplementation with polyamines is effective in preventing cardiovascular diseases. Soda et al.42) reported that negatively associated dietary polyamines with cardiovascular disease mortality using data gathered from the WHO and the International Monetary Fund in 48 different European and other Western countries. Eisenberg et al.26) reported that the intake of dietary spermidine (or that of spermidine and spermine combined) was inversely correlated with the incidence of cardiovascular disease and death in the Bruneck cohort. Inflammation is a factor in the onset and development of atherosclerosis. Polyamines, especially spermine, inhibit inflammatory activity by suppressing proinflammatory cytokine synthesis in macrophages.13) Spermine suppresses LFA-1, which binds to endothelial cells through interaction with Intercellular Adhesion Molecule 1 (ICAM-1), and is involved in the transmigration of inflammatory cells to sites of inflammation on human lymphocytes.14) LaRocca et al.43) reported that spermidine exerts a potent anti-aging influence on arteries by increasing nitric oxide bioavailability, reducing oxidative stress, modifying structural factors, and enhancing autophagy. Recently, it was reported that cardiovascular diseases, including atherosclerosis, can be prevented by autophagy that is induced by spermidine in rodent experiments. Eisenberg et al.26) found that oral spermidine supplementation extended the lifespan of mice, exerted cardioprotective effects, reduced systemic blood pressure, and induced a delay in the progression to heart failure by enhancing autophagy in aged mice and salt-sensitive hypertensive rats. Michiels et al.44) reported that oral spermidine supplementation reduced the formation of atherosclerotic plaques by inducing autophagy in apolipoprotein E-deficient (ApoE − /−) mice. Furthermore, it is known that polyamines exhibit antagonist action in platelet aggregation.45) Together, exogenous polyamine supplementation via intestinal microbiome is also likely effective in preventing atherosclerosis.
5.2. Clinical TrialWe evaluated the effects of putrescine produced by the intestinal microbiome, following B. animalis ssp. lactis and Arg administration, on vascular endothelial dysfunction in a clinical trial.46) Vascular endothelial dysfunction is a very early symptom of arteriosclerosis, and the disease is considered recoverable at this stage by intervention or improvement of lifestyle habits.
Healthy individuals with a body mass index near the maximum value of the “healthy” range (25 kg/m3; n = 44, average age 45 years) consumed normal yogurt containing B. animalis ssp. lactis and Arg (Bifal + Arg YG) or placebo (normal yogurt alone) for 12 weeks in this randomized, double-blind, placebo-controlled, parallel-group comparative study. The primary outcome was the vascular endothelial function, which was expressed as reactive hyperemia index (RHI) measured with an EndoPAT 2000 system (Itamar Medical, Caesarea, Israel).
At the end of the 12-week period, the change in RHI in the Bifal + Arg YG group was significantly higher than that in the placebo group (Fig. 4a). Furthermore, the RHI increased significantly in the Bifal + Arg YG group, but not in the placebo group (Fig. 4b). RHI values <1.67 indicate vascular endothelial dysfunction.47,48) Here, the RHI increased from 1.50 (week 0) to 1.81 (week 12) after Bifal + Arg YG consumption, suggesting that Bifal + Arg YG intake restored vascular endothelial function to a normal (safe) level. To support the improvement of vascular endothelial function, the change in serum platelet concentration during the clinical test in the Bifal + Arg YG group was significantly lower (i.e., improved) than that in the placebo group (Fig. 4c), and the systolic blood pressure at week 12 in the Bifal + Arg YG group tended to be lower than that in the placebo group, although there had been no difference between the two groups at week 0. The improvements in platelet level and blood pressure were probably caused by the decrease in platelet aggregation frequency due to the suppression of rupture/disruption of plaques and the restoration of vascular flexibility, respectively. In addition, as expected, the Bifal + Arg YG group had a significantly higher fecal putrescine concentration (no effect in fecal spermidine) and, at the same time, a significantly higher serum spermidine concentration than the placebo group (Fig. 4d). Colonic cells can absorb putrescine,49) which is utilized for spermidine and spermine biosynthesis.50) Therefore, the improvement in endothelial function might be mediated by a putrescine-induced increase in serum spermidine biosynthesis. The data indicate that Bifal + Arg YG intake induces microbial putrescine production. The putrescine is absorbed from the intestinal lumen and transported in the bloodstream. Consequently, spermidine is biosynthesized, which promotes autophagy in vascular endothelial cells, thereby improving vascular endothelial function.
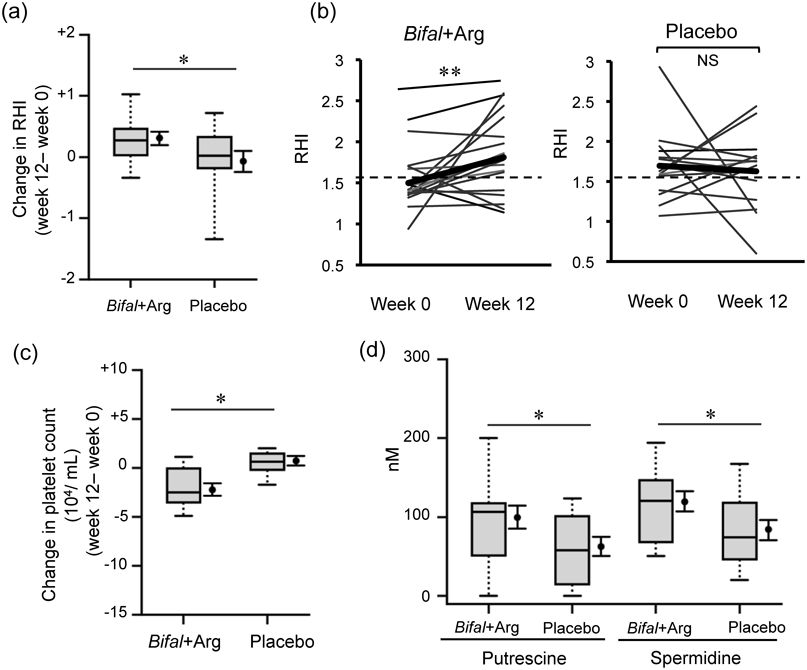
Boxplots represent the 5th percentile, 95th percentile, interquartile range (25–75%), and median. The black circle and error bars on the side of the boxplots represent the average value and SEM, respectively. (a) Change in RHI during the clinical test period (* p < 0.05 by two-way ANOVA). (b) Change in RHI within each group. The bold line and horizon dot line represent the average value and RHI 1.67 used as a value of vascular endothelial dysfunction, respectively; ** p < 0.01 by paired t-test, NS: Not significant. (c) Change in platelet count during the clinical test period; * p < 0.05 by two-way ANOVA. (d) Serum putrescine and spermidine concentrations at week 12; * p < 0.05, Student’s t-test.
Regarding fecal microbiota at week 12, the relative abundance of Citrobacter and Escherichia/Shigella, representing bacteria possessing an Arg-dependent acid resistance system (using adiA and adiC),40) and Enterococcus, representing bacteria possessing an energy production system (using aguA and aguD),40) was higher in the Bifal + Arg YG group than in the placebo group, indicating that Bifal + Arg YG intake might enhance the intestinal microbiota composition, making it more suitable for putrescine production by acid production of B. animalis ssp. lactis.
Multiple clinical studies have reported the efficacy of probiotics in improving the blood lipid profile.51) However, to our knowledge, the direct improvement of endothelial function by probiotics has not been reported. Therefore, this new technology (Bifal + Arg YG) is the first functional food to demonstrate efficacy in improving endothelial function during the early process of atherosclerosis development in humans. Controlling intestinal bacterial metabolites is a novel strategy to maintain host health. While it is challenging to control bacterial metabolites because of the vast individual differences in human microbiomes and diets, the yogurt supplementation strategy was effective in controlling target metabolites derived from intestinal microbiota and enhancing vascular endothelial function via altered physiological activity (autophagy). The data provide insights into the development of atherosclerosis, and highlight a novel treatment option for at-risk patients.
My thanks to Dr. Yoshimi Benno (RIKEN) for constructive comments and warm encouragement. I would also like to express my gratitude to Dr. Shin Kurihara (Kindai University) for many discussions in a series of studies, including the provision of genetically engineered bacteria. I would like to thank Dr. Yuji Naito (Kyoto Prefectural University of Medicine, Kyoto) for insightful comments and suggestions during the clinical trial. I am deeply grateful to Human Metabolome Technologies, Inc. (Tsuruoka, Japan) and imeQ RD Co., Ltd. (Tokyo, Japan) for generous support in fecal metabolomics, and for their enthusiastic work during the clinical trial, respectively. Special thanks to Mr. Koji Muramatsu, Mrs. Emiko Sawaki, Dr. Ayano Yamashita, Mr. Atsuo Nakamura, Mr. Yusuke Kitada, and Dr. Yumi Shimomura (Laboratories of Kyodo Milk Industry Co., Ltd.) for performing each part of experiment. Part of this study was supported by the program for the Promotion of Basic and Applied Research for Innovations in Bio-oriented Industry by the Bio-oriented Technology Research Advancement Institution, NARO, Japan.
M. Matsumoto is an employee of Kyodo Milk Industry Co., Ltd., which funded part of this research and supplies commercial products (yogurt) related to the content of this study.